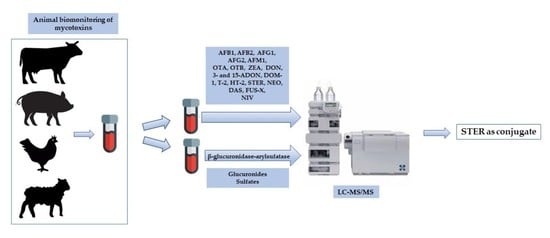
Biomonitoring of 19 Mycotoxins in Plasma from Food-Producing Animals (Cattle, Poultry, Pigs, and Sheep)
Abstract
1. Introduction
2. Results
2.1. Analytical Method Revalidation
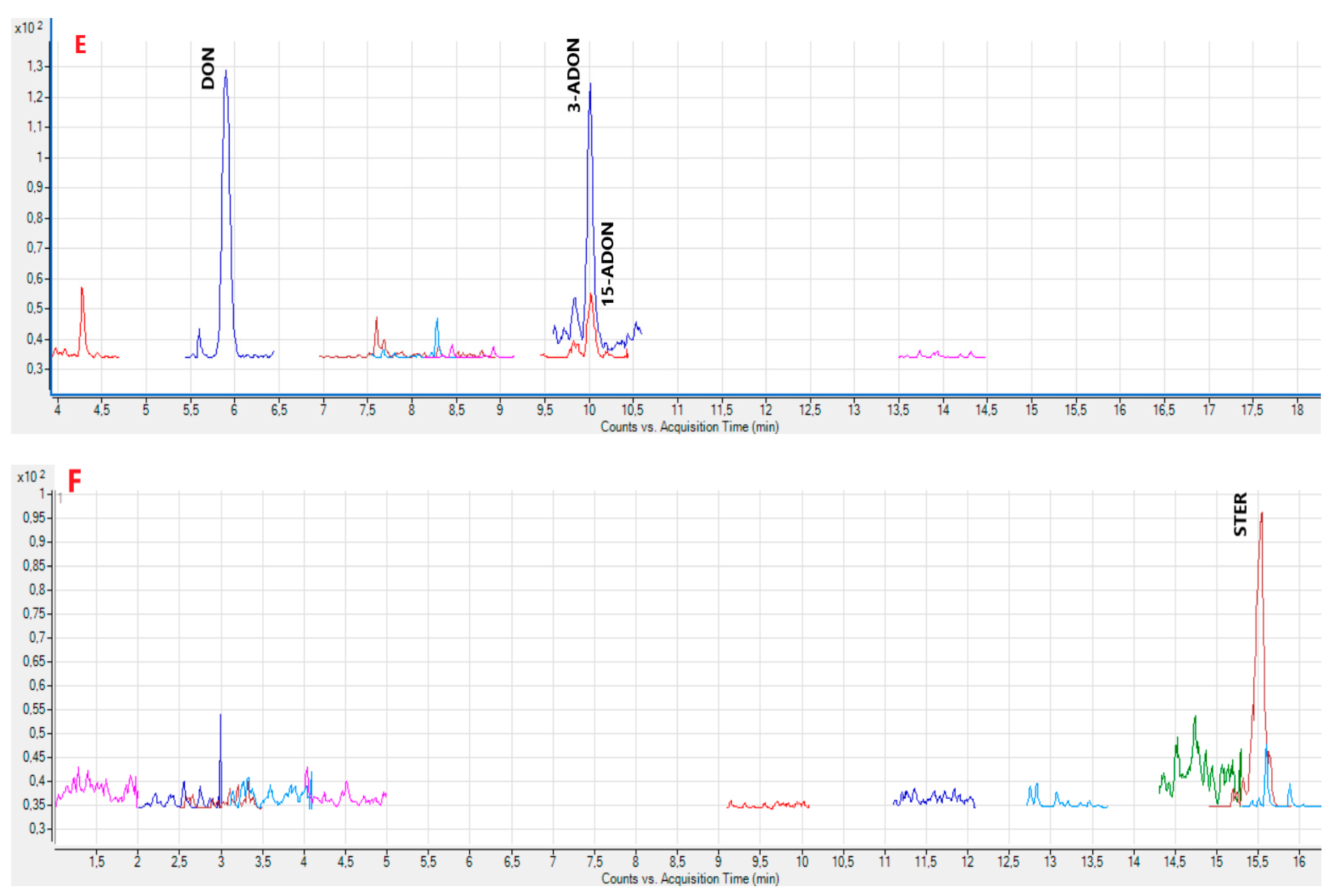
2.2. Analysis of the Samples
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemical Reagents and Materials
5.2. Mycotoxin Standards
5.3. Safety Precautions
5.4. Animal Plasma Samples
5.5. Preparation of Mycotoxin Solutions and Calibrators
5.6. Sample Preparation
5.7. Analysis of Samples
5.8. Method Revalidation
5.9. Identification of Mycotoxins in Samples
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 15-ADON | 15-acetyldeoxynivalenol |
| 3-ADON | 3-acetyldeoxynivalenol |
| ABM | Animal biomonitoring |
| ACN | Acetonitrile |
| AFB1 | Aflatoxin B1 |
| AFB2 | Aflatoxin B2 |
| AFG1 | Aflatoxin G1 |
| AFG2 | Aflatoxin G2 |
| AFM1 | Aflatoxin M1 |
| AFs | Aflatoxins |
| DAS | Diacetoxyscirpenol |
| DOM-1 | Deepoxy-deoxynivalenol |
| DON | Deoxynivalenol |
| EMA | European Medicines Agency |
| FUS-X | Fusarenon-X |
| HBM | Human biomonitoring |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| ME | Matrix effect |
| MeOH | Methanol |
| NEO | Neosolaniol |
| NIV | Nivalenol |
| OTA | Ochratoxin A |
| OTA-d5 | Ochratoxin A-(phenyl-d5) |
| OTB | Ochratoxin B |
| q | Transition of qualification |
| Q | Transition of quantification |
| RE | Relative error |
| RT | Retention Time |
| STER | Sterigmatocystin |
| ZEA | Zearalenone |
References
- European Commission. White Paper on Food Safety; The European Commission, Ed.Brussels; Commision of the European Communities: Brussels, Belgium, 2000. [Google Scholar]
- The Council of the European Communities. Council Regulation (EEC) No 315/93 of 8 February 1993 Laying down Community Procedures for Contaminants in Food. Off. J. Eur. Communities 1993, L 37, 1–3. [Google Scholar]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; De Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked Mycotoxins: A Review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Mattson, J. Mixtures in the Real World: The Importance of Plant Self-Defense Toxicants, Mycotoxins, and the Human Diet. Toxicol. Appl. Pharmacol. 2007, 223, 125–132. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Mycotoxins Fact Sheets. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 2 February 2023).
- Buszewska-Forajta, M. Mycotoxins, Invisible Danger of Feedstuff with Toxic Effect on Animals. Toxicon 2020, 182, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.-X.; Marchal, J.L.M.; van der Poel, A.F.B. Strategies to Prevent and Reduce Mycotoxins for Compound Feed Manufacturing. Anim. Feed Sci. Technol. 2018, 237, 129–153. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Reddy, P.R.K.; Chilaka, C.A.; Balogun, O.B.; Elghandour, M.M.M.Y.; Rivas-Caceres, R.R.; Salem, A.Z.M. Mycotoxin Toxicity and Residue in Animal Products: Prevalence, Consumer Exposure and Reduction Strategies—A Review. Toxicon 2020, 177, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, G.; Lim, W. Effects of Mycotoxin-Contaminated Feed on Farm Animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Kępińska-Pacelik, J.; Biel, W. Alimentary Risk of Mycotoxins for Humans and Animals. Toxins 2021, 13, 822. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Tolosa, J.; Rodríguez-Carrasco, Y.; Ruiz, M.J.; Vila-Donat, P. Multi-Mycotoxin Occurrence in Feed, Metabolism and Carry-over to Animal-Derived Food Products: A Review. Food Chem. Toxicol. 2021, 158, 112661. [Google Scholar] [CrossRef] [PubMed]
- Pulina, G.; Battacone, G.; Brambilla, G.; Cheli, F.; Danieli, P.P.; Masoero, F.; Pietri, A.; Ronchi, B. An Update on the Safety of Foods of Animal Origin and Feeds. Ital. J. Anim. Sci. 2014, 13, 3571. [Google Scholar] [CrossRef]
- Lauwers, M.; De Baere, S.; Letor, B.; Rychlik, M.; Croubels, S.; Devreese, M. Multi LC-MS/MS and LC-HRMS Methods for Determination of 24 Mycotoxins Including Major Phase I and II Biomarker Metabolites in Biological Matrices from Pigs and Broiler Chickens. Toxins 2019, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- BIOMIN. World Mycotoxin Survey 2019: Annual Report. 2020. Available online: https://cdn2.hubspot.net/hubfs/5480243/BIOMIN/Downloads/MAG_MTXSurveyReport_2019_EN.pdf (accessed on 3 February 2023).
- Twarużek, M.; Skrzydlewski, P.; Kosicki, R.; Grajewski, J. Mycotoxins Survey in Feed Materials and Feedingstuffs in Years 2015–2020. Toxicon 2021, 202, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Den Hollander, D.; Croubels, S.; Lauwers, M.; Caekebeke, N.; Ringenier, M.; De Meyer, F.; Reisinger, N.; Van Immerseel, F.; Dewulf, J.; Antonissen, G. Applied Research Note: Biomonitoring of Mycotoxins in Blood Serum and Feed to Assess Exposure of Broiler Chickens. J. Appl. Poult. Res. 2021, 30, 100111. [Google Scholar] [CrossRef]
- Arce-López, B.; Lizarraga, E.; Vettorazzi, A.; González-Peñas, E. Human Biomonitoring of Mycotoxins in Blood, Plasma and Serum in Recent Years: A Review. Toxins 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Torres, D.; Lopes, C.; Correia, D.; Goios, A.; Assunção, R.; Alvito, P.; Vidal, A.; De Boevre, M.; De Saeger, S.; et al. Deoxynivalenol Exposure Assessment through a Modelling Approach of Food Intake and Biomonitoring Data—A Contribution to the Risk Assessment of an Enteropathogenic Mycotoxin. Food Res. Int. 2021, 140, 109863. [Google Scholar] [CrossRef] [PubMed]
- Marín, S.; Cano-Sancho, G.; Sanchis, V.; Ramos, A.J. The Role of Mycotoxins in the Human Exposome: Application of Mycotoxin Biomarkers in Exposome-Health Studies. Food Chem. Toxicol. 2018, 121, 504–518. [Google Scholar] [CrossRef]
- Louro, H.; Heinälä, M.; Bessems, J.; Buekers, J.; Vermeire, T.; Woutersen, M.; van Engelen, J.; Borges, T.; Rousselle, C.; Ougier, E.; et al. Human Biomonitoring in Health Risk Assessment in Europe: Current Practices and Recommendations for the Future. Int. J. Hyg. Environ. Health 2019, 222, 727–737. [Google Scholar] [CrossRef]
- Choi, J.; Aarøe Mørck, T.; Polcher, A.; Knudsen, L.E.; Joas, A. Review of the State of the Art of Human Biomonitoring for Chemical Substances and Its Application to Human Exposure Assessment for Food Safety. EFSA Support. Publ. 2015, 12, EN-724. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Jedziniak, P. Mycotoxin Biomarkers in Pigs—Current State of Knowledge and Analytics. Toxins 2021, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Arce-López, B.; Lizarraga, E.; Flores-Flores, M.; Irigoyen, Á.; González-Peñas, E. Development and Validation of a Methodology Based on Captiva EMR-Lipid Clean-up and LC-MS/MS Analysis for the Simultaneous Determination of Mycotoxins in Human Plasma. Talanta 2020, 206, 120193. [Google Scholar] [CrossRef] [PubMed]
- Jouany, J.P. Methods for Preventing, Decontaminating and Minimizing the Toxicity of Mycotoxins in Feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Devreese, M.; De Baere, S.; De Backer, P.; Croubels, S. Quantitative Determination of Several Toxicological Important Mycotoxins in Pig Plasma Using Multi-Mycotoxin and Analyte-Specific High Performance Liquid Chromatography–Tandem Mass Spectrometric Methods. J. Chromatogr. A 2012, 1257, 74–80. [Google Scholar] [CrossRef]
- De Baere, S.; Croubels, S.; Novak, B.; Bichl, G.; Antonissen, G. Development and Validation of a UPLC-MS/MS and UPLC-HR-MS Method for the Determination of Fumonisin B1 and Its Hydrolysed Metabolites and Fumonisin B2 in Broiler Chicken Plasma. Toxins 2018, 10, 62. [Google Scholar] [CrossRef]
- De Baere, S.; Ochieng, P.E.; Kemboi, D.C.; Scippo, M.-L.; Okoth, S.; Lindahl, J.F.; Gathumbi, J.K.; Antonissen, G.; Croubels, S. Development of High-Throughput Sample Preparation Procedures for the Quantitative Determination of Aflatoxins in Biological Matrices of Chickens and Cattle Using UHPLC-MS/MS. Toxins 2023, 15, 37. [Google Scholar] [CrossRef]
- De Baere, S.; Goossens, J.; Osselaere, A.; Devreese, M.; Vandenbroucke, V.; De Backer, P.; Croubels, S. Quantitative Determination of T-2 Toxin, HT-2 Toxin, Deoxynivalenol and Deepoxy-Deoxynivalenol in Animal Body Fluids Using LC–MS/MS Detection. J. Chromatogr. B 2011, 879, 2403–2415. [Google Scholar] [CrossRef]
- Broekaert, N.; Devreese, M.; De Mil, T.; Fraeyman, S.; De Baere, S.; De Saeger, S.; De Backer, P.; Croubels, S. Development and Validation of an LC–MS/MS Method for the Toxicokinetic Study of Deoxynivalenol and Its Acetylated Derivatives in Chicken and Pig Plasma. J. Chromatogr. B 2014, 971, 43–51. [Google Scholar] [CrossRef]
- European Commission. Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (2002/657/EC). Off. J. Eur. Communities 2002, 221, 8–36. [Google Scholar]
- European Medicines Agency. Committee for Medicinal Products for Human Use. Guideline on Bioanalytical Method Validation. EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2** 2012, 23p. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 11 April 2023).
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef] [PubMed]
- Devreese, M.; Croubels, S.; De Baere, S.; Gehring, R.; Antonissen, G. Comparative Toxicokinetics and Plasma Protein Binding of Ochratoxin A in Four Avian Species. J. Agric. Food Chem. 2018, 66, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Solano, B.; González-Peñas, E. Co-Occurrence of Mycotoxins in Feed for Cattle, Pigs, Poultry, and Sheep in Navarra, a Region of Northern Spain. Toxins 2023, 15, 172. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and Metabolism of Deoxynivalenol in Animals and Humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef]
- Schelstraete, W.; Devreese, M.; Croubels, S. Comparative Toxicokinetics of Fusarium Mycotoxins in Pigs and Humans. Food Chem. Toxicol. 2020, 137, 111140. [Google Scholar] [CrossRef]
- Dänicke, S.; Brüssow, K.-P.; Valenta, H.; Ueberschär, K.-H.; Tiemann, U.; Schollenberger, M. On the Effects of Graded Levels of Fusarium Toxin Contaminated Wheat in Diets for Gilts on Feed Intake, Growth Performance and Metabolism of Deoxynivalenol and Zearalenone. Mol. Nutr. Food Res. 2005, 49, 932–943. [Google Scholar] [CrossRef]
- Fushimi, Y.; Takagi, M.; Uno, S.; Kokushi, E.; Nakamura, M.; Hasunuma, H.; Shinya, U.; Deguchi, E.; Fink-Gremmels, J. Measurement of Sterigmatocystin Concentrations in Urine for Monitoring the Contamination of Cattle Feed. Toxins 2014, 6, 3117–3128. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific Opinion on the Risk for Public and Animal Health Related to the Presence of Sterigmatocystin in Food and Feed. EFSA J. 2013, 11, 3254. [Google Scholar] [CrossRef]
- Viegas, S.; Assunção, R.; Martins, C.; Nunes, C.; Osteresch, B.; Twarużek, M.; Kosicki, R.; Grajewski, J.; Ribeiro, E.; Viegas, C. Occupational Exposure to Mycotoxins in Swine Production: Environmental and Biological Monitoring Approaches. Toxins 2019, 11, 78. [Google Scholar] [CrossRef]
- Flores-Flores, M.E.; González-Peñas, E. An LC–MS/MS Method for Multi-Mycotoxin Quantification in Cow Milk. Food Chem. 2017, 218, 378–385. [Google Scholar] [CrossRef]
- Flores-Flores, M.E.; González-Peñas, E. Development and Validation of a High Performance Liquid Chromatographic–Mass Spectrometry Method for the Simultaneous Quantification of 10 Trichothecenes in Ultra-High Temperature Processed Cow Milk. J. Chromatogr. A 2015, 1419, 37–44. [Google Scholar] [CrossRef] [PubMed]

| Mycotoxin | Cattle | Pigs | Poultry | Sheep | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Equation | RE * (%) | R2 | Equation | RE (%) | R2 | Equation | RE (%) | R2 | Equation | RE (%) | |
| DOM-1 | 0.9983 | y = 15.86x − 31.47 | <14.6 | 0.9989 | y = 16.76x − 25.10 | <13.1 | 0.9950 | y = 15.47x − 26.37 | <16.0 | 0.9997 | y = 15.69x − 24.00 | <19.6 |
| AFG2 | 0.9987 | y = 146.91x − 11.44 | <16.1 | 0.9983 | y = 152.77x − 51.19 | <19.4 | 0.9970 | y = 150.42x − 19.02 | <10.3 | 0.9992 | y = 145.06x + 4.12 | <16.8 |
| AFM1 | 0.9992 | y = 108.30x + 5.20 | <13.0 | 0.9983 | y = 143.67x + 15.50 | <15.4 | 0.9995 | y = 146.32x − 13.84 | <19.4 | 0.9955 | y = 145.44x − 15.21 | <15.8 |
| AFG1 | 0.9996 | y = 324.75x − 36.60 | <19.6 | 0.9956 | y = 401.38x − 41.55 | <17.0 | 0.9987 | y = 394.98x + 13.83 | <16.2 | 0.9973 | y = 395.46x − 12.15 | <16.1 |
| AFB2 | 0.9993 | y = 415.65x − 21.48 | <11.8 | 0.9982 | y = 373.74x + 12.14 | <16.9 | 0.9976 | y = 389.17x − 10.79 | <12.6 | 0.9990 | y = 374.46x − 45.24 | <11.8 |
| AFB1 | 0.9994 | y = 561.42x + 2.56 | <19.9 | 0.9991 | y = 603.48x − 59.23 | <16.1 | 0.9987 | y = 560.27x + 18.73 | <18.6 | 0.9978 | y = 592.19x − 0.10 | <16.9 |
| HT-2 | 0.9977 | y = 20.19x − 37.72 | <14.8 | 0.9991 | y = 17.81x + 38.29 | <18.4 | 0.9957 | y = 18.94x + 22.20 | <12.5 | 0.9989 | y = 19.06x − 29.50 | <17.8 |
| OTB | 0.9965 | y = 112.87x + 7.69 | <15.4 | 0.9977 | y = 122.41x − 19.96 | <14.0 | 0.9973 | y = 109.23x + 2.71 | <14.3 | 0.9979 | y = 118.62x − 16.35 | <12.9 |
| T-2 | 0.9990 | y = 100.99x + 3.81 | <18.0 | 0.9987 | y = 113.49x + 21.60 | <17.3 | 0.9986 | y = 120.12x − 21.19 | <14.3 | 0.9967 | y = 111.35x − 11.23 | <12.7 |
| ZEA | 0.9990 | y = 25.43x − 38.81 | <16.7 | 0.9987 | y = 28.71x − 39.60 | <16.2 | 0.9961 | y = 26.63x + 2.15 | <13.8 | 0.9969 | y = 29.68x + 8.34 | <13.1 |
| OTA-d5 | 0.9996 | y = 74.91x − 52.75 | <18.8 | 0.9968 | y = 56.95x + 1.14 | <13.0 | 0.9973 | y = 62.52x − 18.50 | <10.4 | 0.9937 | y = 58.67x − 31.63 | <13.7 |
| STER | 0.9998 | y = 191.70x − 65.30 | <16.9 | 0.9959 | y = 111.19x + 50.23 | <19.5 | 0.9936 | y = 120.34x − 35.22 | <14.5 | 0.9983 | y = 123.31x − 35.46 | <19.4 |
| NIV | 0.9978 | y = 3.67x − 9.42 | <15.7 | 0.9967 | y = 3.63x + 13.43 | <13.8 | 0.9968 | y = 3.41x + 24.66 | <18.6 | 0.9970 | y = 3.80x − 10.51 | <11.4 |
| DON | 0.9960 | y = 8.04x + 21.82 | <18.4 | 0.9971 | y = 8.78x − 9.97 | <11.7 | 0.9997 | y = 8.78x − 16.46 | <8.8 | 0.9988 | y = 9.38x − 30.31 | <12.5 |
| FUS-X | 0.9990 | y = 11.59x − 29.51 | <15.3 | 0.9941 | y = 11.54x − 55.12 | <18.6 | 0.9967 | y = 11.32x − 14.72 | <5.7 | 0.9906 | y = 10.75x + 1.42 | <19.1 |
| NEO | 0.9957 | y = 99.31x − 11.48 | <14.3 | 0.9989 | y = 90.43x + 12.09 | <12.8 | 0.9938 | y = 94.17x + 7.16 | <11.5 | 0.9934 | y = 103.78x − 24.38 | <8.9 |
| 3-ADON | 0.9918 | y = 44.18x − 19.72 | <15.2 | 0.9946 | y = 42.82x − 6.24 | <16.5 | 0.9979 | y = 46.71x − 13.94 | <17.5 | 0.9934 | y = 46.65x − 8.59 | <11.8 |
| 15-ADON | 0.9971 | y = 30.49x − 34.99 | <13.6 | 0.9984 | y = 28.24x − 31.74 | <10.5 | 0.9961 | y = 27.39x − 0.51 | <14.2 | 0.9945 | y = 29.33x − 10.39 | <10.7 |
| DAS | 0.9941 | y = 107.62x − 6.98 | <14.1 | 0.9988 | y = 111.37x − 13.16 | <8.0 | 0.9990 | y = 107.56x − 19.55 | <17.6 | 0.9986 | y = 116.46x − 7.33 | <16.0 |
| Mycotoxin | ME (%) | Recovery (%) | Precision (RSD%) (n = 3) | Accuracy (RE%) (n = 3) | ||||
|---|---|---|---|---|---|---|---|---|
| LOQ | 6× LOQ | 30× LOQ | LOQ | 6× LOQ | 30× LOQ | |||
| DOM-1 | 93.2 | 97.0 | 5.4 | 4.1 | 2.7 | 5.8 | 1.9 | 1.1 |
| AFG2 | 98.8 | 90.7 | 5.5 | 6.3 | 1.8 | 12.7 | 3.3 | 0.9 |
| AFM1 | 103.8 | 91.8 | 9.6 | 2.6 | 1.7 | 5.6 | 2.1 | 1.1 |
| AFG1 | 107.7 | 88.6 | 4.5 | 4.0 | 1.4 | 15.7 | 4.0 | 2.0 |
| AFB2 | 93.5 | 100.3 | 6.3 | 3.0 | 2.0 | 10.6 | 2.6 | 1.6 |
| AFB1 | 99.7 | 94.0 | 4.3 | 2.8 | 1.3 | 12.7 | 8.4 | 0.4 |
| HT-2 | 94.5 | 95.8 | 13.2 | 4.9 | 2.9 | 4.0 | 2.0 | 1.0 |
| OTB | 92.3 | 96.9 | 9.7 | 4.9 | 1.9 | 2.7 | 3.8 | 1.3 |
| T-2 | 93.0 | 90.9 | 7.9 | 2.8 | 1.4 | 9.3 | 2.3 | 1.1 |
| ZEA | 110.7 | 96.3 | 7.7 | 5.8 | 2.2 | 7.1 | 0.4 | 0.7 |
| OTA-d5 | 89.8 | 83.7 | 11.0 | 4.2 | 3.3 | 1.4 | 8.0 | 2.0 |
| STER | 91.7 | 81.2 | 8.8 | 2.6 | 2.1 | 10.0 | 1.3 | 3.0 |
| NIV | 107.5 | 92.5 | 9.7 | 1.9 | 3.8 | 18.9 | 5.0 | 2.5 |
| DON | 92.5 | 86.8 | 9.2 | 4.9 | 5.5 | 8.8 | 6.8 | 2.5 |
| FUS-X | 108.2 | 104.0 | 3.2 | 4.2 | 5.1 | 14.4 | 2.9 | 2.0 |
| NEO | 83.4 | 102.9 | 11.6 | 4.3 | 2.6 | 7.4 | 3.2 | 0.5 |
| 3-ADON | 116.4 | 95.5 | 7.0 | 3.3 | 3.2 | 9.8 | 9.5 | 0.8 |
| 15-ADON | 96.2 | 108.9 | 6.5 | 6.6 | 4.4 | 13.3 | 1.0 | 1.0 |
| DAS | 82.9 | 102.6 | 4.3 | 4.4 | 2.2 | 10.5 | 3.5 | 1.8 |
| Mycotoxin | Mean q/Q Ratio Calibrators (%) | Mean q/Q Ratio Samples (%) | RE(%) | Mean RT Calibrators (min) | Mean RT Samples (min) | RE(%) |
|---|---|---|---|---|---|---|
| Poultry (without enzymatic treatment) (sample identification: 20) | ||||||
| DON | 90.43 | 93.27 | 3.0 | 2.38 | 2.36 | 0.8 |
| 3-ADON | 76.97 | 75.25 | 2.2 | 9.92 | 9.92 | 0.1 |
| 15-ADON | 91.14 | 87.95 | 3.5 | 10.11 | 10.12 | 0.1 |
| Poultry (with enzymatic treatment) (sample identification: 20) | ||||||
| DON | 91.22 | 85.13 | 6.7 | 2.38 | 2.37 | 0.4 |
| STER | 86.50 | 88.31 | 2.0 | 15.75 | 15.74 | 0.1 |
| Pigs (with enzymatic treatment) | ||||||
| STER | 90.15 | 86.94 | 3.6 | 15.74 | 15.74 | 0.0 |
| Cattle (with enzymatic treatment) | ||||||
| STER | 87.00 | 88.17 | 1.3 | 15.75 | 15.74 | 0.1 |
| Sheep (with enzymatic treatment) | ||||||
| STER | 84.69 | 88.25 | 4.0 | 15.74 | 15.74 | 0.0 |
| Parameter | Cattle | Poultry | Pigs | Sheep |
|---|---|---|---|---|
| % Positive samples (>LOD) | 100 | 100 | 100 | 100 |
| % Positive samples (>LOQ) | 95 | 80 | 95 | 80 |
| Mean value of positive samples (>LOD) (ng/mL) | 2.4 | 2.0 | 2.9 | 2.4 |
| Median value (ng/mL) | 2.0 | 1.7 | 2.6 | 2.3 |
| 1st Quartile (ng/mL) | 1.4 | 1.1 | 1.8 | 1.1 |
| 3rd Quartile (ng/mL) | 3.5 | 2.8 | 4.4 | 3.6 |
| Maximum level found (ng/mL) | 4.3 | 3.9 | 5.0 | 4.9 |
| Group I | Group II | ||||
|---|---|---|---|---|---|
| Mycotoxin | ng/mL | Mycotoxin | ng/mL | Mycotoxin | ng/mL |
| DOM-1 | 300 | T2 | 50 | NIV | 1020 |
| AFG2 | 40 | ZEA | 200 | DON | 435 |
| AFM1 | 40 | OTA-d5 | 100 | Fus-X | 350 |
| AFG1 | 15 | STER | 50 | NEO | 40 |
| AFB2 | 15 | HT2 | 300 | 3-ADON | 87 |
| AFB1 | 10 | 15-ADON | 136 | ||
| OTB | 50 | DAS | 35 | ||
| Mycotoxin | LOD (ng/mL) | LOQ (ng/mL) | Calibration Range (ng/mL) |
|---|---|---|---|
| AFG2 | 0.35 | 0.8 | 0.8–24.0 |
| AFG1 | 0.07 | 0.3 | 0.3–9.0 |
| AFB2 | 0.07 | 0.3 | 0.3–9.0 |
| AFB1 | 0.04 | 0.2 | 0.2–6.0 |
| AFM1 | 0.18 | 0.8 | 0.8–24.0 |
| DOM-1 | 1.35 | 6.0 | 6.0–180.0 |
| 3-ADON | 0.70 | 1.8 | 1.8–52.5 |
| 15-ADON | 1.20 | 2.7 | 2.7–81.6 |
| T-2 | 0.20 | 1.0 | 1.0–30.0 |
| HT-2 | 2.70 | 6.0 | 6.0–180.0 |
| DAS | 0.15 | 0.7 | 0.7–21.0 |
| NEO | 0.18 | 0.8 | 0.8–24.0 |
| FUS-X | 1.95 | 8.7 | 8.7–261.6 |
| OTA-d5 | 0.40 | 2.0 | 2.0–60.0 |
| OTB | 0.40 | 1.0 | 1.0–30.0 |
| ZEA | 1.80 | 4.0 | 4.0–120.0 |
| STER | 0.20 | 1.0 | 1.0–30.0 |
| NIV | 9.10 | 20.4 | 20.4–612.0 |
| DON | 1.94 | 8.7 | 8.72–261.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Solano, B.; González-Peñas, E. Biomonitoring of 19 Mycotoxins in Plasma from Food-Producing Animals (Cattle, Poultry, Pigs, and Sheep). Toxins 2023, 15, 295. https://doi.org/10.3390/toxins15040295
Muñoz-Solano B, González-Peñas E. Biomonitoring of 19 Mycotoxins in Plasma from Food-Producing Animals (Cattle, Poultry, Pigs, and Sheep). Toxins. 2023; 15(4):295. https://doi.org/10.3390/toxins15040295
Chicago/Turabian StyleMuñoz-Solano, Borja, and Elena González-Peñas. 2023. "Biomonitoring of 19 Mycotoxins in Plasma from Food-Producing Animals (Cattle, Poultry, Pigs, and Sheep)" Toxins 15, no. 4: 295. https://doi.org/10.3390/toxins15040295
APA StyleMuñoz-Solano, B., & González-Peñas, E. (2023). Biomonitoring of 19 Mycotoxins in Plasma from Food-Producing Animals (Cattle, Poultry, Pigs, and Sheep). Toxins, 15(4), 295. https://doi.org/10.3390/toxins15040295