Functional Profiling of the A-Family of Venom Peptides from the Wolf Spider Lycosa shansia
Abstract
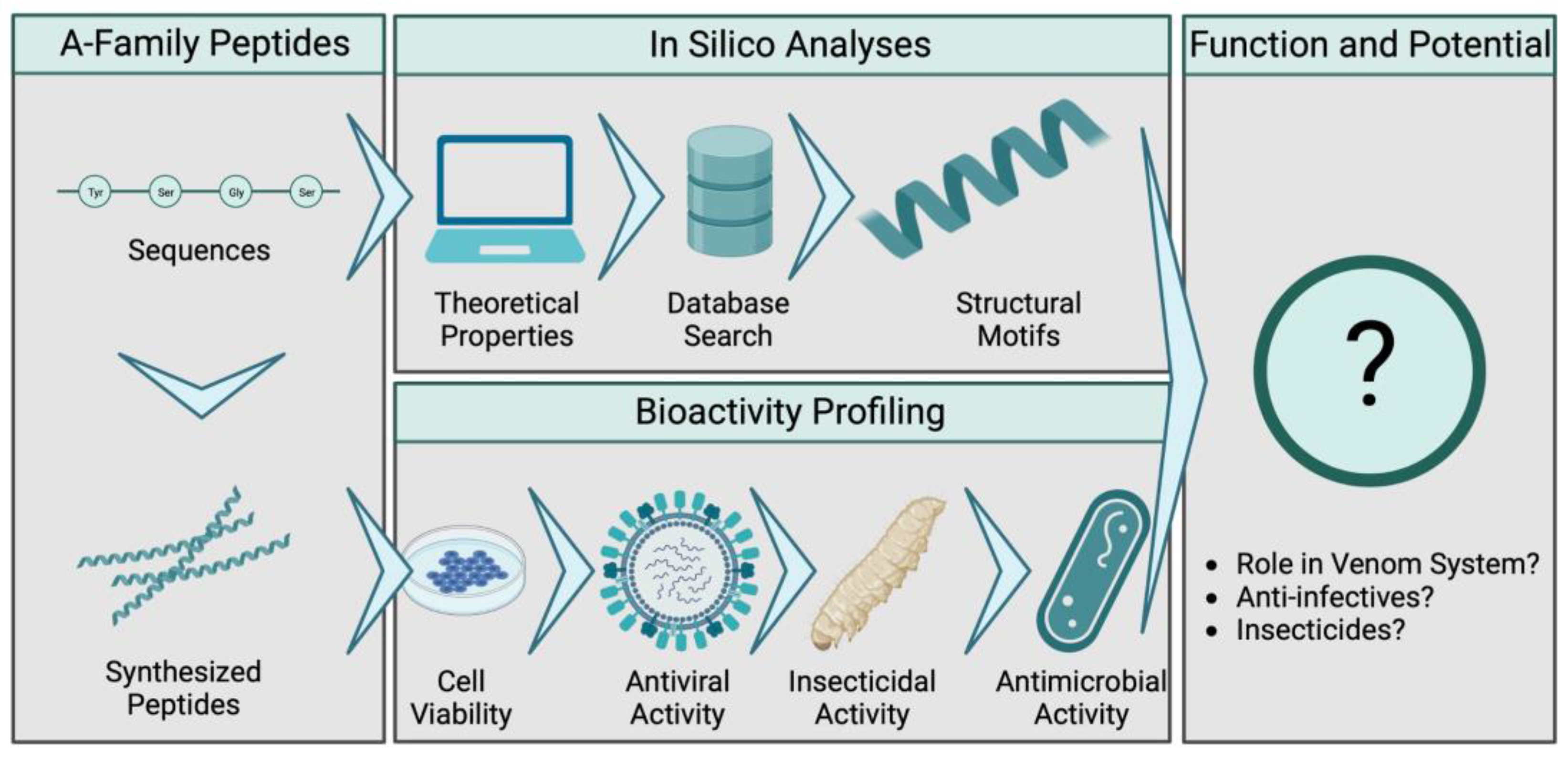
1. Introduction
2. Results
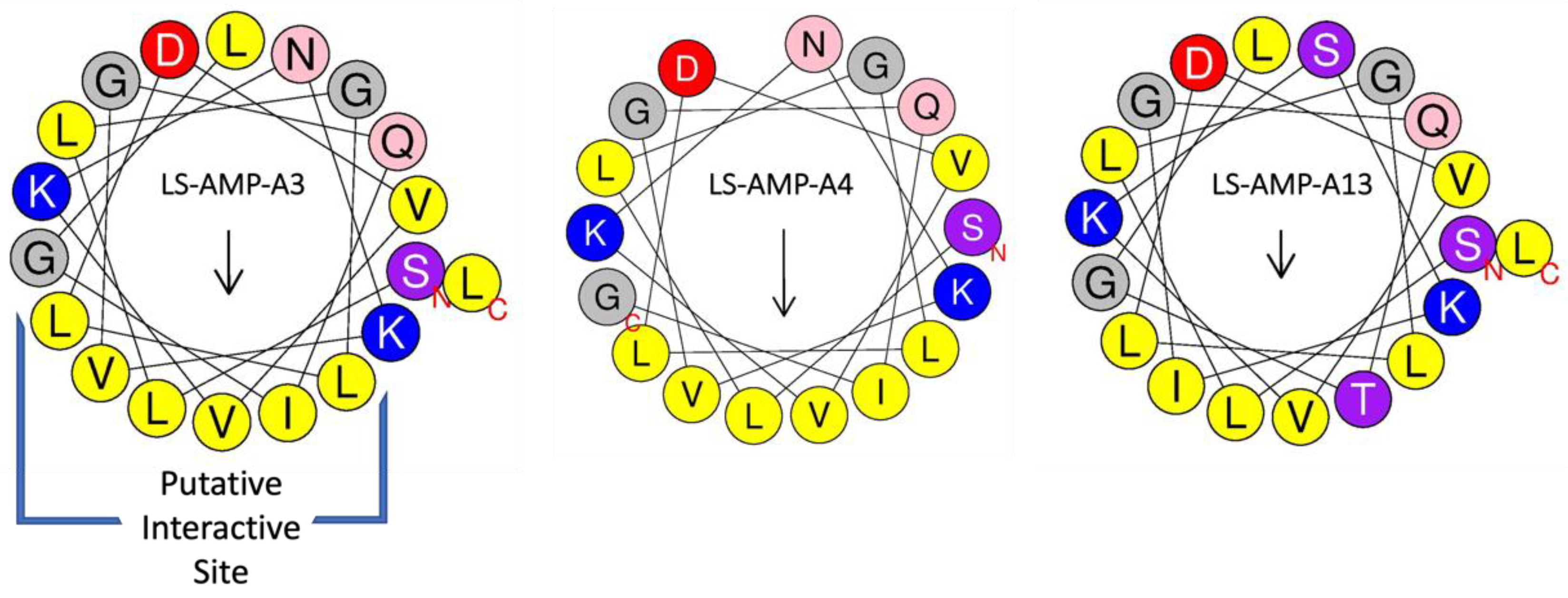
2.1. Physicochemical Properties and Structures of A-Family Peptides
2.2. A-Family Peptides Resemble AMPs from Frog Poisons
2.3. Evaluation of Cytotoxicity
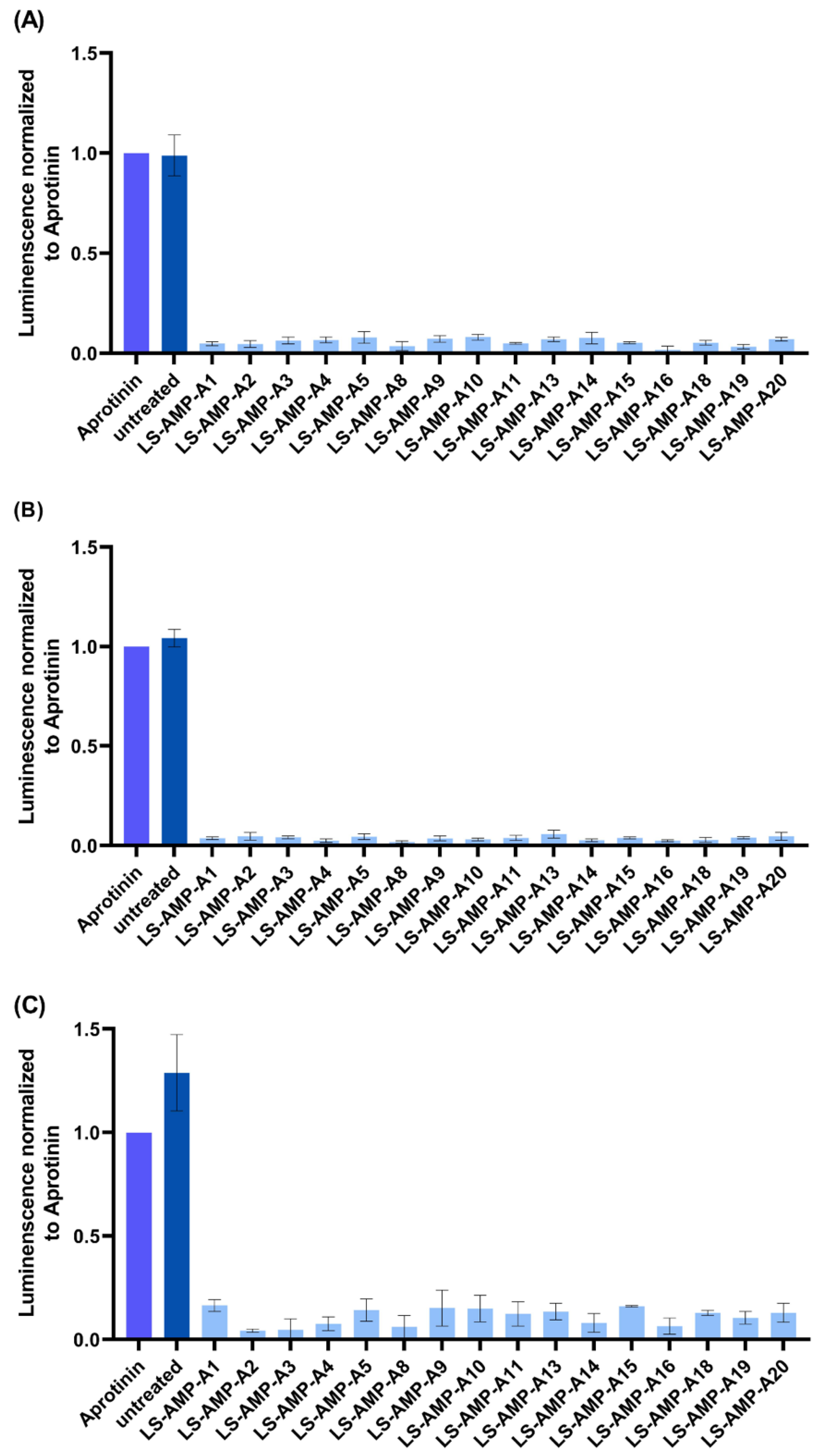
2.4. Evaluation of Antiviral Activity
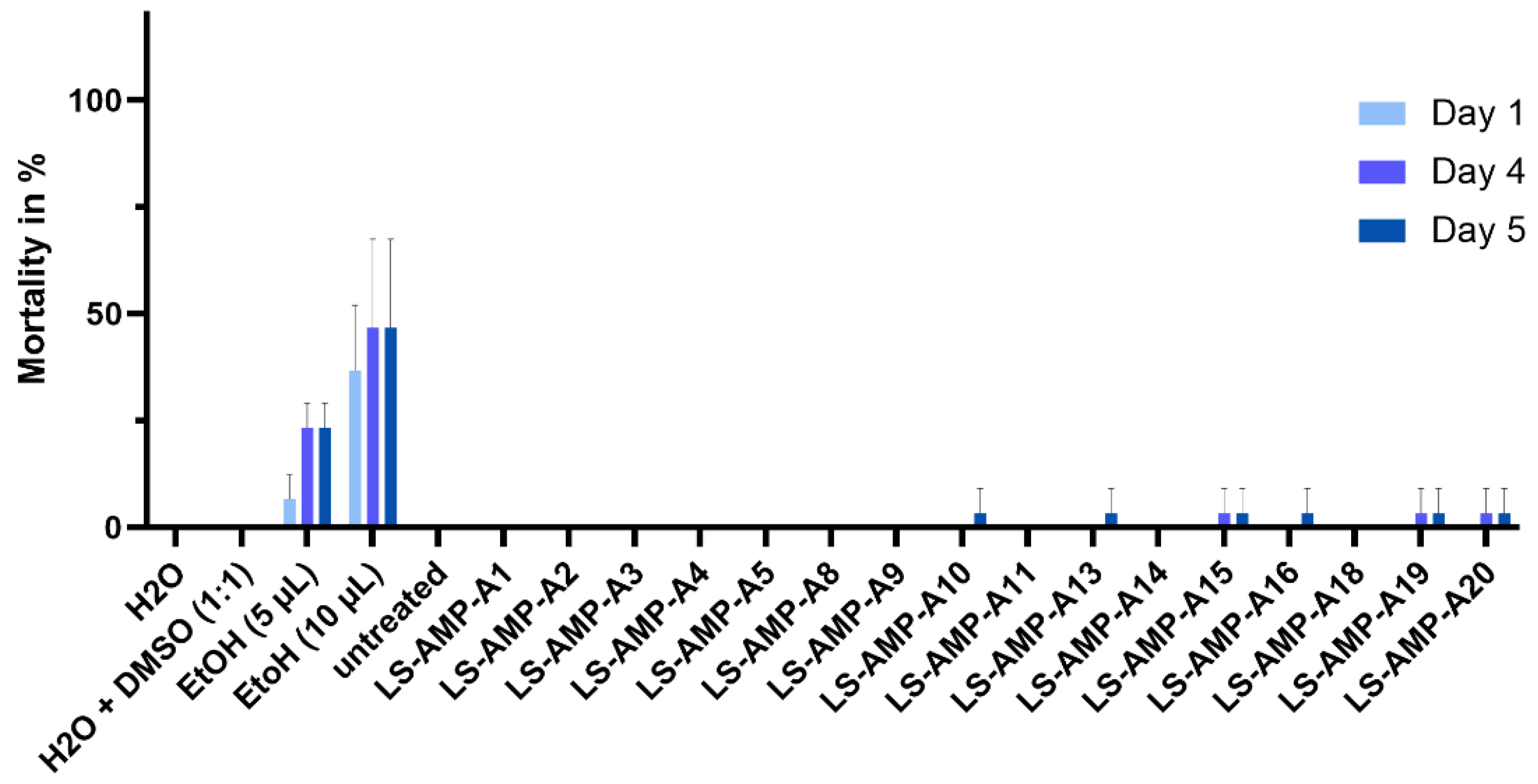
2.5. Insecticidal Activity Evaluation against G. mellonella Larvae
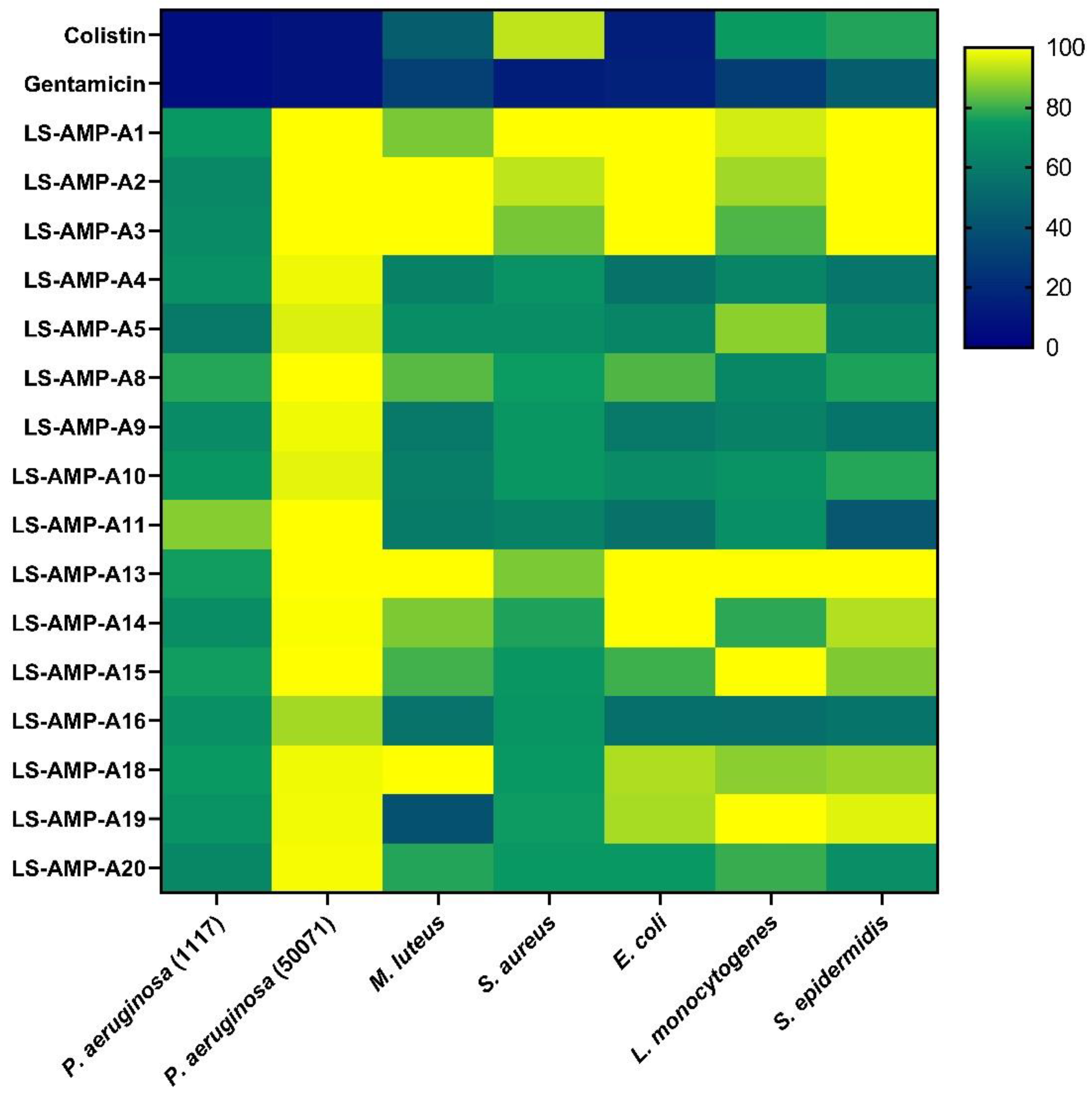
2.6. Antibacterial Activity
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sequence Analysis
5.2. Peptide Synthesis
5.3. Cell Culture
5.4. Cytotoxicity Assays
5.5. Antiviral Activity
5.6. Insecticidal Activity
5.7. Antibacterial Activity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lüddecke, T.; Herzig, V.; von Reumont, B.M.; Vilcinskas, A. The biology and evolution of spider venoms. Biol. Rev. Camb. Philos. Soc. 2022, 97, 163–178. [Google Scholar] [CrossRef]
- Windley, M.J.; Herzig, V.; Dziemborowicz, S.A.; Hardy, M.C.; King, G.F.; Nicholson, G.M. Spider-venom peptides as bioinsecticides. Toxins 2012, 4, 191–227. [Google Scholar] [CrossRef] [PubMed]
- Langenegger, N.; Nentwig, W.; Kuhn-Nentwig, L. Spider venom: Components, modes of action, and novel strategies in transcriptomic and proteomic analyses. Toxins 2019, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, B.; Kuhn-Nentwig, L.; Tromp, J.; Kämpfer, U.; Schaller, J.; Schürch, S.; Nentwig, W. CSTX-13, a highly synergistically acting two-chain neurotoxic enhancer in the venom of the spider Cupiennius salei (Ctenidae). Proc. Natl. Acad. Sci. USA 2004, 101, 11251–11256. [Google Scholar] [CrossRef] [PubMed]
- Clémençon, B.; Kuhn-Nentwig, L.; Langenegger, N.; Kopp, L.; Peigneur, S.; Tytgat, J.; Nentwig, W.; Lüscher, B.P. Neurotoxin merging: A Strategy Deployed by the Venom of the Spider Cupiennius salei to Potentiate Toxicity on Insects. Toxins 2020, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Kuhn-Nentwig, L.; Langenegger, N.; Heller, M.; Koua, D.; Nentwig, W. The dual prey-inactivation strategy of spiders—In-depth venomic analysis of Cupiennius salei. Toxins 2019, 11, 167. [Google Scholar] [CrossRef]
- Zhang, A.H.; Sharma, G.; Undheim, E.A.B.; Jia, X.; Mobli, M. A complicated complex: Ion channels, voltage sensing, cell membranes and peptide inhibitors. Neurosci. Lett. 2018, 679, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.L.; Milligan, C.J.; Richardson, R.J.; Jancovski, N.; Grunnet, M.; Jacobson, L.H.; Undheim, E.A.B.; Mobli, M.; Chow, C.Y.; Herzig, V.; et al. Selective NaV1.1 activation rescues Dravet syndrome mice from seizures and premature death. Proc. Natl. Acad. Sci. USA 2018, 115, E8077–E8085. [Google Scholar] [CrossRef] [PubMed]
- Pineda, S.S.; Undheim, E.A.B.; Rupasinghe, D.B.; Ikonomopoulou, M.P.; King, G.F. Spider venomics: Implications for drug discovery. Future Med. Chem. 2014, 6, 1699–1714. [Google Scholar] [CrossRef]
- Chassagnon, I.R.; McCarthy, C.A.; Chin, Y.K.Y.; Pineda, S.S.; Keramidas, A.; Mobli, M.; Pham, V.; De Silva, T.M.; Lynch, J.W.; Widdop, R.E.; et al. Potent neuroprotection after stroke afforded by a double-knot spider-venom peptide that inhibits acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA 2017, 114, 3750–3755. [Google Scholar] [CrossRef]
- King, G.F.; Hardy, M.C. Spider-Venom Peptides: Structure, Pharmacology, and Potential for Control of Insect Pests. Annu. Rev. Entomol. 2013, 58, 475–496. [Google Scholar] [CrossRef]
- Lüddecke, T.; von Reumont, B.M.; Förster, F.; Billion, A.; Timm, T.; Lochnit, G.; Vilcinskas, A.; Lemke, S. An Economic Dilemma between Molecular Weapon Systems May Explain an Arachnological-atypical Venom in Wasp Spiders (Argiope bruennichi). Biomolecules 2020, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Zobel-Thropp, P.A.; Mullins, J.; Kristensen, C.; Kronmiller, B.A.; David, C.L.; Breci, L.A.; Binford, G.J. Not so Dangerous After All? Venom Composition and Potency of the Pholcid (Daddy Long-Leg) Spider Physocyclus mexicanus. Front. Ecol. Evol. 2019, 7, 256. [Google Scholar] [CrossRef]
- Garb, J.E.; Hayashi, C.Y. Molecular evolution of α-latrotoxin, the exceptionally potent vertebrate neurotoxin in black widow spider venom. Mol. Biol. Evol. 2013, 30, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Lüddecke, T.; Vilcinskas, A.; Lemke, S. Phylogeny-guided selection of priority groups for venom bioprospecting: Harvesting toxin sequences in tarantulas as a case study. Toxins 2019, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- von Reumont, B.M.; Anderluh, G.; Antunes, A.; Ayvazyan, N.; Beis, D.; Caliskan, F.; Crnković, A.; Damm, M.; Dutertre, S.; Ellgaard, L.; et al. Modern venomics—Current insights, novel methods, and future perspectives in biological and applied animal venom research. Gigascience 2022, 11, giac048. [Google Scholar] [PubMed]
- Brogden, K.A.; Bates, A.M.; Fischer, C.L. Antimicrobial Peptides in Host Defense: Functions Beyond Antimicrobial Activity. In Birkhäuser Advances in Infectious Diseases; Springer: Cham, Switzerland, 2016; pp. 129–146. [Google Scholar]
- Kuhn-Nentwig, L.; Lischer, H.E.L.; Pekár, S.; Langenegger, N.; Albo, M.J.; Isaia, M.; Nentwig, W. Linear Peptides—A Combinatorial Innovation in the Venom of Some Modern Spiders. Front. Mol. Biosci. 2021, 8, 620. [Google Scholar] [CrossRef]
- Megaly, A.M.A.; Yoshimoto, Y.; Tsunoda, Y.; Miyashita, M.; Abdel-Wahab, M.; Nakagawa, Y.; Miyagawa, H. Characterization of 2 linear peptides without disulfide bridges from the venom of the spider Lycosa poonaensis (Lycosidae). Biosci. Biotechnol. Biochem. 2021, 85, 1348–1356. [Google Scholar] [CrossRef]
- Won, A.; Ruscito, A.; Ianoul, A. Imaging the membrane lytic activity of bioactive peptide latarcin 2a. Biochim. Biophys. Acta 2012, 1818, 3072–3080. [Google Scholar] [CrossRef]
- Perumal Samy, R.; Stiles, B.G.; Franco, O.L.; Sethi, G.; Lim, L.H.K. Animal venoms as antimicrobial agents. Biochem. Pharmacol. 2017, 134, 127–138. [Google Scholar] [CrossRef]
- Garcia, F.; Villegas, E.; Espino-Solis, G.P.; Rodriguez, A.; Paniagua-Solis, J.F.; Sandoval-Lopez, G.; Possani, L.D.; Corzo, G. Antimicrobial peptides from arachnid venoms and their microbicidal activity in the presence of commercial antibiotics. J. Antibiot. 2013, 66, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Adams, M.E. Lycotoxins, antimicrobial peptides from venom of the wolf spider Lycosa carolinensis. J. Biol. Chem. 1998, 273, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Jing, Y.; Zhigui, D.; Liping, J.; Zhonghua, L.; Songping, L. Molecular diversification of antimicrobial peptides from the wolf spider Lycosa sinensis venom based on peptidomic, transcriptomic, and bioinformatic analyses. Acta Biochim. Biophys. Sin. 2020, 52, 1274–1280. [Google Scholar] [CrossRef]
- Utkin, Y.; Siniavin, A.; Kasheverov, I.; Tsetlin, V. Antiviral Effects of Animal Toxins: Is There a Way to Drugs? Int. J. Mol. Sci. 2022, 23, 3634. [Google Scholar] [CrossRef]
- Lima, W.G.; Maia, C.Q.; de Carvalho, T.S.; Leite, G.O.; Brito, J.C.M.; Godói, I.P.D.; de Lima, M.E.; Ferreira, J.M.S. Animal venoms as a source of antiviral peptides active against arboviruses: A systematic review. Arch. Virol. 2022, 167, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Eichberg, J.; Maiworm, E.; Oberpaul, M.; Czudai-Matwich, V.; Lüddecke, T.; Vilcinskas, A.; Hardes, K. Antiviral Potential of Natural Resources against Influenza Virus Infections. Viruses 2022, 14, 2452. [Google Scholar] [CrossRef] [PubMed]
- Schendel, V.; Rash, L.D.; Jenner, R.A.; Undheim, E.A.B. The diversity of venom: The importance of behavior and venom system morphology in understanding its ecology and evolution. Toxins 2019, 11, 666. [Google Scholar] [CrossRef]
- Santos, D.M.; Verly, R.M.; Piló-Veloso, D.; De Maria, M.; De Carvalho, M.A.R.; Cisalpino, P.S.; Soares, B.M.; Diniz, C.G.; Farias, L.M.; Moreira, D.F.F.; et al. LyeTx I, a potent antimicrobial peptide from the venom of the spider Lycosa erythrognatha. Amino Acids 2010, 39, 135–144. [Google Scholar] [CrossRef]
- Yacoub, T.; Rima, M.; Marc, K.; Jean-Marc, S.; Ziad, F. Antimicrobials from Venomous Animals: An Overview. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef]
- Krämer, J.; Lüddecke, T.; Marner, M.; Maiworm, E.; Eichberg, J.; Hardes, K.; Schäberle, T.F.; Vilcinskas, A.; Predel, R. Antimicrobial, Insecticidal and Cytotoxic Activity of Linear Venom Peptides from the Pseudoscorpion Chelifer cancroides. Toxins 2022, 14, 58. [Google Scholar] [CrossRef]
- Schaefer, K.; Austhof, E.; Boyd, K.; Armstrong, A.; Hoffman, S.; Pogreba-Brown, K. Septicemia Due to Listeria monocytogenes Infection: A Systematic Review and Meta-Analysis. Foodborne Pathog. Dis. 2022, 19, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Grima, P.; Urciuoli, C.; Simone, G.; Palazzo, A.G.; Nuzzo, M.; Quarta, M.; Carraturo, I.; Maci, A.M.; Marinaci, S.; Portaccio, G.; et al. Fatal Listeria monocytogenes septicemia and meningitis complicated by Candida glabrata fungemia: A case report. Curr. Med. Res. Opin. 2022, 38, 2119–2121. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Hatasaki, K.; Ueno, K.; Fujita, S.; Igarashi, N.; Kuroda, M.; Wada, T. One-year-old boy with refractory Listeria monocytogenes meningitis due to persistent hypercytokinemia. J. Infect. Chemother. 2022, 28, 1682–1686. [Google Scholar] [CrossRef]
- Apponyi, M.A.; Pukala, T.L.; Brinkworth, C.S.; Maselli, V.M.; Bowie, J.H.; Tyler, M.J.; Booker, G.W.; Wallace, J.C.; Carver, J.A.; Separovic, F.; et al. Host-defence peptides of Australian anurans: Structure, mechanism of action and evolutionary significance. Peptides 2004, 25, 1035–1054. [Google Scholar] [CrossRef]
- Rozek, T.; Wegener, K.L.; Bowie, J.H.; Olver, I.N.; Carver, J.A.; Wallace, J.C.; Tyler, M.J. The antibiotic and anticancer active aurein peptides from the Australian Bell Frogs Litoria aurea and Litoria raniformis. Eur. J. Biochem. 2000, 267, 5330–5341. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.J.M.; Waugh, R.J.; Bowie, J.H.; Wallace, J.C.; Tyler, M.J. Peptides from Australian frogs. Structures of the caerins and caeridin 1 from Litoria splendida. J. Chem. Soc. Perkin Trans. 1 1992, 23, 3173–3178. [Google Scholar] [CrossRef]
- Yang, X.; Lee, W.H.; Zhang, Y. Extremely abundant antimicrobial peptides existed in the skins of nine kinds of Chinese odorous frogs. J. Proteome Res. 2012, 11, 306–319. [Google Scholar] [CrossRef]
- Ali, M.F.; Soto, A.M.; Knoop, F.C.; Conlon, J.M. Antimicrobial peptides isolated from skin secretions of the diploid frog, Xenopus tropicalis (Pipidae). Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2001, 1550, 81–89. [Google Scholar] [CrossRef]
- Pukala, T.L.; Bertozzi, T.; Donnellan, S.C.; Bowie, J.H.; Surinya-Johnson, K.H.; Liu, Y.; Jackway, R.J.; Doyle, J.R.; Llewellyn, L.E.; Tyler, M.J. Host-defence peptide profiles of the skin secretions of interspecific hybrid tree frogs and their parents, female Litoria splendida and male Litoria caerulea. FEBS J. 2006, 273, 3511–3519. [Google Scholar] [CrossRef]
- Lüddecke, T.; Schulz, S.; Steinfartz, S.; Vences, M. A salamander’s toxic arsenal: Review of skin poison diversity and function in true salamanders, genus Salamandra. Sci. Nat. 2018, 105, 56. [Google Scholar] [CrossRef]
- Silva, L.M.; Carvalho Botelho, A.C.; Nacif-Pimenta, R.; Martins, G.F.; Alves, L.C.; Brayner, F.A.; Fortes-Dias, C.L.; Paolucci Pimenta, P.F. Structural analysis of the venom glands of the armed spider Phoneutria nigriventer (Keyserling, 1891): Microanatomy, fine structure and confocal observations. Toxicon 2008, 51, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Schmidtberg, H.; von Reumont, B.M.; Lemke, S.; Vilcinskas, A.; Lüddecke, T. Morphological Analysis Reveals a Compartmentalized Duct in the Venom Apparatus of the Wasp Spider (Argiope bruennichi). Toxins 2021, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- Ul-Hasan, S.; Rodríguez-Román, E.; Reitzel, A.M.; Adams, R.M.M.; Herzig, V.; Nobile, C.J.; Saviola, A.J.; Trim, S.A.; Stiers, E.E.; Moschos, S.A.; et al. The emerging field of venom-microbiomics for exploring venom as a microenvironment, and the corresponding Initiative for Venom Associated Microbes and Parasites (iVAMP). Toxicon X 2019, 4, 100016. [Google Scholar] [CrossRef] [PubMed]
- Gaver-Wainwright, M.M.; Zack, R.S.; Foradori, M.J.; Lavine, L.C. Misdiagnosis of spider bites: Bacterial associates, mechanical pathogen transfer, and hemolytic potential of venom from the hobo spider, Tegenaria agrestis (Araneae: Agelenidae). J. Med. Entomol. 2011, 48, 382–388. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific alpha-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Nongoierma, A.B.; FitzGerald, R.J. Inhibition of dipeptidyl peptidase IV (DPP-IV) by tryptophan containing dipeptides. Food Funct. 2013, 12, 1843–1849. [Google Scholar] [CrossRef]
- Cobbold, M.; De La Pena, H.; Norris, A.; Polefrone, J.M.; Qian, J.; English, A.M.; Cummings, K.L.; Penny, S.; Turner, J.E.; Cottine, J.; et al. MHC class-I associated phosphopeptides are the targets of memory-like immunity in Leukemia. Sci. Transl. Med. 2013, 5, 203ra125. [Google Scholar] [CrossRef]
- Chongzhao, Y.; Yumu, Z.; Youwei, X.; Peiyu, X.; Zhen, L.; Huadong, L.; Sijie, H.; Zecai, C.; Jingru, L.; Eric, X.H.; et al. Structural basis for motilin and erythromycin recognition by motilin receptor. Sci. Adv. 2023, 9, eade9020. [Google Scholar] [CrossRef]
- Hurka, S.; Lüddecke, T.; Paas, A.; Dersch, L.; Schulte, L.; Eichberg, J.; Hardes, K.; Brinkrolf, K.; Vilcinskas, A. Bioactivity Profiling of In Silico Predicted Linear Toxins from the Ants Myrmica rubra and Myrmica ruginodis. Toxins 2022, 14, 846. [Google Scholar] [CrossRef]


| Peptide | Sequence | #AAs | Hydrophobicity | Charge |
|---|---|---|---|---|
| LS-AMP-A1 | SLFGLLDLVKSKVGQTGLL | 19 | 47% | +1 |
| LS-AMP-A2 | SLFGLLDLVKNKVGQTGLL | 19 | 47% | +1 |
| LS-AMP-A3 | SLLGLLDVVKNKVGQIGLL | 19 | 53% | +1 |
| LS-AMP-A4 | SLLGLLDVVKNKVGQIG | 17 | 47% | +1 |
| LS-AMP-A5 * | SLLGLLDVVKNKVGQI | 16 | 44% | +2 |
| LS-AMP-A8 | SLLGLLDVVKNTVGQTGLL | 19 | 47% | 0 |
| LS-AMP-A9 | SLLGLLDVVKNTVGQTG | 17 | 41% | 0 |
| LS-AMP-A10 * | SLLGLLDVVKNTVGQT | 16 | 50% | +2 |
| LS-AMP-A11 | SLLGLLDVVKNTVGQ | 15 | 47% | 0 |
| LS-AMP-A13 | SLLGLLDVVKSKIGQTGLL | 19 | 47% | +1 |
| LS-AMP-A14 | SLLGLLDVVKSKIGQTG | 17 | 41% | +1 |
| LS-AMP-A15 * | SLLGLLDVVKSKIGQT | 16 | 44% | +1 |
| LS-AMP-A16 | LLDVVKSKIGQTGLL | 15 | 47% | +1 |
| LS-AMP-A18 | SLLGLLDVVKSKVGQTGLL | 19 | 47% | +1 |
| LS-AMP-A19 | GSLLGLLDVVKSKVGQTGLL | 20 | 45% | +1 |
| LS-AMP-A20 * | DVVKSKVGQT | 10 | 30% | +2 |
| Name | Hit | Similarity | Species |
|---|---|---|---|
| LS-AMP-A1 | Aurein 2.1 | 55% | Litoria aurea |
| LS-AMP-A2 | Aurein 2.1 | 55% | Litoria aurea |
| LS-AMP-A3 | Aurein 2.1 | 55% | Litoria aurea |
| LS-AMP-A4 | Aurein 2.1 | 50% | Litoria aurea |
| LS-AMP-A5 | Caerin 2.1 | 46% | Litoria splendida |
| LS-AMP-A8 | Aurein 2.1 | 53% | Litoria aurea |
| LS-AMP-A9 | Aurein 2.1 | 47% | Litoria aurea |
| LS-AMP-A10 | Maculatin 1.3 | 43% | Litoria eucnemis |
| LS-AMP-A11 | Maculatin 1.3 | 43% | Litoria eucnemis |
| LS-AMP-A13 | Aurein 2.1 | 50% | Litoria aurea |
| LS-AMP-A14 | Caerin 2.1 | 50% | Litoria splendida |
| LS-AMP-A15 | Caerin 2.6 | 50% | Litoria caerulea |
| LS-AMP-A16 | Aurein 2.1 | 53% | Litoria aurea |
| LS-AMP-A18 | Aurein 2.1 | 55% | Litoria aurea |
| LS-AMP-A19 | Aurein 2.1 | 52% | Litoria aurea |
| LS-AMP-A20 | Aurein 2.4 | 39% | Litoria aurea |
| Peptide | MW calc. | MW. det. | Purity |
|---|---|---|---|
| LS-AMP-A1 | 1988.38 | 1989.2 | 82.0% |
| LS-AMP-A2 | 2015.41 | 2015.1 | 93.5% |
| LS-AMP-A3 | 1979.42 | 1979.2 | 96.8% |
| LS-AMP-A4 | 1753.10 | 1753.0 | 97.3% |
| LS-AMP-A5 | 1695.06 | 1695.2 | 87.8% |
| LS-AMP-A8 | 1940.29 | 1940.0 | 70.5% |
| LS-AMP-A9 | 1713.98 | 1714.0 | 82.4% |
| LS-AMP-A10 | 1655.94 | 1656.0 | 95.7% |
| LS-AMP-A11 | 1555.82 | 1555.8 | 93.4% |
| LS-AMP-A13 | 1954.36 | 1954.2 | 83.9% |
| LS-AMP-A14 | 1728.05 | 1727.8 | 90.6% |
| LS-AMP-A15 | 1670.01 | 1670.0 | 80.3% |
| LS-AMP-A16 | 1583.92 | 1583.8 | 92.0% |
| LS-AMP-A18 | 1940.34 | 1940.2 | 95.4% |
| LS-AMP-A19 | 1997.39 | 1997.1 | 89.4% |
| LS-AMP-A20 | 1059.22 | 1059.6 | 98.6% |
| Name | Unique Identifier | OD600 for Assay |
|---|---|---|
| Listeria monocytogenes | DSM 20600 | 0.000625 |
| Micrococcus luteus | DSM 20030 | 0.005 |
| Pseudomonas aeruginosa 50071 | DSM 50071 | 0.00125 |
| Pseudomonas aeruginosa 1117 | DSM 1117 | 0.005 |
| Staphylococcus aureus | DSM 2569 | 0.00125 |
| Staphylococcus epidermidis | ATCC 35984; DSM 28319 | 0.000625 |
| Escherichia coli DE3 | BL21(DE3) | 0.000325 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lüddecke, T.; Dersch, L.; Schulte, L.; Hurka, S.; Paas, A.; Oberpaul, M.; Eichberg, J.; Hardes, K.; Klimpel, S.; Vilcinskas, A. Functional Profiling of the A-Family of Venom Peptides from the Wolf Spider Lycosa shansia. Toxins 2023, 15, 303. https://doi.org/10.3390/toxins15050303
Lüddecke T, Dersch L, Schulte L, Hurka S, Paas A, Oberpaul M, Eichberg J, Hardes K, Klimpel S, Vilcinskas A. Functional Profiling of the A-Family of Venom Peptides from the Wolf Spider Lycosa shansia. Toxins. 2023; 15(5):303. https://doi.org/10.3390/toxins15050303
Chicago/Turabian StyleLüddecke, Tim, Ludwig Dersch, Lennart Schulte, Sabine Hurka, Anne Paas, Markus Oberpaul, Johanna Eichberg, Kornelia Hardes, Sven Klimpel, and Andreas Vilcinskas. 2023. "Functional Profiling of the A-Family of Venom Peptides from the Wolf Spider Lycosa shansia" Toxins 15, no. 5: 303. https://doi.org/10.3390/toxins15050303
APA StyleLüddecke, T., Dersch, L., Schulte, L., Hurka, S., Paas, A., Oberpaul, M., Eichberg, J., Hardes, K., Klimpel, S., & Vilcinskas, A. (2023). Functional Profiling of the A-Family of Venom Peptides from the Wolf Spider Lycosa shansia. Toxins, 15(5), 303. https://doi.org/10.3390/toxins15050303






