Defensive Molecules Momilactones A and B: Function, Biosynthesis, Induction and Occurrence
Abstract
1. Introduction
2. Defense Function against Pathogens, Microbes and Insects
2.1. Rice Blast Fungal Pathogen
2.2. Other Fungal Pathogens
2.3. Anti-Microbe Activity
2.4. Insect Attack
3. Function in Allelopathy
3.1. Activities of Momilactones A and B as Allelochemicals
3.2. Concentration and Secretion of Momilactones
3.3. Contribution of Momilactones to Rice Allelopathy
3.4. Genetic Evidence for Momilactones in Rice Allelopathy
3.5. Inhibitory Mechanism
3.6. Induction of Rice Allelopathy and Momilactone
4. Pharmacological Activity
4.1. Anticancer Activity
4.2. Anti-Inflammatory Activity
4.3. Anti-Diabetic Activity
4.4. Anti-Ketosis Activity
4.5. Anti-Melanogenic Activity
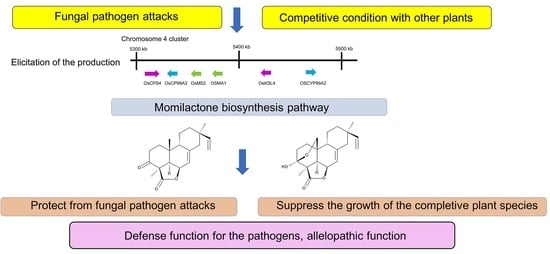
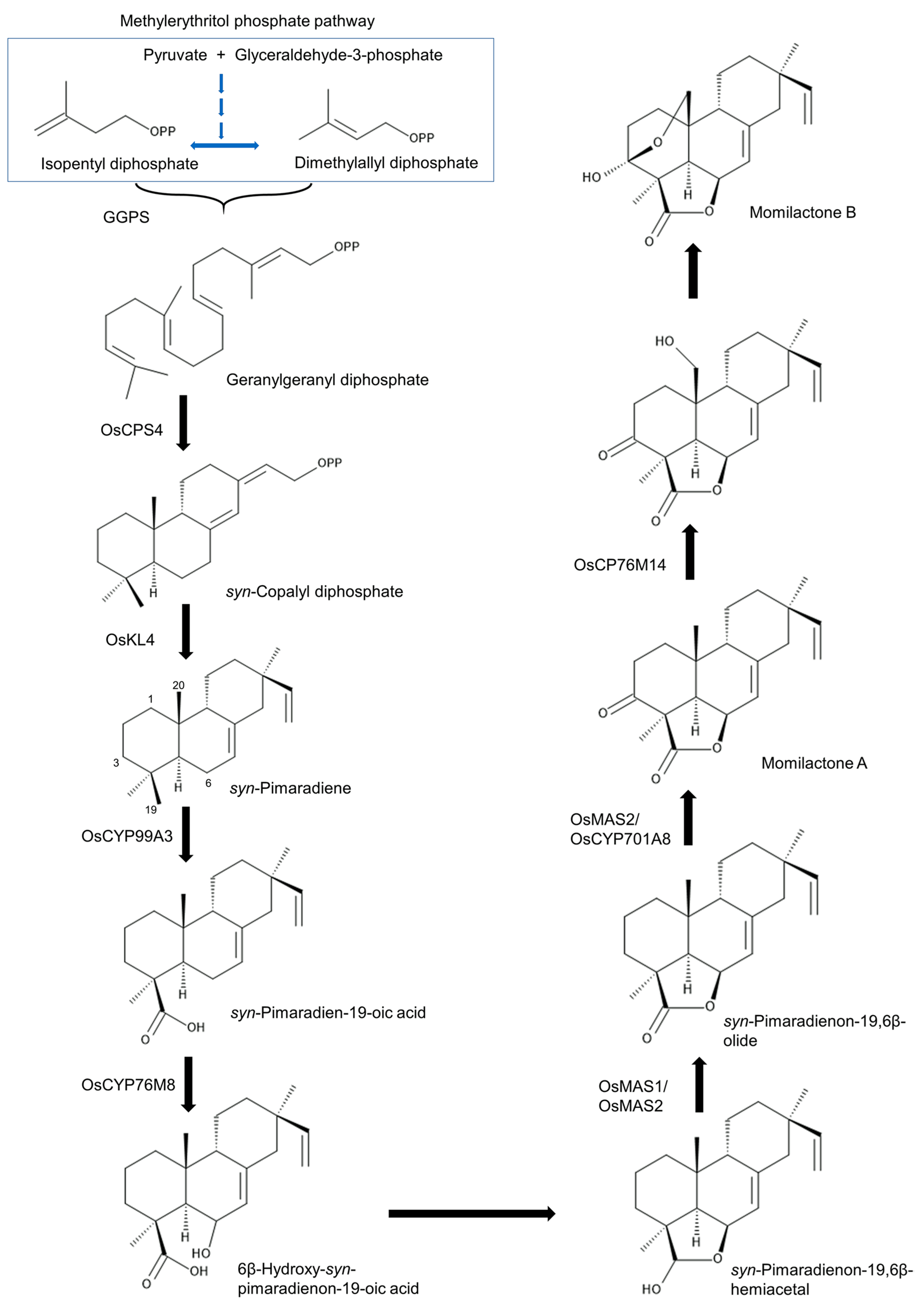
5. Biosynthesis and Related Genes
6. Momilactone Induction
6.1. Biotic Elicitors
6.2. Abiotic Elicitors
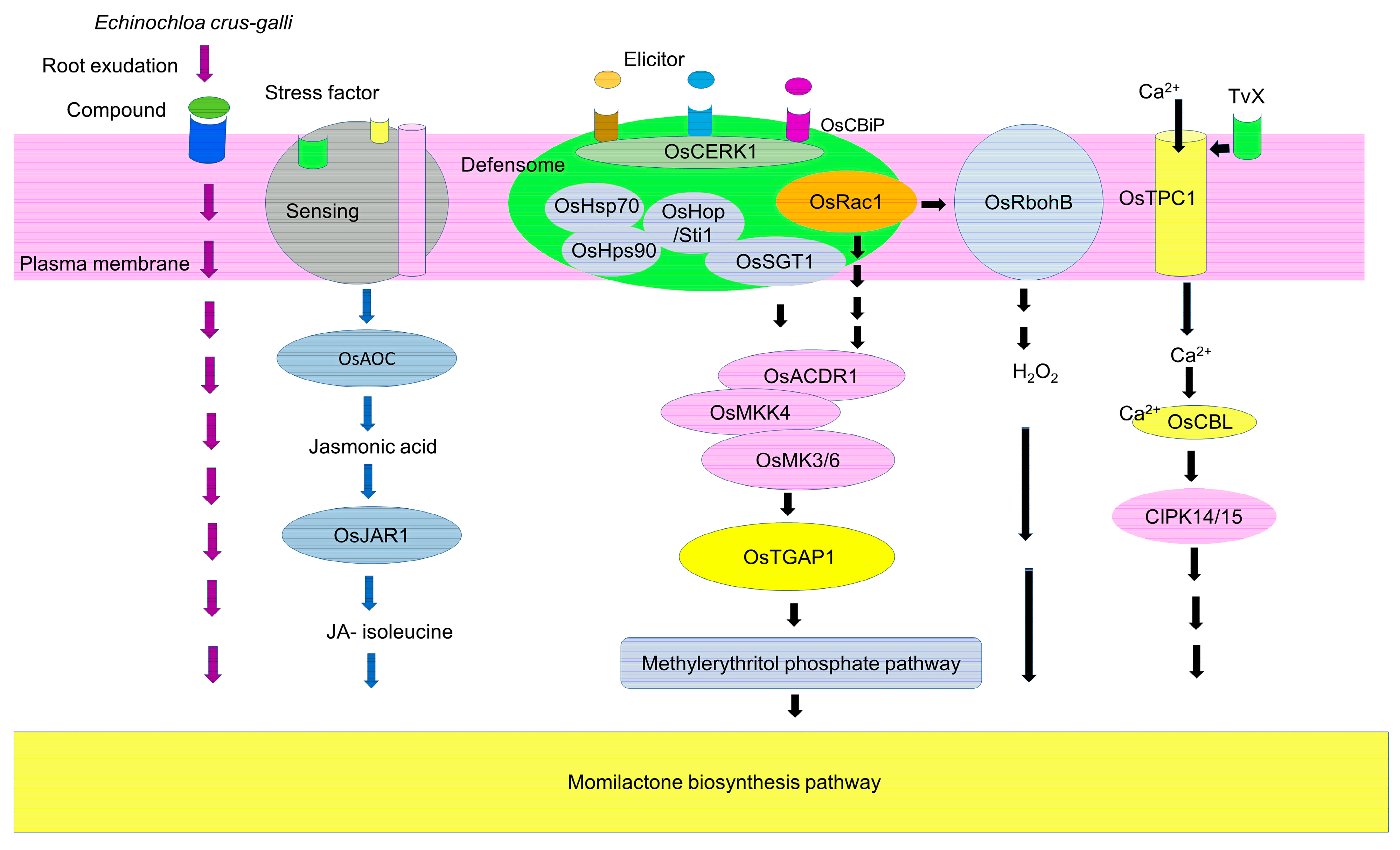
7. Induction Signaling
8. Occurrence of Momilactone
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
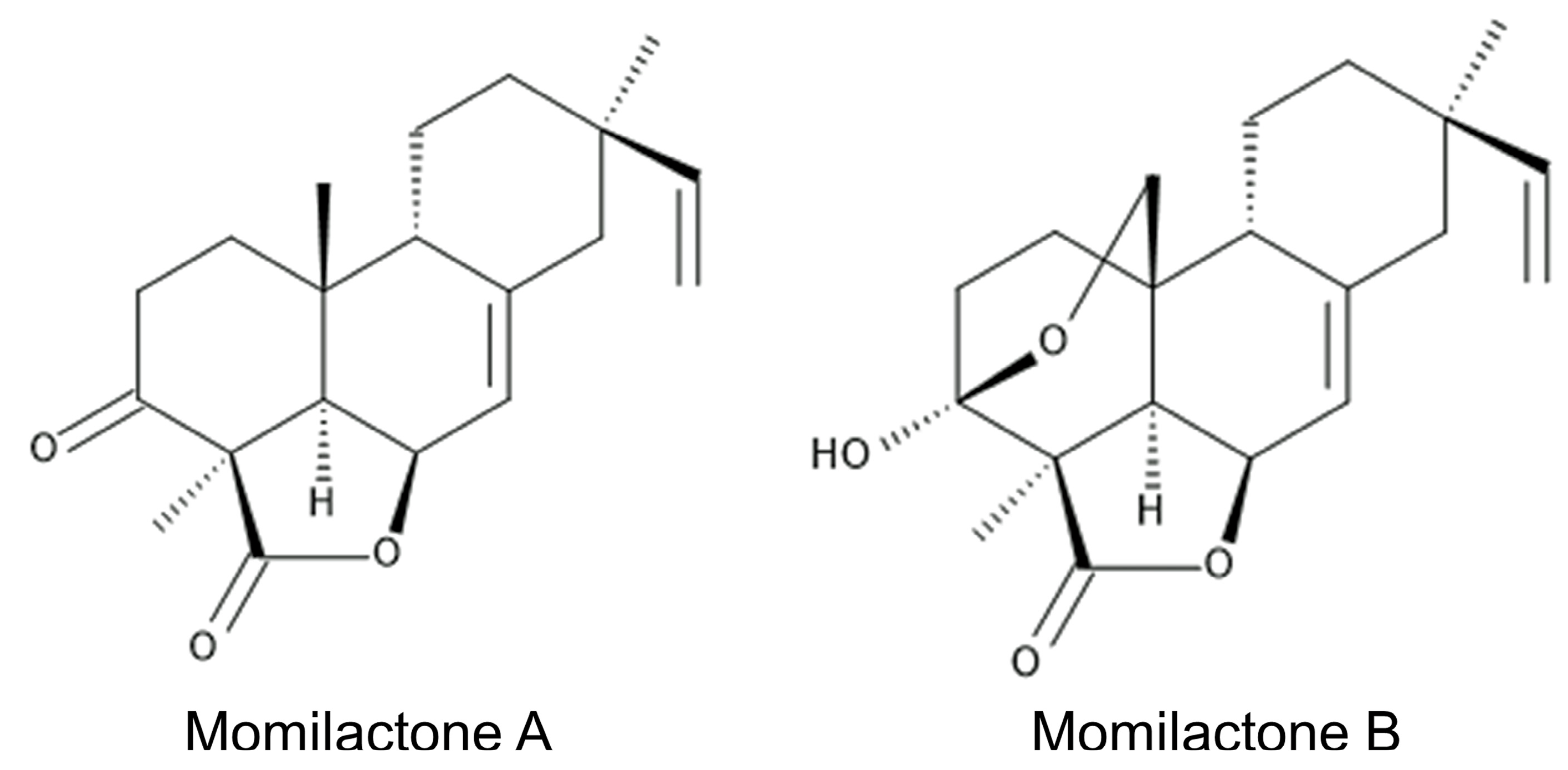
- Kato, T.; Kabuto, C.; Sasaki, N.; Tsunagawa, M.; Aizawa, H.; Fujita, K.; Kato, Y.; Takahashi, N. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett. 1973, 39, 3861–3864. [Google Scholar] [CrossRef]
- Cartwright, D.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Chemical activation of host defense mechanisms as a basis for crop protection. Nature 1977, 267, 511–513. [Google Scholar] [CrossRef]
- Cartwright, D.W.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry 1981, 20, 535–537. [Google Scholar] [CrossRef]
- Takahashi, A.; Kawasaki, T.; Henmi, K.; Shii, K.; Kodama, O.; Satoh, H.; Shimamoto, K. Lesion mimic mutants of rice with alterations in early signaling events of defense. Plant J. 1999, 17, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Mitsuhara, I.; Seo, S.; Imai, T.; Koga, J.; Okada, K.; Yamane, H.; Ohashi, Y. Phytoalexin accumulation in the interaction between rice and the blast fungus. Mol. Plant-Micro. Intrac. 2010, 23, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.K.; Rakwal, R.; Tamogami, S.; Yonekura, M.; Kubo, A.; Saji, H. Chitosan activates defense/stress response(s) in the leaves of Oryza sativa seedlings. Plant Physiol. Biochem. 2002, 40, 1061–1069. [Google Scholar] [CrossRef]
- Shimizu, T.; Jikumaru, Y.; Okada, A.; Okada, K.; Koga, J.; Umemura, K.; Minami, E.; Shibuya, N.; Hasegawa, M.; Kodama, O.; et al. Effects of a bile acid elicitor, cholic acid, on the biosynthesis of diterpenoid phytoalexins in suspension-cultured rice cells. Phytochemistry 2008, 69, 973–981. [Google Scholar] [CrossRef]
- Tamogami, S.; Kodama, O. Coronatine elicits phytoalexin production in rice leaves (Oryza sativa L.) in the same manner as jasmonic acid. Phytochemistry 2000, 54, 689–694. [Google Scholar]
- Jung, Y.H.; Lee, J.H.; Agrawal, G.K.; Rakwal, R.; Kim, J.A.; Shim, J.K.; Lee, S.K.; Jeon, J.S.; Koh, H.J.; Lee, Y.H.; et al. The rice (Oryza sativa) blast lesion mimic mutant, blm, may confer resistance to blast pathogens by triggering multiple defense-associated signaling pathways. Plant Physiol. Biochem. 2005, 43, 397–406. [Google Scholar] [CrossRef]
- Okada, A.; Shimizu, T.; Okada, K.; Kuzuyama, T.; Koga, J.; Shibuya, N.; Nojiri, H.; Yamane, H. Elicitor induced activation of the methylerythritol phosphate pathway toward phytoalexins biosynthesis in rice. Plant Mol. Biol. 2007, 65, 177–187. [Google Scholar] [CrossRef]
- Dilday, R.H.; Nastasi, P.; Smith, R.J., Jr. Allelopathic observations in rice (Oryza sativa L.) to ducksalad (Heteranthera limosa). Proc. Arkansas. Acad. Sci. 1989, 43, 21–22. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422. [Google Scholar]
- Putnam, A.R.; Tang, C.S. Allelopathy: State of the science. In The Science of Allelopathy; Putnam, A.R., Tang, C.S., Eds.; John Wiley and Sons: Ithaca, NY, USA, 1986; pp. 1–19. [Google Scholar]
- Inderjit. Plant phenolics in allelopathy. Bot. Rev. 1996, 62, 186–202. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Dilday, R.H.; Lin, J.; Yan, W. Identification of allelopathy in the USDA-ARS rice germplasm collection. Aust. J. Exp. Agric. 1994, 34, 907–910. [Google Scholar] [CrossRef]
- Dilday, R.H.; Yan, W.G.; Moldenhauer, K.A.K.; Gravois, K.A. Allelopathic activity in rice for controlling major aquatic weeds. In Allelopathy in Rice; Olofsdotter, M., Ed.; International Rice Research Institute: Manila, Philippines, 1998; pp. 7–26. [Google Scholar]
- Hassan, S.M.; Aidy, I.R.; Bastawisi, A.O.; Draz, A.E. Weed management using allelopathic rice varieties in Egypt. In Allelopathy in Rice; Olofsdotter, M., Ed.; International Rice Research Institute: Manila, Philippines, 1998; pp. 27–37. [Google Scholar]
- Kim, K.U.; Shin, D.H. Rice allelopathy research in Korea. In Allelopathy in Rice; Olofsdotter, M., Ed.; International Rice Research Institute: Manila, Philippines, 1998; pp. 39–43. [Google Scholar]
- Olofsdotter, M.; Navarez, D.; Rebulanan, M.; Streibig, J.C. Weed-suppressing rice cultivars: Does allelopathy play a role? Weed Res. 1999, 39, 441–454. [Google Scholar] [CrossRef]
- Pheng, S.; Adkins, S.; Olofsdotter, M.; Jahn, G. Allelopathic effects of rice (Oryza sativa L.) on the growth of awnless barnyardgrass (Echinochloa colona (L.) Link): A new form for weed management. Cambodian J. Agri. 1999, 2, 42–49. [Google Scholar]
- Kato-Noguchi, H.; Ino, T. Assessment of allelopathic potential of root exudate of rice seedlings. Biol. Plant. 2001, 44, 635–638. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ino, T.; Sata, N.; Yamamura, S. Isolation and identification of a potent allelopathic substance in rice root exudates. Physiol. Plant. 2002, 115, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Ino, T.; Ota, K. Secretion of momilactone A from rice roots to the rhizosphere. J. Plant Physiol. 2008, 165, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Allelopathic substance in rice root exudates: Rediscovery of momilactone B as an allelochemical. J. Plant Physiol. 2004, 161, 271–276. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Peters, R.J. The role of momilactones in rice allelopathy. J. Chem. Ecol. 2013, 39, 175–185. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Huffaker, A.; Sims, J.W.; Christensen, S.A.; Lu, X.; Okada, K.; Peters, R.J. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 2014, 79, 659–678. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Convergent or parallel molecular evolution of momilactone A and B: Potent allelochemicals, momilactones have been found only in rice and the moss Hypnum plumaeforme. J. Plant Physiol. 2011, 168, 1511–1516. [Google Scholar] [CrossRef]
- Serra, N.S.; Shanmuganathan, R.; Becker, C. Allelopathy in rice: A story of momilactones, kin recognition, and weed management. J. Exp. Bot. 2021, 72, 4022–4037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peters, R.J. Why are momilactones always associated with biosynthetic gene clusters in plants? Proc. Natl. Acad. Sci. USA 2020, 117, 13867–13869. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Shigemori, H.; Kato-Noguch, H. Allelopathic potential of Hypnum plumaeforme L. and its allelopathic substances. In Proceedings of the 4th Asia-Pacific Conference on Chemical Ecology, from Biomolecules to Ecosystems an Interactive Chemical Message for our Future, Tsukuba, Japan, 10–14 September 2007; p. 77. [Google Scholar]
- Nozaki, H.; Hayashi, K.I.; Nishimura, N.; Kawaide, H.; Matsuo, A.; Takaoka, D. Momilactone A and B as allelochemicals from moss Hypnum plumaeforme: First occurrence in bryophytes. Biosci. Biotech. Biochem. 2007, 71, 3127–3130. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kobayashi, K.; Shigemori, H. Allelopathy of the moss Hypnum plumaeforme by the production of momilactone A and B. Weed Res. 2009, 49, 621–627. [Google Scholar] [CrossRef]
- Umemura, K.; Ogawa, N.; Shimura, M.; Koga, J.; Usami, H.; Kono, T. Possible role of phytocassane, rice phytoalexin, in disease resistance of rice against the blast fungus Magnaporthe grisea. Biosci. Biotech. Biochem. 2003, 67, 899–902. [Google Scholar] [CrossRef]
- Dillon, V.M.; Overton, J.; Grayer, R.J.; Harborne, J.B. Differences in phytoalexin response among rice cultivars of different resistance to blast. Phytochemistry 1997, 44, 599–603. [Google Scholar] [CrossRef]
- Toyomasu, T.; Usui, M.; Sugawara, C.; Otomo, K.; Hirose, Y.; Miyao, A.; Hirochik, H.; Okad, K.; Shimizu, T.; Koga, J.; et al. Reverse-genetic approach to verify physiological roles of rice phytoalexins: Characterization of a knockdown mutant of OsCPS4 phytoalexin biosynthetic gene in rice. Physiol. Plant. 2014, 150, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, M.; Xuan, T.D.; Deba, F.; Tawata, S.; Dang Khanh, T.; Chung, M.I. Comparative efficacies in vitro of antibacterial, fungicidal, antioxidant, and herbicidal activities of momilatones A and B. J. Plant Interac. 2007, 2, 245–251. [Google Scholar] [CrossRef]
- Gu, C.Z.; Xia, X.M.; Lv, J.; Tan, J.W.; Baerson, S.R.; Pan, Z.Q.; Song, Y.Y.; Zeng, R.S. Diterpenoids with herbicidal and antifungal activities from hulls of rice (Oryza sativa). Fitoterapia 2019, 136, 104183. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, X.; Xiao, J.; Wang, S. A convenient method for simultaneous quantification of multiple phytohormones and metabolites: Application in study of rice-bacterium interaction. Plant Methods 2012, 8, 1–12. [Google Scholar] [CrossRef]
- Howe, G.A.; Major, I.T.; Koo, A.J. Modularity in jasmonate signaling for multistress resilience. Ann. Rev. Plant Biol. 2018, 69, 387–415. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant responses to herbivory, wounding, and infection. Int. J. Mol. Sci. 2022, 23, 7031. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Goossens, A.; Lacchini, E. Jasmonate: A hormone of primary importance for plant metabolism. Curr. Opin. Plant Biol. 2022, 67, 102197. [Google Scholar] [CrossRef] [PubMed]
- Hanai, H.; Ishida, S.; Saito, C.; Maita, T.; Kusano, M.; Tamogami, S.; Noma, M. Stimulation of mycelia growth in several mushroom species by rice husks. Biosci. Biotech. Biochem. 2005, 69, 123–127. [Google Scholar] [CrossRef]
- Chung, I.M.; Ali, M.; Ahmad, A.; Chun, S.C.; Kim, J.T.; Sultana, S.; Kim, J.S.; Seo, B.R. Steroidal constituents of rice (Oryza sativa) hulls with Algicidal and Herbicidal activity against blue-green algae and duckweed. Phytochem. Anal. 2007, 18, 133–145. [Google Scholar] [CrossRef]
- Kanno, H.; Hasegawa, M.; Kodama, O. Accumulation of salicylic acid, jasmonic acid and phytoalexins in rice, Oryza sativa, infested by the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Appl. Entomol. Zool. 2012, 47, 27–34. [Google Scholar] [CrossRef]
- Wari, D.; Alamgir, K.M.; Mujiono, K.; Hojo, Y.; Tani, A.; Shinya, T.; Nakatania, H.; Galis, I. Brown planthopper honeydew-associated symbiotic microbes elicit momilactones in rice. Plant Signal. Behav. 2019, 14, 1655335. [Google Scholar] [CrossRef] [PubMed]
- Azmi, M.; Abdullah, M.Z.; Fujii, Y. Exploratory study on allelopathic effect of selected Malaysian rice varieties and rice field weed species. J. Trop. Agric. Food Sci. 2000, 28, 39–54. [Google Scholar]
- Rao, A.N.; Johnson, D.E.; Sivaprasad, B.; Ladaha, J.K.; Mortimer, A.M. Weed management in direct-seeded rice. Adv. Agron. 2007, 93, 153–255. [Google Scholar]
- Kong, C.H. Rice allelopathy. Allelopathy J. 2008, 22, 261–278. [Google Scholar]
- Kato-Noguchi, H.; Ota, K.; Ino, T. Release of momilactone A and B from rice plants into the rhizosphere and its bioactivities. Allelopathy J. 2008, 22, 321–328. [Google Scholar]
- Kato-Noguchi, H.; Hasegawa, M.; Ino, T.; Ota, K.; Kujime, H. Contribution of momilactone A and B to rice allelopathy. J. Plant Physiol. 2010, 167, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Ota, K.; Kujime, H. Absorption of momilactone A and B by Arabidopsis thaliana L. and the growth inhibitory effects. J. Plant Physiol. 2012, 169, 1471–1476. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ota, K. Biological activities of rice allelochemicals momilactone A and B. Rice Res. 2013, 1, 2. [Google Scholar] [CrossRef]
- Takahashi, N.; Kato, T.; Tsunagawa, M.; Sasaki, N.; Kitahara, Y. Mechanisms of dormancy in rice seeds. II. New growth inhibitors, momilactone-A and -B isolated from the hulls of rice seeds. Jpn. J. Breed. 1976, 26, 91–98. [Google Scholar] [CrossRef]
- Kato, T.; Tsunakawa, M.; Sasaki, N.; Aizawa, H.; Fujita, K.; Kitahara, Y.; Takahashi, N. Growth and germination inhibitors in rice husks. Phytochemistry 1977, 16, 45–48. [Google Scholar] [CrossRef]
- Chung, I.M.; Hahh, S.J.; Ahmad, A. Confirmation of potential herbicidal agents in hulls of rice, Oryza sativa. J. Chem. Ecol. 2005, 31, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Toyomasu, T.; Kagahara, T.; Okada, K.; Koga, J.; Hasegawa, M.; Mitsuhashi, W.; Sassa, T.; Yamane, H. Diterpene phytoalexins are biosynthesized in and exuded from the roots of rice seedlings. Biosci. Biotechnol. Biochem. 2008, 72, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Yoneyama, K.; Takeuchi, Y.; Konnai, M.; Tamogami, S.; Kodama, O. Momilactones A and B in rice straw harvested at different growth stages. Biosci. Biotechnol. Biochem. 1999, 63, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Kim, T.K.; Kim, S.H. Evaluation of allelopathic potential and quantification of momilactone A, B from rice hull extracts and assessment of inhibitory bioactivity on paddy field weeds. J. Agric. Food. Chem. 2006, 54, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Ino, T. Rice seedlings release momilactone B into the environment. Phytochemistry 2003, 63, 551–554. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ino, T.; Ichii, M. Changes in release level of momilactone B into the environment from rice throughout its life cycle. Func. Plant Biol. 2003, 30, 995–997. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ino, T. Possible involvement of momilactone B in rice allelopathy. J. Plant Physiol. 2005, 162, 718–721. [Google Scholar] [CrossRef]
- Hawes, M.C.; Gunawardena, U.; Miyasaka, S.; Zhao, X. The role of root border cells in plant defense. Trends Plant Sci. 2000, 5, 128–133. [Google Scholar] [CrossRef]
- Bais, H.P.; Park, S.W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004, 9, 26–32. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Ino, T. Concentration and release level of momilactone B in the seedlings of eight rice cultivars. J. Plant Physiol. 2005, 162, 965–969. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ino, T.; Kujime, H. The relation between growth inhibition and secretion level of momilactone B from rice root. J. Plant Interact. 2010, 5, 87–90. [Google Scholar] [CrossRef]
- Mennan, H.; Ngouajio, M.; Sahin, M.; Isik, D.; Altop, E.K. Quantification of momilactone B in rice hulls and the phytotoxic potential of rice extracts on the seed germination of Alisma plantago-aquatica. Weed Biol. Manag. 2012, 12, 29–39. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Wu, C.; Xiong, L.; Chen, G.; Zhang, Q.; Wang, S. RMD: A rice mutant database for functional analysis of the rice genome. Nucleic Acids Res. 2006, 34, D745–D748. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.S.; Lee, S.; Jung, K.H.; Jun, S.H.; Jeong, D.H.; Lee, J.; Kim, C.; Jang, S.; Yang, K.; Nam, J.; et al. T-DNA insertional mutagenesis for functional genomics in rice. Plant J. 2000, 22, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Galhano, R.; Wiemann, P.; Bueno, E.; Tiernan, M.; Wu, W.; Chung, I.M.; Gershenzon, J.; Sesma, A.T.; Peters, R.J. Genetic evidence for natural product-mediated plant-plant allelopathy in rice (Oryza sativa). New Phytol. 2012, 193, 570–575. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ota, K.; Kujime, H.; Ogawa, M. Effects of momilactone on the protein expression in Arabidopsis germination: Arabidopsis and momilactone. Weed Biol. Manag. 2013, 13, 19–23. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Tenbarge, K.M.; Shumway, J.E.; Crouch, M.L. Role of ABA in maturation of rapeseed embryos. Plant Physiol. 1985, 78, 630–636. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed; Springer: New York, NY, USA, 2012; pp. 1–408. [Google Scholar]
- Job, C.; Rajjou, L.; Lovigny, Y.; Belghazi, M.; Job, D. Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiol. 2005, 138, 790–802. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kitajima, S. Momilactone sensitive proteins in Arabidopsis thaliana. Nat. Prod. Commun. 2015, 10, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Suzuki, M.; Kikuchi, J.; Saito, K.; Muranaka, T. Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 725–730. [Google Scholar] [CrossRef]
- Kuroha, T.; Okuda, A.; Arai, M.; Komatsu, Y.; Sato, S.; Kato, T.; Tabata, S.; Satoh, S. Identification of Arabidopsis subtilisin-like serine protease specifically expressed in root stele by gene trapping. Physiol. Plant. 2009, 137, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, K.; Job, C.; Groot, S.P.C.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomics of Arabidopsis seed germination. A comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol. 2002, 129, 823–837. [Google Scholar] [CrossRef]
- Eastmond, P.J.; Graham, I.A. Re-examining the role of the glyoxylate cycle in oilseeds. Trends Plant Sci. 2001, 6, 72–77. [Google Scholar] [CrossRef]
- Stacy, R.A.P.; Nordeng, T.W.; Culianez-Macia, F.A.; Reidunn, B.; Aalen, R.B. The dormancy-related peroxiredoxin anti-oxidant, PER1, is localized to the nucleus of barley embryo and aleurone cells. Plant J. 1999, 19, 1–8. [Google Scholar] [CrossRef]
- Kim, S.Y.; Paeng, S.K.; Nawkar, G.M.; Maibam, P.; Lee, E.S.; Kim, K.S.; Lee, D.H.; Park, D.J.; Kang, S.B.; Kim, M.R.; et al. The 1-cys peroxiredoxin, a regulator of seed dormancy, functions as a molecular chaperone under oxidative stress conditions. Plant Sci. 2011, 181, 119–124. [Google Scholar] [CrossRef]
- Fanucchi, F.; Alpi, E.; Olivieri, S.; Cannistraci, C.V.; Bachi, A.; Alpi, A.; Alessio, M. Acclimation increases freezing stress response of Arabidopsis thaliana at proteome level. Biochim. Biophys. Acta 2012, 1824, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Neuefeind, T.; Reinemer, P.; Bieseler, B. Plant glutathione S-transferases and herbicide detoxification (Review). Biol. Chem. 1997, 378, 199–205. [Google Scholar] [PubMed]
- Haslekås, C.; Viken, M.K.; Grini, P.E.; Nygaard, V.; Nordgard, S.H.; Meza, T.J.; Aalen, R.B. Seed 1-cysteine peroxiredoxin antioxidants are not involved in dormancy, but contribute to inhibition of germination during stress. Plant Physiol. 2003, 133, 1148–1157. [Google Scholar] [CrossRef]
- Kim, K.U.; Shin, D.H.; Lee, I.J.; Kim, H.Y.; Kim, K.U.; Shin, D.H. Rice allelopathy in Korea. In Rice Allelopathy; Kim, K.U., Shin, D.H., Eds.; Kyungpook National University: Taegu, Korea, 2000; pp. 57–82. [Google Scholar]
- Song, B.; Xiong, J.; Fang, C.; Qiu, L.; Lin, R.; Liang, Y.; Lin, W. Allelopathic enhancement and differential gene expression in rice under low nitrogen treatment. J. Chem. Ecol. 2008, 34, 688–695. [Google Scholar] [CrossRef]
- Shen, L.; Lin, W. Effects of phosphorus levels on allelopathic potential of rice co-cultured with barnyardgrass. Allelopathy J. 2007, 19, 393–402. [Google Scholar]
- Kato-Noguchi, H. Barnyard grass-induced rice allelopathy and momilactone B. J. Plant Physiol. 2011, 168, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, H.; Kong, C.; Xu, X.; Liang, W. Chemical response of allelopathic rice seedlings under varying environmental conditions. Allelopathy J. 2005, 15, 105–110. [Google Scholar]
- Kong, C.H.; Li, H.B.; Hu, F.; Xu, X.H.; Wang, P. Allelochemicals released by rice roots and residues in soil. Plant Soil 2006, 288, 47–56. [Google Scholar] [CrossRef]
- Li, L.L.; Zhao, H.H.; Kong, C.H. (–)-Loliolide, the most ubiquitous lactone, is involved in barnyardgrass-induced rice allelopathy. J. Exp. Bot. 2020, 71, 1540–1550. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. The chemical cross talk between rice and barnyardgrass. Plant Signal. Behav. 2011, 6, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Ino, T. The chemical-mediated allelopathic interaction between rice and barnyard grass. Plant Soil 2013, 370, 267–275. [Google Scholar] [CrossRef]
- Yang, X.F.; Kong, C.H. Interference of allelopathic rice with paddy weeds at the root level. Plant Biol. 2017, 19, 584–591. [Google Scholar] [CrossRef]
- Bi, H.H.; Zeng, R.Z.; Su, L.M.; An, M.; Luo, S.H. Rice allelopathy induced by methyl jasmonate and methyl salicylate. J. Chem. Ecol. 2007, 33, 1089–1103. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kujime, H.; Ino, T. UV-induced momilactone B accumulation in rice rhizosphere. J. Plant Physiol. 2007, 164, 1548–1551. [Google Scholar] [CrossRef]
- Chung, I.M.; Ali, M.; Hahn, S.J.; Siddiqui, N.A.; Lim, Y.H.; Ahmad, A. Chemical constituents from the hulls of Oryza sativa with cytotoxic activity. Chem. Nat. Compd. 2005, 41, 182–189. [Google Scholar] [CrossRef]
- Anh, L.H.; Lam, V.Q.; Takami, A.; Khanh, T.D.; Quan, N.V.; Xuan, T.D. Cytotoxic mechanism of momilactones A and B against acute promyelocytic leukemia and multiple myeloma cell Lines. Cancers 2022, 14, 4848. [Google Scholar] [CrossRef]
- Park, C.; Jeong, N.Y.; Kim, G.Y.; Han, M.H.; Chung, I.M.; Kim, W.J.; Yoo, Y.Y.; Choi, Y.H. Momilactone B induces apoptosis and G1 arrest of the cell cycle in human monocytic leukemia U937 cells through downregulation of pRB phosphorylation and induction of the cyclin-dependent kinase inhibitor p21Waf1/Cip1. Oncol. Rep. 2014, 31, 1653–1660. [Google Scholar] [CrossRef]
- Lee, S.C.; Chung, I.M.; Jin, Y.J.; Song, Y.S.; Seo, S.Y.; Park, B.S.; Vho, K.H.; Yoo, K.S.; Yee, S.B.; Yoo, Y.H. Momilactone B, an allelochemical of rice hulls, induces apoptosis on human lymphoma cells (Jurkat) in a micromolar concentration. Nutr. Cancer 2008, 60, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Joung, Y.H.; Lim, E.J.; Kim, M.S.; Lim, S.D.; Yoon, S.Y.; Lim, Y.C.; Yoo, Y.B.; Ye, S.K.; Park, T.; Chung, I.M.; et al. Enhancement of hypoxia-induced apoptosis of human breast cancer cells via STAT5b by momilactone B. Int. J. Oncol. 2008, 33, 477–484. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, H.R.; Park, E.; Lee, S.C. Cytotoxic and antitumor activity of momilactone B from rice hulls. J. Agric. Food Chem. 2007, 55, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.G.; Cha, B.J.; Lee, S.M.; Shrestha, S.; Jeong, R.H.; Lee, D.S.; Kim, Y.C.; Lee, D.G.; Kang, H.C.; Jiyoung Kima, J.; et al. Diterpenes from the roots of Oryza sativa L. and their inhibition activity on NO production in LPS-stimulated RAW264. 7 macrophages. Chem. Biodivers. 2015, 12, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.V.; Tran, H.D.; Xuan, T.D.; Ahmad, A.; Dat, T.D.; Khanh, T.D.; Teschke, R. Momilactones A and B are α-amylase and α-glucosidase inhibitors. Molecules 2019, 24, 482. [Google Scholar] [CrossRef]
- Quan, N.; Xuan, T.D.; Tran, H.D.; Ahmad, A.; Khanh, T.D.; Dat, T.D. Contribution of momilactones A and B to diabetes inhibitory potential of rice bran: Evidence from in vitro assays. Saudi Pharm. J. 2019, 27, 643–649. [Google Scholar] [CrossRef]
- Kang, D.Y.; SP, N.; Darvin, P.; Joung, Y.H.; Byun, H.J.; Do, C.H.; Park, K.D.; Cho, K.H.; Yang, Y.M. Momilactone B inhibits ketosis in vitro by regulating the ANGPTL3-LPL pathway and inhibiting HMGCS2. Anim. Biotechnol. 2017, 28, 189–197. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, B.; Jun, H.J.; Seo, W.D.; Kim, D.W.; Cho, K.J.; Lee, S.J. Momilactione B inhibits protein kinase A signaling and reduces tyrosinase-related proteins 1 and 2 expression in melanocytes. Biotechnol. Let. 2012, 4, 805–812. [Google Scholar] [CrossRef]
- Vranova, E.; Coman, D.; Gruissem, W. Network analysis of the VA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 4, 665–700. [Google Scholar] [CrossRef]
- Nemoto, T.; Cho, E.M.; Okada, A.; Okada, K.; Otomo, K.; Kanno, Y.; Toyomasu, T.; Mitsuhashi, W.; Sassa, T.; Minami, E.; et al. Stemar-13-ene synthase, a diterpene cyclase involved in the biosynthesis of the phytoalexin oryzalexin S in rice. FEBS Lett. 2004, 571, 182–186. [Google Scholar] [CrossRef]
- Otomo, K.; Kanno, Y.; Motegi, A.; Kenmoku, H.; Yamane, H.; Mitsuhashi, W.; Okikawa, H.; Toshima, H.; Itoh, H.; Matsuoka, M.; et al. Diterpene cyclases responsible or the biosynthesis of phytoalexins, momilactones A, B, and oryzalexins A-F in rice. Biosci. Biotechnol. Biochem. 2004, 68, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Hillwig, M.L.; Prisic, S.; Coates, R.M.; Peters, R.J. Functional identification of rice syn-copalyl diphosphate synthase and its role in initiating biosynthesis of diterpenoid phytoalexin/allelopathic natural products. Plant J. 2004, 39, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Otomo, K.; Kenmoku, H.; Oikawa, H.; Konig, W.A.; Toshima, H.; Mitsuhashi, W.; Yamane, H.; Sassa, T.; Toyomasu, T. Biological functions of ent- and syn-copalyl diphosphate synthases in rice: Key enzymes for the branch point of gibberellin and phytoalexin biosynthesis. Plant J. 2004, 39, 886–893. [Google Scholar] [CrossRef]
- Sakamoto, T.; Miura, K.; Itoh, H.; Tatsumi, T.; Ueguchi-Tanaka, M.; Ishiyama, K.; Kobayashi, M.; Agrawal, G.K.; Takeda, S.; Abe, K.; et al. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 2004, 134, 1642–1653. [Google Scholar] [CrossRef]
- Wilderman, P.R.; Xu, M.; Jin, Y.; Coates, R.M.; Peters, R.J. Identification of syn-pimara-7,15-diene synthase reveals functional clustering of terpene synthases involved in rice phytoalexin/allelochemical biosynthesis. Plant Physiol. 2004, 135, 2098–2105. [Google Scholar] [CrossRef]
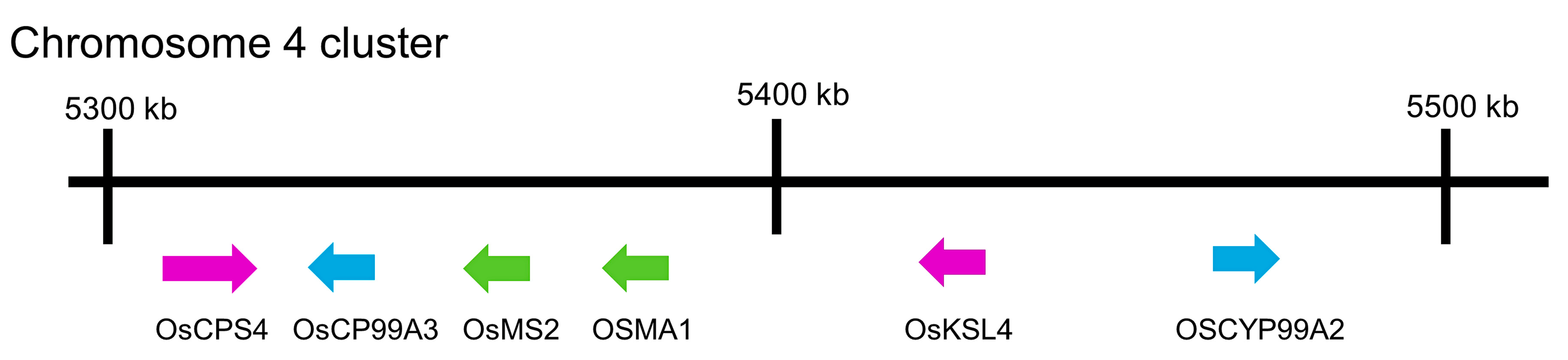
- Shimura, K.; Okada, A.; Okada, K.; Jikumaru, Y.; Ko, K.W.; Toyomasu, T.; Sassa, T.; Hasegawa, M.; Kodama, O.; Shibuya, N.; et al. Identification of a biosynthetic gene cluster in rice for momilactones. J. Biol. Chem. 2007, 282, 34013–34018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hillwig, M.L.; Peters, R.J. CYP99A3: Functional identification of a diterpene oxidase from the momilactone biosynthetic gene cluster in rice. Plant J. 2011, 65, 87–95. [Google Scholar] [CrossRef]
- Kitaoka, N.; Zhang, J.; Oyagbenro, R.K.; Brown, B.; Wu, Y.; Yang, B.; Li, Z.; Peters, R.J. Interdependent evolution of biosynthetic gene clusters for momilactone production in rice. Plant Cell 2021, 33, 290–305. [Google Scholar] [CrossRef] [PubMed]
- De La Peña, R.; Sattely, E.S. Rerouting plant terpene biosynthesis enables momilactone pathway elucidation. Nat. Chem. Biol. 2020, 17, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, J.; Li, Z.; Peters, R.J.; Yang, B. Dissecting the labdane-related diterpenoid biosynthetic gene clusters in rice reveals directional cross-cluster phytotoxicity. New Phytologist. 2022, 233, 878–889. [Google Scholar] [CrossRef]
- Walters, D.; Walsh, D.; Newton, A.; Lyon, G. Induced resistance for plant disease control: Maximizing the effcacy of resistance elicitors. Phytopathology 2005, 95, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Langner, T.; Kamoun, S.; Belhaj, K. CRISPR crops: Plant genome editing toward disease resistance. Annu. Rev. Phytopathol. 2018, 56, 479–512. [Google Scholar] [CrossRef]
- Van, D.E.; Koornneef, A.; Ton, J.; Pieterse, C.M. Induced resistance-orchestrating defence mechanisms through crosstalk and priming. Annu. Plant Rev. 2018, 34, 334–370. [Google Scholar]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Bowman, S.M.; Free, S.J. The structure and synthesis of the fungal cell wall. BioEssays 2006, 28, 799–808. [Google Scholar] [CrossRef]
- Ma, B.; Wang, J.; Liu, C.; Hu, J.; Tan, K.; Zhao, F.; Yuan, M.; Zhang, J.; Gai, Z. Preventive effects of fluoro-substituted benzothiadiazole derivatives and chitosan oligosaccharide against the rice seedling blight induced by Fusarium oxysporum. Plants 2019, 8, 538. [Google Scholar] [CrossRef]
- Yamada, A.; Shibuya, N.; Kodama, O.; Akatsuka, T. Induction of phytoalexin formation in suspension-cultured rice cells by N-acetylchitooligosaccharides. Biosci. Biotech. Biochem. 1993, 57, 405–409. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yamada, A.; Hong, N.; Ogawa, T.; Ishii, T.; Shibuya, N. Differences in the recognition of glucan elicitor signals between rice and soybean: B-glucan fragments from the rice blast disease fungus Pyricularia oryzae that elicit phytoalexin biosynthesis in suspension-cultured rice cells. Plant Cell 2000, 12, 817–826. [Google Scholar] [PubMed]
- MacKintosh, C.; Lyon, G.D.; MacKintosh, R.W. Protein phosphatase inhibitors activate anti-fungal defense responses of soybean cotyledons and cell cultures. Plant J. 1994, 5, 137–147. [Google Scholar] [CrossRef]
- Rakwal, R.; Shii, K.; Agrawal, G.K.; Yonekura, M. Protein phosphatase inhibitors activate defense responses in rice (Oryza sativa) leaves. Physiol. Plant. 2001, 111, 151–157. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kobayashi, K. Jasmonic acid, protein phosphatase inhibitor, metals and UV-irradiation increased momilactone A and B concentrations in the moss Hypnum plumaeforme. J. Plant Physiol. 2009, 166, 1118–1122. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Stress-induced allelopathic activity and momilactone B in rice. Plant Growth Regul. 2009, 59, 153–158. [Google Scholar] [CrossRef]
- Koga, J.; Yamauchi, T.; Shimura, M.; Ogawa, N.; Oshima, K.; Umemura, K.; Kikuchi, M.; Ogasawara, N. Cerebrosides A and C, sphingolipid elicitors of hypersensitive cell death and phytoalexin accumulation in rice plants. J. Biol. Chem. 1998, 273, 31985–31991. [Google Scholar] [CrossRef]
- Nakazato, Y.; Tamogami, S.; Kawai, H.; Hasegawa, M.; Kodama, O. Methionine-induced phytoalexin production in rice leaves. Biosci. Biotech. Biochem. 2000, 64, 577–583. [Google Scholar] [CrossRef]
- Kodama, O.; Suzuki, T.; Miyakawa, J.; Akatsuka, T. Ultraviolet-induced accumulation of phytoalexins in rice leaves. Agric. Biol. Chem. 1988, 52, 2469–2473. [Google Scholar]
- Kodama, O.; Yamada, A.; Yamamoto, A.; Takemoto, T.; Akatsuka, T. Induction of phytoalexins with heavy metal ions in rice leaves. J. Pesticide Sci. 1988, 13, 615–617. [Google Scholar] [CrossRef]
- Rakwal, R.; Tamogami, S.; Kodama, O. Role of jasmonic acid as a signaling molecule in copper chloride-elicited rice phytoalexin production. Biosci. Biotech. Biochem. 1996, 60, 1046–1048. [Google Scholar] [CrossRef]
- Qin, J.; Wand, Y.; He, G. Induction of phytoalexins (PA) formation in suspension-cultured rice cells by metal and nonmetal Ions. Chin. J. Appl. Environ. Biol. 2006, 12, 322. [Google Scholar]
- Rakwal, R.; Agrawal, G.K.; Kubo, A.; Yonekura, M.; Tamogami, S.; Saji, H.; Iwahashi, H. Defense/stress responses elicited in rice seedlings exposed to the gaseous air pollutant sulfur dioxide. Environ. Exp. Bot. 2003, 49, 223–235. [Google Scholar] [CrossRef]
- Tamogami, S.; Kodama, O.; Hirose, K.; Akatsuka, T. Pretilachlor [2-chloro-N-(2, 6-diethylphenyl)-N-(2-propoxyethyl) acetamide]-and butachlor [N-(butoxymethyl)-2-chloro-N-(2, 6-diethylphenyl) acetamide]-induced accumulation of phytoalexin in rice (Oryza sativa) plants. J. Agric. Food Chem. 1995, 43, 1695–1697. [Google Scholar] [CrossRef]
- Ji, C.; Norton, R.A.; Wicklow, D.T.; Dowd, P.F. Isoform patterns of chitinase and b-1,3-glucanase in maturing corn kernels (Zea mays L.) associated with Aspergillus flavus milk stage infection. J. Agric. Food Chem. 2000, 48, 507–511. [Google Scholar] [CrossRef]
- Rodriguez, V.M.; Santiago, R.; Malvar, R.A.; Butron, A. Inducible maize defense mechanisms against the corn borer Sesamia nonagrioides: A transcriptome and biochemical approach. Mol. Plant-Microbe Interact. 2012, 25, 61–68. [Google Scholar] [CrossRef]
- Shimizu, T.; Nakano, T.; Takamizawa, D.; Desaki, Y.; Ishii-Minami, N.; Nishizawa, Y.; Minami, E.; Okada, K.; Yamane, H.; Kaku, H.; et al. Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J. 2010, 64, 204–214. [Google Scholar] [CrossRef]
- Thao, N.P.; Chen, L.; Nakashima, A.; Hara, S.I.; Umemura, K.; Takahashi, A.; Shirasu, K.; Kawasaki, T.; Shimamoto, K. RAR1 and HSP90 form a complex with Rac/Rop GTPase and function in innate-immune responses in rice. Plant Cell 2007, 19, 4035–4045. [Google Scholar] [CrossRef]
- Seo, N.S.; Lee, S.K.; Song, M.Y.; Suh, J.P.; Hahn, T.R.; Ronald, P.; Jeon, J.S. The HSP90–SGT1–RAR1 molecular chaperone complex: A core modulator in plant immunity. J. Plant Biol. 2008, 51, 1–10. [Google Scholar] [CrossRef]
- Chen, L.; Hamada, S.; Fujiwara, M.; Zhu, T.; Thao, N.P.; Wong, H.L.; Krishna, P.; Ueda, T.; Kaku, H.; Naoto Shibuya, N.; et al. The Hop/Sti1–Hsp90 chaperone complex facilitates the maturation and transport of a PAMP receptor in rice innate immunity. Cell Host Microbe 2010, 7, 185–196. [Google Scholar] [CrossRef]
- Lieberherr, D.; Thao, N.P.; Nakashima, A.; Umemura, K.; Kawasaki, T.; Shimamoto, K. A sphingolipid elicitor-inducible mitogen-activated protein kinase is regulated by the small GTPase OsRac1 and heterotrimeric G-protein in rice. Plant Physiol. 2005, 138, 1644–1652. [Google Scholar] [CrossRef]
- Kim, J.A.; Cho, K.; Singh, R.; Jung, Y.H.; Jeong, S.H.; Kim, S.H.; Kim, S.H.; Lee, J.E.; Cho, Y.S.; Agrawal, G.K.; et al. Rice OsACDR1 (Oryza sativa accelerated cell death and resistance 1) is a potential positive regulator of fungal disease resistance. Mol. Cells 2009, 28, 431–439. [Google Scholar] [CrossRef]
- Kishi-Kaboshi, M.; Okada, K.; Kurimoto, L.; Murakami, S.; Umezawa, T.; Shibuya, N.; Yamane, H.; Miyao, A.; Takatsuji, H.; Takahashi, A.; et al. A rice fungal MAMP-responsive MAPK cascade regulates metabolic flow to antimicrobial metabolite synthesis. Plant J. 2010, 63, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Okada, K.; Miyamoto, K.; Koga, J.; Shibuya, N.; Nojiri, H.; Yamane, H. OsTGAP1, a bZIP transcription factor, coordinately regulates the inductive production of diterpenoid phytoalexins in rice. J. Biol. Chem. 2009, 284, 26510–26518. [Google Scholar] [CrossRef] [PubMed]
- Yokotani, N.; Sato, Y.; Tanabe, S.; Chujo, T.; Shimizu, T.; Okada, K.; Yamane, H.; Shimono, M.; Sugano, S.; Takatsuji, H.; et al. OsWRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J. Exp. Bot. 2013, 64, 5085–5097. [Google Scholar] [CrossRef]
- Wong, H.L.; Pinontoan, R.; Hayashi, K.; Tabata, R.; Yaeno, T.; Hasegawa, K.; Kojima, C.; Yoshioka, H.; Iba, L.; Kawasaki, T.; et al. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell 2007, 19, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Wong, H.L.; Kawasaki, T.; Hasegawa, M.; Kodama, O.; Shimamoto, K. Essential role of the small GTPase Rac in disease resistance of rice. Proc. Natl Acad. Sci. USA 2001, 98, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Ron, M.; Avni, A. The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell 2004, 16, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Kurusu, T.; Hamada, J.; Nokajima, H.; Kitagawa, Y.; Kiyoduka, M.; Takahashi, A.; Hanamata, S.; Ohno, R.; Hayashi, T.; Okada, K.; et al. Regulation of microbe-associated molecular pattern-induced hypersensitive cell death, phytoalexin production, and defense gene expression by calcineurin B-like protein-interacting protein kinases, OsCIPK14/15, in rice cultured cells. Plant Physiol. 2010, 153, 678–692. [Google Scholar] [CrossRef]
- Hamada, H.; Kurusu, T.; Okuma, E.; Nokajima, H.; Kiyoduka, M.; Koyano, T.; Sugiyama, Y.; Okada, K.; Koga, J.; Saji, H.; et al. Regulation of a proteinaceous elicitor-induced Ca2+ influx and production of phytoalexins by a putative voltage-gated cation channel, OsTPC1, in cultured rice cells. J. Biol. Chem. 2012, 287, 9931–9939. [Google Scholar] [CrossRef]
- Riemann, M.; Haga, K.; Shimizu, T.; Okada, K.; Ando, S.; Mochizuki, S.; Nishizawa, Y.; Yamanouchi, U.; Nick, P.; Yano, M.; et al. Identification of rice allene oxide cyclase mutants and the function of jasmonate for defense against Magnaporthe oryzae. Plant J. 2013, 74, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Daw, B.D.; Zhang, L.H.; Wang, Z.Z. Salicylic acid enhances antifungal resistance to Magnaporthe grisea in rice plants. Aust. Plant Pathol. 2008, 37, 637–644. [Google Scholar] [CrossRef]
- Kariya, K.; Ube, N.; Ueno, M.; Teraishi, M.; Okumoto, Y.; Mori, N.; Ueno, L.; Ishihara, A. Natural variation of diterpenoid phytoalexins in cultivated and wild rice species. Phytochemistry 2020, 180, 112518. [Google Scholar] [CrossRef]
- Miyamoto, K.; Fujita, M.; Shenton, M.R.; Akashi, S.; Sugawara, C.; Sakai, A.; Horie, K.; Hasegawa, M.; Kawaide, H.; Mitsuhashi, W.; et al. Evolutionary trajectory of phytoalexin biosynthetic gene clusters in rice. Plant J. 2016, 87, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Qiu, J.; Ye, C.; Jin, G.; Mao, L.; Zhang, H.; Yang, X.; Peng, Q.; Wang, Y.; Jia, L.; et al. Echinochloa crus-galli genome analysis provides insight into its adaptation and invasiveness as a weed. Nat. Commun. 2017, 8, 1031. [Google Scholar] [CrossRef]
- Ando, H.; Matsuo, A. Applied bryology. In Advance in Bryology, vol. 2.; W. Schultze-Motel, W., Ed.; International Association of Bryologists: Vaduz, Liechtenstein, 1984; pp. 133–224. [Google Scholar]
- Tsubota, H.; Kuroda, A.; Masuzaki, H.; Nakahara, M.; Deguchi, H. Preliminary study on allelopathic activity of bryophytes under laboratory conditions using the sandwich method. J. Hattori Bot. Lab. 2006, 100, 517–525. [Google Scholar]
- Okada, K.; Kawaide, H.; Miyamoto, K.; Miyazaki, S.; Kainuma, R.; Kimura, H.; Fujiwara, K.; Natsume, M.; Nojiri, H.; Nakajima, M.; et al. HpDTC1, a stress-inducible bifunctional diterpene cyclase involved in momilactone biosynthesis, functions in chemical defense in the moss Hypnum plumaeforme. Sci. Rep. 2006, 6, 1–12. [Google Scholar]
- Mao, L.; Kawaide, H.; Higuchi, T.; Chen, M.; Miyamoto, K.; Hirata, Y.; Kimura, H.; Miyazaki, S.; Teruya, M.; Fujiwara, K.; et al. Genomic evidence for convergent evolution of gene clusters for momilactone biosynthesis in land plants. Proc. Natl Acad. Sci. USA 2020, 117, 12472–12480. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Secretion of momilactone A and B by the moss Hypnum plumaeforme. Plant Signal. Behav. 2009, 4, 737–739. [Google Scholar] [CrossRef]
| Momilactone A | Momilactone B | Reference | |||
|---|---|---|---|---|---|
| Target Plant Species | Roots | Shoots | Roots | Shoots | |
| Echinochola crus-gall | 28.7 | 46.4 | 6.1 | 6.3 | [53] |
| Echinochloa colonum | 65.4 | 240 | 5.04 | 12.5 | [52] |
| Phleum pratense | 76.5 | 157 | 5.6 | 7.9 | [55] |
| Digitaria sanguinalis | 98.5 | 275 | 9.5 | 12.4 | [55] |
| Lolium multiflorum | 91.9 | 138 | 6.9 | 6.5 | [55] |
| Arabidopsis thiliana | 203 | 84.4 | 12 | 6.5 | [54] |
| Lepidium sativum | 425 | 285 | 6.3 | 4.6 | [35] |
| Lactuca sativa | 472 | 395 | 54.3 | 77.9 | [55] |
| Medicago sativa | 379 | 315 | 67.8 | 82.4 | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato-Noguchi, H. Defensive Molecules Momilactones A and B: Function, Biosynthesis, Induction and Occurrence. Toxins 2023, 15, 241. https://doi.org/10.3390/toxins15040241
Kato-Noguchi H. Defensive Molecules Momilactones A and B: Function, Biosynthesis, Induction and Occurrence. Toxins. 2023; 15(4):241. https://doi.org/10.3390/toxins15040241
Chicago/Turabian StyleKato-Noguchi, Hisashi. 2023. "Defensive Molecules Momilactones A and B: Function, Biosynthesis, Induction and Occurrence" Toxins 15, no. 4: 241. https://doi.org/10.3390/toxins15040241
APA StyleKato-Noguchi, H. (2023). Defensive Molecules Momilactones A and B: Function, Biosynthesis, Induction and Occurrence. Toxins, 15(4), 241. https://doi.org/10.3390/toxins15040241