Challenges and Opportunities in Clinical Diagnostic Routine of Envenomation Using Blood Plasma Proteomics
Abstract
1. Introduction
2. Diagnostic and Monitoring Tools for Envenomation: An Urgent Need
2.1. Snakebite
2.2. Scorpion Envenomation
2.3. Honey Bee Stings
2.4. Spider Envenomation
3. Biomarkers for Envenomation by Venomous Animals: What to Look For?
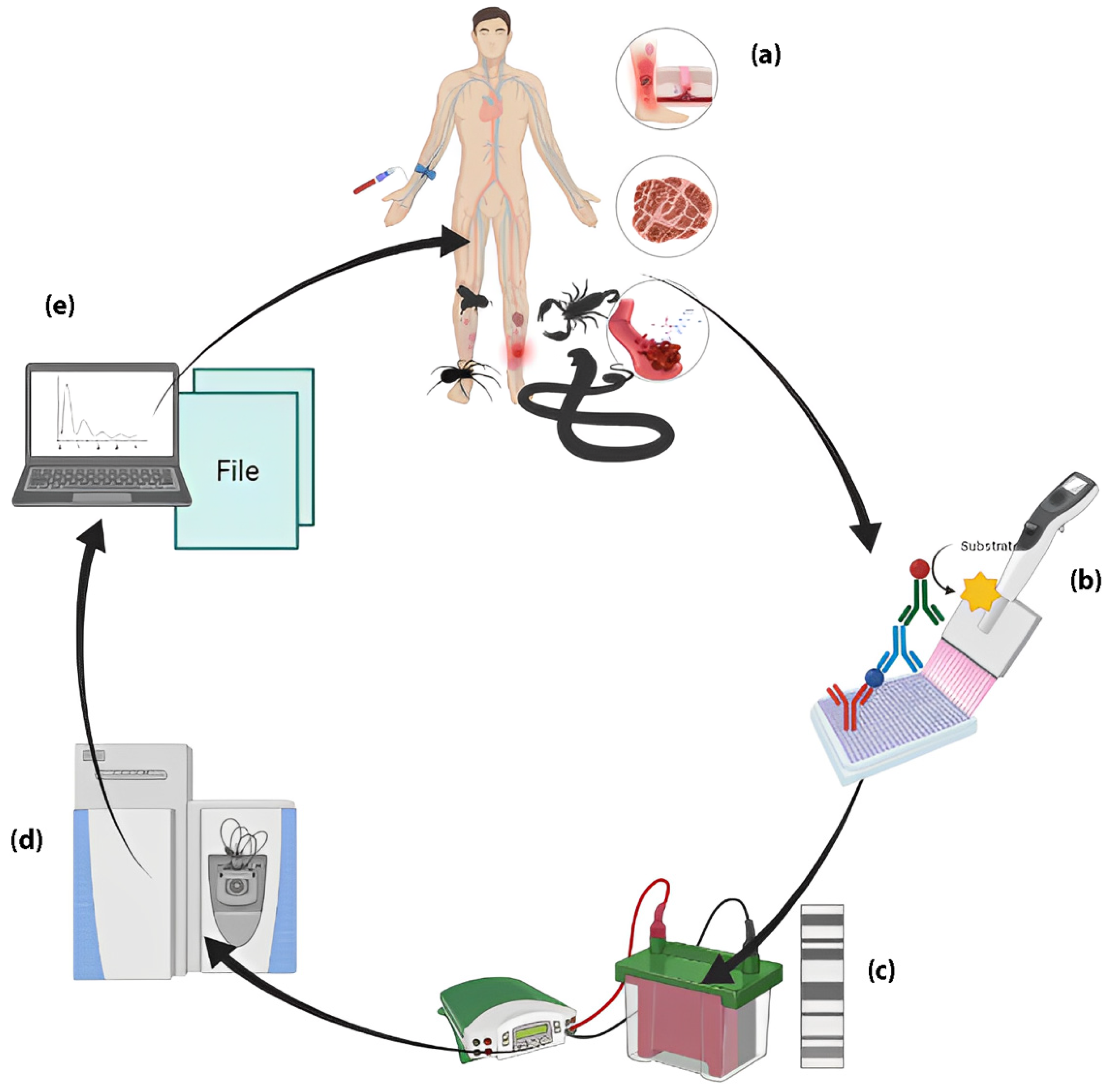
4. Biomarker’s Development Phase
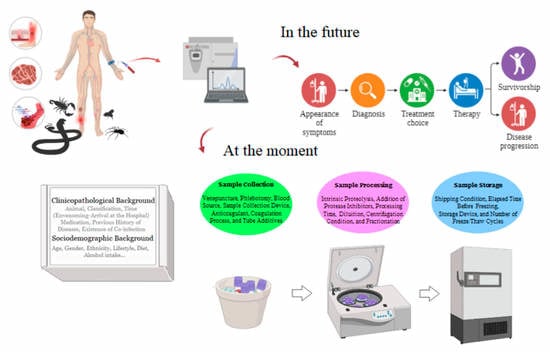
5. Preanalytical Variables: Sample Preparation Challenges
6. Sociodemographic Background
7. Clinicopathological Background
8. Other Directions for Studies of Biomarkers in Envenomations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, H.F.; Layfield, H.J.; Vallance, T.; Patel, K.; Bicknell, A.B.; Trim, S.A.; Vaiyapuri, S. The Urgent Need to Develop Novel Strategies for the Diagnosis and Treatment of Snakebites. Toxins 2019, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Abroug, F.; Ouanes-Besbes, L.; Tilouche, N.; Elatrous, S. Scorpion Envenomation: State of the Art. Intensive Care Med. 2020, 46, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Kono, I.S.; Freire, R.L.; Caldart, E.T.; de Souza Rodrigues, F.; Santos, J.A.; Freire, L.G.D.; Faccin, T.C. Bee Stings in Brazil: Epidemiological Aspects in Humans. Toxicon 2021, 201, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.C.; Min, K.; Hamrick, P.N.; Montebello, L.R.; Ranieri, T.M.; Mardini, L.; Camara, V.M.; Raggio Luiz, R.; Liese, B.; Vuckovic, M.; et al. Overview of Snakebite in Brazil: Possible Drivers and a Tool for Risk Mapping. PLoS Negl. Trop. Dis. 2021, 15, e0009044. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Cerni, F.A.; Oliveira, I.S.; Jenkins, T.P.; Argemí, L.; Sørensen, C.V.; Ahmadi, S.; Barbosa, J.E.; Laustsen, A.H. Bee Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Front. Immunol. 2019, 10, 2090. [Google Scholar] [CrossRef]
- Teixeira, C.; Fernandes, C.M.; Leiguez, E.; Chudzinski-Tavassi, A.M. Inflammation Induced by Platelet-Activating Viperid Snake Venoms: Perspectives on Thromboinflammation. Front. Immunol. 2019, 10, 2082. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Cavalcante, J.; Nogueira Júnior, F.A.; Bezerra Jorge, R.J.; Almeida, C. Pain Modulated by Bothrops Snake Venoms: Mechanisms of Nociceptive Signaling and Therapeutic Perspectives. Toxicon 2021, 201, 105–114. [Google Scholar] [CrossRef]
- Pucca, M.B.; Cerni, F.A.; Janke, R.; Bermúdez-Méndez, E.; Ledsgaard, L.; Barbosa, J.E.; Laustsen, A.H. History of Envenoming Therapy and Current Perspectives. Front. Immunol. 2019, 10, 1598. [Google Scholar] [CrossRef]
- Laustsen, A.H.; María Gutiérrez, J.; Knudsen, C.; Johansen, K.H.; Bermúdez-Méndez, E.; Cerni, F.A.; Jürgensen, J.A.; Ledsgaard, L.; Martos-Esteban, A.; Øhlenschlæger, M.; et al. Pros and Cons of Different Therapeutic Antibody Formats for Recombinant Antivenom Development. Toxicon 2018, 146, 151–175. [Google Scholar] [CrossRef]
- de Castañeda, R.R.; Durso, A.M.; Ray, N.; Fernández, J.L.; Williams, D.J.; Alcoba, G.; Chappuis, F.; Salathé, M.; Bolon, I. Snakebite and Snake Identification: Empowering Neglected Communities and Health-Care Providers with AI. Lancet Digit. Health 2019, 1, e202–e203. [Google Scholar] [CrossRef]
- Pucca, M.B.; Knudsen, C.; Oliveira, I.S.; Rimbault, C.; Cerni, F.A.; Wen, F.H.; Sachett, J.; Sartim, M.A.; Laustsen, A.H.; Monteiro, W.M. Current Knowledge on Snake Dry Bites. Toxins 2020, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Daswani, B. Comparison of Different Dosing Protocols of Anti-Snake Venom (ASV) in Snake Bite Cases. J. Clin. Diagn. Res. 2017, 11, FC17–FC21. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Patel, S.K.; Kumar, V.; Damahe, J.; Srivastava, S. Differential Expression of Serum/Plasma Proteins in Various Infectious Diseases: Specific or Nonspecific Signatures. Proteom. Clin. Appl. 2014, 8, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, C.; Jürgensen, J.A.; Føns, S.; Haack, A.M.; Friis, R.U.W.; Dam, S.H.; Bush, S.P.; White, J.; Laustsen, A.H. Snakebite Envenoming Diagnosis and Diagnostics. Front. Immunol. 2021, 12, 661457. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, L.; Huang, C.; Li, Q.; Nice, E.C. Proteomic Profiling of Human Plasma for Cancer Biomarker Discovery. Proteomics 2017, 17, 1600240. [Google Scholar] [CrossRef]
- Suvarna, K.; Biswas, D.; Pai, M.G.J.; Acharjee, A.; Bankar, R.; Palanivel, V.; Salkar, A.; Verma, A.; Mukherjee, A.; Choudhury, M.; et al. Proteomics and Machine Learning Approaches Reveal a Set of Prognostic Markers for COVID-19 Severity With Drug Repurposing Potential. Front. Physiol. 2021, 12, 652799. [Google Scholar] [CrossRef]
- Kumar, V.; Ray, S.; Aggarwal, S.; Biswas, D.; Jadhav, M.; Yadav, R.; Sabnis, S.V.; Banerjee, S.; Talukdar, A.; Kochar, S.K.; et al. Multiplexed Quantitative Proteomics Provides Mechanistic Cues for Malaria Severity and Complexity. Commun. Biol. 2020, 3, 683. [Google Scholar] [CrossRef]
- Theakston, R.; Laing, G. Diagnosis of Snakebite and the Importance of Immunological Tests in Venom Research. Toxins 2014, 6, 1667–1695. [Google Scholar] [CrossRef]
- Hifumi, T.; Sakai, A.; Kondo, Y.; Yamamoto, A.; Morine, N.; Ato, M.; Shibayama, K.; Umezawa, K.; Kiriu, N.; Kato, H.; et al. Venomous Snake Bites: Clinical Diagnosis and Treatment. J. Intensive Care 2015, 3, 16. [Google Scholar] [CrossRef]
- Lamb, T.; Abouyannis, M.; de Oliveira, S.S.; Shenoy, K.R.; Geevar, T.; Zachariah, A.; Sharma, S.K.; Bhatt, N.; Mukaka, M.; Harriss, E.; et al. The 20-Minute Whole Blood Clotting Test (20WBCT) for Snakebite Coagulopathy—A Systematic Review and Meta-Analysis of Diagnostic Test Accuracy. PLoS Negl. Trop. Dis. 2021, 15, e0009657. [Google Scholar] [CrossRef]
- Hamza, M.; Knudsen, C.; Gnanathasan, C.A.; Monteiro, W.; Lewin, M.R.; Laustsen, A.H.; Habib, A.G. Clinical Management of Snakebite Envenoming: Future Perspectives. Toxicon X 2021, 11, 100079. [Google Scholar] [CrossRef] [PubMed]
- Bucaretchi, F.; Pimenta, M.M.B.; Borrasca-Fernandes, C.F.; Prado, C.C.; de Capitani, E.M.; Hyslop, S. Thrombotic Microangiopathy Following Bothrops Jararaca Snakebite: Case Report. Clin. Toxicol. 2019, 57, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Malaque, C.M.S.; Duayer, I.F.; Santoro, M.L. Acute Kidney Injury Induced by Thrombotic Microangiopathy in Two Cases of Bothrops Envenomation. Clin. Toxicol. 2019, 57, 213–216. [Google Scholar] [CrossRef]
- Mota, S.M.B.; Albuquerque, P.L.M.M.; da Silva Júnior, G.B.; Daher, E.D.F. Thrombotic Microangiopathy Due to Bothrops Erythromelas: A Case Report in Northeast Brazil. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e53. [Google Scholar] [CrossRef]
- Noutsos, T.; Currie, B.J.; Isbister, G.K. Snakebite Associated Thrombotic Microangiopathy: A Protocol for the Systematic Review of Clinical Features, Outcomes, and Role of Interventions. Syst. Rev. 2019, 8, 212. [Google Scholar] [CrossRef]
- Luciano, P.M.; Silva, G.E.B.; Azevedo-Marques, M.M. Acidente Botrópico Fatal. Medicina 2009, 42, 61–65. [Google Scholar] [CrossRef]
- Valle, L.A.; Silva, D.D.F.R.; Magalhães, P.H.; Mattos, P.A.; Leal, J.A. Bilateral amputation of inferior extremities due to serious Bothrops accident: A case report. Arq. Med. Hosp. Fac. Cienc. Med. Santa Casa São Paulo 2008, 53, 81–84. [Google Scholar]
- Graciano, A.R.; De Carvalho, K.C.N. Compartimental syndrome associated to snake bite of the Bothrops gender: Case report. Rev Pesq Saúde 2017, 18, 54–56. [Google Scholar]
- Lippi, G.; Schena, F.; Ceriotti, F. Diagnostic Biomarkers of Muscle Injury and Exertional Rhabdomyolysis. Clin. Chem. Lab. Med. 2018, 57, 175–182. [Google Scholar] [CrossRef]
- Delanghe, J.R.; Speeckaert, M.M.; de Buyzere, M.L. Is Creatine Kinase an Ideal Biomarker in Rhabdomyolysis? Reply to Lippi et al.: Diagnostic Biomarkers of Muscle Injury and Exertional Rhabdomyolysis (https://doi.org/10.1515/cclm-2018-0656). Clin. Chem. Lab. Med. 2019, 57, e75–e76. [Google Scholar] [CrossRef]
- Kularatne, S.A.M.; Budagoda, B.D.S.S.; Gawarammana, I.B.; Kularatne, W.K.S. Epidemiology, Clinical Profile and Management Issues of Cobra (Naja Naja) Bites in Sri Lanka: First Authenticated Case Series. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Nicoleti, A.F.; de Medeiros, C.R.; Duarte, M.R.; de Siqueira França, F.O. Comparison of Bothropoides Jararaca Bites with and without Envenoming Treated at the Vital Brazil Hospital of the Butantan Institute, State of São Paulo, Brazil. Rev. Soc. Bras. Med. Trop. 2010, 43, 657–661. [Google Scholar] [CrossRef]
- Spano, S.; Macias, F.; Snowden, B.; Vohra, R. Snakebite Survivors Club: Retrospective Review of Rattlesnake Bites in Central California. Toxicon 2013, 69, 38–41. [Google Scholar] [CrossRef]
- Valenta, J.; Stach, Z.; Stříteský, M.; Michálek, P. Common Viper Bites in the Czech Republic—Epidemiological and Clinical Aspects during 15 Year Period (1999–2013). Prague Med. Rep. 2014, 115, 120–127. [Google Scholar] [CrossRef]
- Roth, B.; Sharma, K.; Onisko, N.; Chen, T. Prospective Evaluation of Pain, Swelling, and Disability from Copperhead Envenomation. Clin. Toxicol. 2016, 54, 271–276. [Google Scholar] [CrossRef]
- Bawaskar, H.; Bawaskar, P. Diagnosis of Envenomation by Russell’s and Echis Carinatus Viper: A Clinical Study at Rural Maharashtra State of India. J. Family Med. Prim. Care 2019, 8, 1386. [Google Scholar] [CrossRef]
- Soares, F.G.S.; Ibiapina, H.N.; Sartim, M.A.; Mendonça-da-Silva, I.; Nascimento, E.F.; Ferreira, L.C.L.; Cerni, F.A.; Malheiro, A.; Pucca, M.B.; Wen, F.H.; et al. Lower Levels of CXCL-8 and IL-2 on Admission as Predictors of Early Adverse Reactions to Bothrops Antivenom in the Brazilian Amazon. Cytokine 2022, 152, 155825. [Google Scholar] [CrossRef]
- Dong, L. Immunogenicity of Venoms from Four Common Snakes in the South of Vietnam and Development of ELISA Kit for Venom Detection. J. Immunol. Methods 2003, 282, 13–31. [Google Scholar] [CrossRef]
- Kulawickrama, S.; O’Leary, M.A.; Hodgson, W.C.; Brown, S.G.A.; Jacoby, T.; Davern, K.; Isbister, G.K. Development of a Sensitive Enzyme Immunoassay for Measuring Taipan Venom in Serum. Toxicon 2010, 55, 1510–1518. [Google Scholar] [CrossRef]
- Liu, C.-C.; Yu, J.-S.; Wang, P.-J.; Hsiao, Y.-C.; Liu, C.-H.; Chen, Y.-C.; Lai, P.-F.; Hsu, C.-P.; Fann, W.-C.; Lin, C.-C. Development of Sandwich ELISA and Lateral Flow Strip Assays for Diagnosing Clinically Significant Snakebite in Taiwan. PLoS Negl. Trop. Dis. 2018, 12, e0007014. [Google Scholar] [CrossRef]
- Macêdo, J.K.A.; Joseph, J.K.; Menon, J.; Escalante, T.; Rucavado, A.; Gutiérrez, J.M.; Fox, J.W. Proteomic Analysis of Human Blister Fluids Following Envenomation by Three Snake Species in India: Differential Markers for Venom Mechanisms of Action. Toxins 2019, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, W.M.; de Oliveira, S.S.; Pivoto, G.; Alves, E.C.; de Almeida Gonçalves Sachett, J.; Alexandre, C.N.; Fé, N.F.; das Graças Vale Barbosa Guerra, M.; da Silva, I.M.; Tavares, A.M.; et al. Scorpion Envenoming Caused by Tityus Cf. Silvestris Evolving with Severe Muscle Spasms in the Brazilian Amazon. Toxicon 2016, 119, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Krifi, M.N.; Kharrat, H.; Zghal, K.; Abdouli, M.; Abroug, F.; Bouchoucha, S.; Dellagi, K.; el Ayeb, M. Development of an ELISA for the Detection of Scorpion Venoms in Sera of Humans Envenomed by Androctonus Australis Garzonii (Aag) and Buthus Occitanus Tunetanus (Bot): Correlation with Clinical Severity of Envenoming in Tunisia. Toxicon 1998, 36, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Olórtegui, C.; Fonseca, S.C.G.; Campolina, D.; Amaral, C.F.S.; Diniz, C.R. ELISA for the Detection of Toxic Antigens in Experimental and Clinical Envenoming by Tityus Serrulatus Scorpion Venom. Toxicon 1994, 32, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- de Rezende, N.A.; Dias, M.B.; Campolina, D.; Chavéz-Olortegui, C.; Amaral, C.F.S. Standardization of an Enzyme Linked Immunosorbent Assay (ELISA) for Detecting Circulating Toxic Venom Antigens in Patients Stung by the Scorpion Tityus Serrulatus. Rev. Inst. Med. Trop. Sao Paulo 1995, 37, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Bahloul, M.; Chaari, A.; Dammak, H.; Samet, M.; Chtara, K.; Chelly, H.; ben Hamida, C.; Kallel, H.; Bouaziz, M. Pulmonary Edema Following Scorpion Envenomation: Mechanisms, Clinical Manifestations, Diagnosis and Treatment. Int. J. Cardiol. 2013, 162, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Abdi, A.; Farshidi, H.; Rahimi, S.; Amini, A.; Tasnim Eftekhari, S.F. Electrocardiologic and Echocardiographic Findings in Patients with Scorpion Sting. Iran. Red. Crescent Med. J. 2013, 15, 446–447. [Google Scholar] [CrossRef]
- Kumar, C.M.; Naveen Prasad, S. v Echocardiologic Evaluation and Follow-up of Cardiovascular Complications in Children with Scorpion Sting in Coastal South India. Indian J. Crit. Care Med. 2015, 19, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.N.; Boyer, L.; Chippaux, J.-P.; Medolago, N.B.; Caramori, C.A.; Paixão, A.G.; Poli, J.P.V.; Mendes, M.B.; dos Santos, L.D.; Ferreira, R.S.; et al. A Clinical Trial Protocol to Treat Massive Africanized Honeybee (Apis mellifera) Attack with a New Apilic Antivenom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 14. [Google Scholar] [CrossRef]
- Barbosa, A.N.; Ferreira, R.S.; de Carvalho, F.C.T.; Schuelter-Trevisol, F.; Mendes, M.B.; Mendonça, B.C.; Batista, J.N.; Trevisol, D.J.; Boyer, L.; Chippaux, J.-P.; et al. Single-Arm, Multicenter Phase I/II Clinical Trial for the Treatment of Envenomings by Massive Africanized Honey Bee Stings Using the Unique Apilic Antivenom. Front. Immunol. 2021, 12, 653151. [Google Scholar] [CrossRef]
- Vetter, R.S.; Isbister, G.K. Medical Aspects of Spider Bites. Annu. Rev. Entomol. 2008, 53, 409–429. [Google Scholar] [CrossRef]
- Dunbar, J.P.; Vitkauskaite, A.; O’Keeffe, D.T.; Fort, A.; Sulpice, R.; Dugon, M.M. Bites by the Noble False Widow Spider Steatoda Nobilis Can Induce Latrodectus -like Symptoms and Vector-Borne Bacterial Infections with Implications for Public Health: A Case Series. Clin. Toxicol. 2022, 60, 59–70. [Google Scholar] [CrossRef]
- Jerusalem, K.; Salavert Lletí, M. Probable Cutaneous Loxoscelism with Mild Systemic Symptoms: A Case Report from Spain. Toxicon 2018, 156, 7–12. [Google Scholar] [CrossRef]
- Langner, T.R.; Ganatra, H.A.; Schwerdtfager, J.; Stoecker, W.; Thornton, S. Viscerocutaneous Loxoscelism Manifesting with Myocarditis: A Case Report. Am. J. Case Rep. 2021, 22, e932378. [Google Scholar] [CrossRef]
- Lopes, P.H.; Squaiella-Baptistão, C.C.; Marques, M.O.T.; Tambourgi, D.V. Clinical Aspects, Diagnosis and Management of Loxosceles Spider Envenomation: Literature and Case Review. Arch. Toxicol. 2020, 94, 1461–1477. [Google Scholar] [CrossRef]
- Stoecker, W.V.; Green, J.A.; Gomez, H.F. Diagnosis of Loxoscelism in a Child Confirmed with an Enzyme-Linked Immunosorbent Assay and Noninvasive Tissue Sampling. J. Am. Acad. Dermatol. 2006, 55, 888–890. [Google Scholar] [CrossRef]
- Keklikci, U.; Akdeniz, S.; Sakalar, Y.B.; Cakmak, S.S.; Unlu, K. Loxosceles Reclusa Bite to the Eyelid. Eur. J. Ophthalmol. 2008, 18, 633–635. [Google Scholar] [CrossRef]
- Krywko, D.M.; Gomez, H.F. Detection of Loxosceles Species Venom in Dermal Lesions: A Comparison of 4 Venom Recovery Methods. Ann. Emerg. Med. 2002, 39, 475–480. [Google Scholar] [CrossRef]
- Miller, M.J.; Gomez, H.F.; Snider, R.J.; Stephens, E.L.; Czop, R.M.; Warren, J.S. Detection of Loxosceles Venom in Lesional Hair Shafts and Skin: Application of a Specific Immunoassay to Identify Dermonecrotic Arachnidism. Am. J. Emerg. Med. 2000, 18, 626–628. [Google Scholar] [CrossRef]
- Chávez-Olórtegui, C.; Zanetti, V.C.; Ferreira, A.P.; Minozzo, J.C.; Mangili, O.C.; Gubert, I.C. ELISA for the Detection of Venom Antigens in Experimental and Clinical Envenoming by Loxosceles Intermedia Spiders. Toxicon 1998, 36, 563–569. [Google Scholar] [CrossRef]
- Chávez-Olórtegui, C.; Bohórquez, K.; Alvarenga, L.M.; Kalapothakis, E.; Campolina, D.; Maria, W.S.; Diniz, C.R. Sandwich-ELISA Detection of Venom Antigens in Envenoming by Phoneutria Nigriventer Spider. Toxicon 2001, 39, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Bucaretchi, F.; Mello, S.M.; Vieira, R.J.; Mamoni, R.L.; Blotta, M.H.S.L.; Antunes, E.; Hyslop, S. Systemic Envenomation Caused by the Wandering Spider Phoneutria Nigriventer, with Quantification of Circulating Venom. Clin. Toxicol. 2008, 46, 885–889. [Google Scholar] [CrossRef]
- Miller, M.; O’Leary, M.A.; Isbister, G.K. Towards Rationalisation of Antivenom Use in Funnel-Web Spider Envenoming: Enzyme Immunoassays for Venom Concentrations. Clin. Toxicol. 2016, 54, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Lan, Y.; Zhu, X.; Li, J.; Chen, T.; Huang, Q.; Ho, C.-T.; Chen, Y.; Cao, Y. Hepatic Lipidomics Analysis Reveals the Antiobesity and Cholesterol-Lowering Effects of Tangeretin in High-Fat Diet-Fed Rats. J. Agric. Food Chem. 2020, 68, 6142–6153. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, H.; Pathak, P.; Kumar, Y.; Jagavelu, K.; Dikshit, M. Modulation of Insulin Resistance, Dyslipidemia and Serum Metabolome in INOS Knockout Mice Following Treatment with Nitrite, Metformin, Pioglitazone, and a Combination of Ampicillin and Neomycin. Int. J. Mol. Sci. 2021, 23, 195. [Google Scholar] [CrossRef]
- Shu, T.; Ning, W.; Wu, D.; Xu, J.; Han, Q.; Huang, M.; Zou, X.; Yang, Q.; Yuan, Y.; Bie, Y.; et al. Plasma Proteomics Identify Biomarkers and Pathogenesis of COVID-19. Immunity 2020, 53, 1108–1122.e5. [Google Scholar] [CrossRef]
- Cavalcante, J.S.; Brito, I.M.d.C.; de Oliveira, L.A.; de Barros, L.C.; Almeida, C.; Rossini, B.C.; Sousa, D.L.; Alves, R.S.; Jorge, R.J.B.; Santos, L.D. dos Experimental Bothrops Atrox Envenomation: Blood Plasma Proteome Effects after Local Tissue Damage and Perspectives on Thromboinflammation. Toxins 2022, 14, 613. [Google Scholar] [CrossRef]
- Cavalcante, J.S.; da Silva, W.R.G.B.; de Oliveira, L.A.; Brito, I.M.C.; Muller, K.S.; Vidal, I.S.J.; dos Santos, L.D.; Jorge, R.J.B.; Almeida, C.; Bicho, C.D.L. Blood Plasma Proteome Alteration after Local Tissue Damage Induced by Bothrops Erythromelas Snake Venom in Mice. J. Proteom. 2022, 269, 104742. [Google Scholar] [CrossRef]
- Cavalcante, J.d.S.; de Almeida, C.A.S.; Clasen, M.A.; da Silva, E.L.; de Barros, L.C.; Marinho, A.D.; Rossini, B.C.; Marino, C.L.; Carvalho, P.C.; Jorge, R.J.B.; et al. A Fingerprint of Plasma Proteome Alteration after Local Tissue Damage Induced by Bothrops Leucurus Snake Venom in Mice. J. Proteom. 2022, 253, 104464. [Google Scholar] [CrossRef]
- Dong, D.; Deng, Z.; Yan, Z.; Mao, W.; Yi, J.; Song, M.; Li, Q.; Chen, J.; Chen, Q.; Liu, L.; et al. Oxidative Stress and Antioxidant Defense in Detoxification Systems of Snake Venom-Induced Toxicity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20200053. [Google Scholar] [CrossRef]
- Luna, K.P.O.; Xavier, E.M.; Pascoal, V.P.M.; Martins-Filho, O.A.; Pereira, V.R.A. Humoral Immune Response of Patients Bitten by the Snake Bothrops Erythromelas. Rev. Soc. Bras. Med. Trop. 2010, 43, 731–732. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Ju, S.; Ma, B.; Huang, W.; Luo, S. Isolation and Characterization of Five Novel Disulfide-Poor Conopeptides from Conus Marmoreus Venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210116. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Lecaer, J.-P.; Nghia, N.D.; Vinh, P.T.K. Isolation and Structural Identification of a New T1-Conotoxin with Unique Disulfide Connectivities Derived from Conus Bandanus. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190095. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.T.F.; Silva, M.F.P.; Porto, R.M.; Lebrun, I.; de Camargo Gonçalves, L.R.; de Fátima Correia Batista, I.; Sandoval, M.R.L.; Abdalla, F.M.F. Effects of Mlx-8, a Phospholipase A2 from Brazilian Coralsnake Micrurus Lemniscatus Venom, on Muscarinic Acetylcholine Receptors in Rat Hippocampus. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190041. [Google Scholar] [CrossRef] [PubMed]
- de Melo-Braga, M.N.; da Silva Moreira, R.; Gervásio, J.H.D.B.; Felicori, L.F. Overview of Protein Posttranslational Modifications in Arthropoda Venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210047. [Google Scholar] [CrossRef]
- Marchi, F.C.; Mendes-Silva, E.; Rodrigues-Ribeiro, L.; Bolais-Ramos, L.G.; Verano-Braga, T. Toxinology in the Proteomics Era: A Review on Arachnid Venom Proteomics. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, 20210034. [Google Scholar] [CrossRef]
- Anita, S.; Sadjuri, A.R.; Rahmah, L.; Nugroho, H.A.; Mulyadi; Trilaksono, W.; Ridhani, W.; Safira, N.; Bahtiar, H.; Maharani; et al. Venom Composition of Trimeresurus slbolabris, T. insularis, T. puniceus and T. purpureomaculatus from Indonesia. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210103. [Google Scholar] [CrossRef]
- Chanhome, L.; Khow, O.; Reamtong, O.; Vasaruchapong, T.; Laoungbua, P.; Tawan, T.; Suntrarachun, S.; Sitprija, S.; Kumkate, S.; Chaiyabutr, N. Biochemical and Proteomic Analyses of Venom from a New Pit Viper, Protobothrops Kelomohy. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210080. [Google Scholar] [CrossRef]
- Nie, X.; He, Q.; Zhou, B.; Huang, D.; Chen, J.; Chen, Q.; Yang, S.; Yu, X. Exploring the Five-Paced Viper (Deinagkistrodon acutus) Venom Proteome by Integrating a Combinatorial Peptide Ligand Library Approach with Shotgun LC-MS/MS. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200196. [Google Scholar] [CrossRef]
- Faisal, T.; Tan, N.H.; Sim, S.M.; Gnanathasan, C.A.; Tan, C.H. Proteomics, Toxicity and Antivenom Neutralization of Sri Lankan and Indian Russell’s Viper (Daboia russelii) Venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200177. [Google Scholar] [CrossRef]
- Sanz, L.; Pérez, A.; Quesada-Bernat, S.; Diniz-Sousa, R.; Calderón, L.A.; Soares, A.M.; Calvete, J.J.; Caldeira, C.A.S. Venomics and Antivenomics of the Poorly Studied Brazil’s Lancehead, Bothrops Brazili (Hoge, 1954), from the Brazilian State of Pará. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Isbister, G. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Macêdo, J.K.A.; Feoli, A.; Escalante, T.; Rucavado, A.; Gutiérrez, J.M.; Fox, J.W. Muscle Tissue Damage Induced by the Venom of Bothrops Asper: Identification of Early and Late Pathological Events through Proteomic Analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004599. [Google Scholar] [CrossRef] [PubMed]
- Brasileiro-Martins, L.M.; Nascimento, T.P.; Silva-Neto, A.V.; Martins, F.; Cavalcante, S.A.; Martins, R.B.; Marques, H.; Colombini, M.; Martins, M.; Sartim, M.A.; et al. The Severity of Acute Kidney Injury Correlates with Plasma Venom Levels in Bothrops Atrox Envenomings. Toxicon 2022, 219, 106924. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef]
- Rashmi, U.; Khochare, S.; Attarde, S.; Laxme, R.R.S.; Suranse, V.; Martin, G.; Sunagar, K. Remarkable Intrapopulation Venom Variability in the Monocellate Cobra (Naja kaouthia) Unveils Neglected Aspects of India’s Snakebite Problem. J. Proteom. 2021, 242, 104256. [Google Scholar] [CrossRef]
- Bourke, L.A.; Zdenek, C.N.; Neri-Castro, E.; Bénard-Valle, M.; Alagón, A.; Gutiérrez, J.M.; Sanchez, E.F.; Aldridge, M.; Fry, B.G. Pan-American Lancehead Pit-Vipers: Coagulotoxic Venom Effects and Antivenom Neutralisation of Bothrops Asper and B. Atrox Geographical Variants. Toxins 2021, 13, 78. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Ainsworth, S.; Lomonte, B.; Kini, R.M.; Chávez-Olórtegui, C. Editorial: Novel Immunotherapies Against Envenomings by Snakes and Other Venomous Animals. Front. Immunol. 2020, 11, 1004. [Google Scholar] [CrossRef]
- Long, C.; Wu, F.; Lu, Q.; Xie, B.; Shen, C.; Li, J.; Deng, Y.; Liang, P.; Yu, Y.; Lai, R. A Strategy for Efficient Preparation of Genus-Specific Diagnostic Antibodies for Snakebites. Front. Immunol. 2021, 12, 775678. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Navarrete, F.; Sala, F.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front. Psychiatry 2020, 11, 432. [Google Scholar] [CrossRef]
- García-Bailo, B.; Brenner, D.R.; Nielsen, D.; Lee, H.-J.; Domanski, D.; Kuzyk, M.; Borchers, C.H.; Badawi, A.; Karmali, M.A.; El-Sohemy, A. Dietary Patterns and Ethnicity Are Associated with Distinct Plasma Proteomic Groups. Am. J. Clin. Nutr. 2012, 95, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Kumar, V.; Bhave, A.; Singh, V.; Gogtay, N.J.; Thatte, U.M.; Talukdar, A.; Kochar, S.K.; Patankar, S.; Srivastava, S. Proteomic Analysis of Plasmodium Falciparum Induced Alterations in Humans from Different Endemic Regions of India to Decipher Malaria Pathogenesis and Identify Surrogate Markers of Severity. J. Proteom. 2015, 127, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Moussa, E.M.; Huang, H.; Thézénas, M.L.; Fischer, R.; Ramaprasad, A.; Sisay-Joof, F.; Jallow, M.; Pain, A.; Kwiatkowski, D.; Kessler, B.M.; et al. Proteomic Profiling of the Plasma of Gambian Children with Cerebral Malaria. Malar J. 2018, 17, 337. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Moiyadi, A.; Srivastava, S. Biorepositories for Cancer Research in Developing Countries. Nat. Rev. Clin. Oncol. 2013, 10, 434–436. [Google Scholar] [CrossRef]
- Ray, S.; Patel, S.K.; Venkatesh, A.; Chatterjee, G.; Ansari, N.N.; Gogtay, N.J.; Thatte, U.M.; Gandhe, P.; Varma, S.G.; Patankar, S.; et al. Quantitative Proteomics Analysis of Plasmodium Vivax Induced Alterations in Human Serum during the Acute and Convalescent Phases of Infection. Sci. Rep. 2017, 7, 4400. [Google Scholar] [CrossRef]
- Vidova, V.; Spacil, Z. A Review on Mass Spectrometry-Based Quantitative Proteomics: Targeted and Data Independent Acquisition. Anal. Chim. Acta 2017, 964, 7–23. [Google Scholar] [CrossRef]
- Masucci, G.V.; Cesano, A.; Hawtin, R.; Janetzki, S.; Zhang, J.; Kirsch, I.; Dobbin, K.K.; Alvarez, J.; Robbins, P.B.; Selvan, S.R.; et al. Validation of Biomarkers to Predict Response to Immunotherapy in Cancer: Volume I—Pre-Analytical and Analytical Validation. J. Immunother. Cancer 2016, 4, 76. [Google Scholar] [CrossRef]
- Santos, F.L.N.; Celedon, P.A.F.; Zanchin, N.I.T.; de Souza, W.V.; da Silva, E.D.; Foti, L.; Krieger, M.A.; de Miranda Gomes, Y. Accuracy of Chimeric Proteins in the Serological Diagnosis of Chronic Chagas Disease—A Phase II Study. PLoS Negl. Trop. Dis. 2017, 11, e0005433. [Google Scholar] [CrossRef]
- Manole, E.; Bastian, A.E.D.; Popescu, I.; Constantin, C.; Mihai, S.; Gaina, G.F.; Codrici, E.; Neagu, M.T. Immunoassay Techniques Highlighting Biomarkers in Immunogenetic Diseases. In Immunogenetics; IntechOpen: London, UK, 2019; Volume 6. [Google Scholar] [CrossRef]
- Manjunath, D.; Kumaraswamy, S.B.; Venkatakrishniah, S.A.; Appaiah, H.N.; Thomas, A.; Banerjee, S.D. Validation and Evaluation of a Common Biomarker in Human Cancers Sera Protein Detected by a Monoclonal Antibody UNIVmAb. BMC Res. Notes 2019, 12, 744. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Liu, S.; Ding, G.; Liu, W.; Zhou, J.; Kuang, M.; Ji, Y.; Kondo, T.; Fan, J. Quantitative Proteomic Analysis Identified Paraoxonase 1 as a Novel Serum Biomarker for Microvascular Invasion in Hepatocellular Carcinoma. J. Proteome Res. 2013, 12, 1838–1846. [Google Scholar] [CrossRef]
- Stewart, T.; Shi, M.; Mehrotra, A.; Aro, P.; Soltys, D.; Kerr, K.F.; Zabetian, C.P.; Peskind, E.R.; Taylor, P.; Shaw, L.M.; et al. Impact of Pre-Analytical Differences on Biomarkers in the ADNI and PPMI Studies: Implications in the Era of Classifying Disease Based on Biomarkers. J. Alzheimer’s Dis. 2019, 69, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S. Exploring the Human Plasma Proteome. Proteomics 2005, 5, 3223–3225. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; States, D.J.; Adamski, M.; Blackwell, T.W.; Menon, R.; Hermjakob, H.; Apweiler, R.; Haab, B.B.; Simpson, R.J.; Eddes, J.S.; et al. Overview of the HUPO Plasma Proteome Project: Results from the Pilot Phase with 35 Collaborating Laboratories and Multiple Analytical Groups, Generating a Core Dataset of 3020 Proteins and a Publicly-Available Database. Proteomics 2005, 5, 3226–3245. [Google Scholar] [CrossRef]
- Mahboob, S.; Ahn, S.B.; Mohamedali, A.; Amirkhani, A.; Tan, S.; Ranganathan, S.; Nice, E.; Baker, M.S. Addition of Protease Inhibitors to EDTA Containing Blood Collection Tubes Does Not Deliver Significant Advantage for Preservation of Plasma for Down-Stream Analysis. Clin. Proteom. Bioinform. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Lan, J.; Núñez Galindo, A.; Doecke, J.; Fowler, C.; Martins, R.N.; Rainey-Smith, S.R.; Cominetti, O.; Dayon, L. Systematic Evaluation of the Use of Human Plasma and Serum for Mass-Spectrometry-Based Shotgun Proteomics. J. Proteome Res. 2018, 17, 1426–1435. [Google Scholar] [CrossRef]
- Hassis, M.E.; Niles, R.K.; Braten, M.N.; Albertolle, M.E.; Ewa Witkowska, H.; Hubel, C.A.; Fisher, S.J.; Williams, K.E. Evaluating the Effects of Preanalytical Variables on the Stability of the Human Plasma Proteome. Anal. Biochem. 2015, 478, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Agashe, C.; Chiang, D.; Grishin, A.; Masilamani, M.; Jones, S.M.; Wood, R.A.; Sicherer, S.H.; Burks, A.W.; Leung, D.Y.M.; Dawson, P.; et al. Impact of Granulocyte Contamination on PBMC Integrity of Shipped Blood Samples: Implications for Multi-Center Studies Monitoring Regulatory T Cells. J. Immunol. Methods 2017, 449, 23–27. [Google Scholar] [CrossRef]
- Bonilauri, B.; Santos, M.D.M.; Camillo-Andrade, A.C.; Bispo, S.; Nogueira, F.C.S.; Carvalho, P.C.; Zanchin, N.I.T.; de S. da G. Fischer, J. The Impact of Blood-Processing Time on the Proteome of Human Peripheral Blood Mononuclear Cells. Biochim. et Biophys. Acta (BBA)—Proteins Proteom. 2021, 1869, 140581. [Google Scholar] [CrossRef]
- Mateos, J.; Carneiro, I.; Corrales, F.; Elortza, F.; Paradela, A.; del Pino, M.S.; Iloro, I.; Marcilla, M.; Mora, M.I.; Valero, L.; et al. Multicentric Study of the Effect of Pre-Analytical Variables in the Quality of Plasma Samples Stored in Biobanks Using Different Complementary Proteomic Methods. J. Proteom. 2017, 150, 109–120. [Google Scholar] [CrossRef]
- Verberk, I.M.W.; Misdorp, E.O.; Koelewijn, J.; Ball, A.J.; Blennow, K.; Dage, J.L.; Fandos, N.; Hansson, O.; Hirtz, C.; Janelidze, S.; et al. Characterization of Pre-analytical Sample Handling Effects on a Panel of Alzheimer’s Disease–Related Blood-based Biomarkers: Results from the Standardization of Alzheimer’s Blood Biomarkers (SABB) Working Group. Alzheimer’s Dement. 2022, 18, 1484–1497. [Google Scholar] [CrossRef]
- Halvey, P.; Farutin, V.; Koppes, L.; Gunay, N.S.; Pappas, D.A.; Manning, A.M.; Capila, I. Variable Blood Processing Procedures Contribute to Plasma Proteomic Variability. Clin. Proteom. 2021, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Roche, S.; Tiers, L.; Provansal, M.; Seveno, M.; Piva, M.-T.; Jouin, P.; Lehmann, S. Depletion of One, Six, Twelve or Twenty Major Blood Proteins before Proteomic Analysis: The More the Better? J. Proteom. 2009, 72, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Rudnick, P.A.; Martinez, M.Y.; Cheek, K.L.; Stein, S.E.; Slebos, R.J.C.; Liebler, D.C. Depletion of Abundant Plasma Proteins and Limitations of Plasma Proteomics. J. Proteome Res. 2010, 9, 4982–4991. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Sandberg, A.; Araújo, J.E.; Cvetkovski, F.; Berglund, E.; Eriksson, L.E.; Pernemalm, M. Evaluation of Spin Columns for Human Plasma Depletion to Facilitate MS-Based Proteomics Analysis of Plasma. J. Proteome Res. 2021, 20, 4610–4620. [Google Scholar] [CrossRef] [PubMed]
- Zolotarjova, N.; Mrozinski, P.; Chen, H.; Martosella, J. Combination of Affinity Depletion of Abundant Proteins and Reversed-Phase Fractionation in Proteomic Analysis of Human Plasma/Serum. J. Chromatogr. A 2008, 1189, 332–338. [Google Scholar] [CrossRef]
- Qian, W.-J.; Kaleta, D.T.; Petritis, B.O.; Jiang, H.; Liu, T.; Zhang, X.; Mottaz, H.M.; Varnum, S.M.; Camp, D.G.; Huang, L.; et al. Enhanced Detection of Low Abundance Human Plasma Proteins Using a Tandem IgY12-SuperMix Immunoaffinity Separation Strategy. Mol. Cell. Proteom. 2008, 7, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Polaskova, V.; Kapur, A.; Khan, A.; Molloy, M.P.; Baker, M.S. High-Abundance Protein Depletion: Comparison of Methods for Human Plasma Biomarker Discovery. Electrophoresis 2010, 31, 471–482. [Google Scholar] [CrossRef]
- Ahmed, N.; Barker, G.; Oliva, K.; Garfin, D.; Talmadge, K.; Georgiou, H.; Quinn, M.; Rice, G. An Approach to Remove Albumin for the Proteomic Analysis of Low Abundance Biomarkers in Human Serum. Proteomics 2003, 3, 1980–1987. [Google Scholar] [CrossRef]
- Colantonio, D.A.; Dunkinson, C.; Bovenkamp, D.E.; van Eyk, J.E. Effective Removal of Albumin from Serum. Proteomics 2005, 5, 3831–3835. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Lin, S.-Y.; Yeh, Y.-Y.; Hsiao, H.-H.; Wu, C.-Y.; Chen, S.-T.; Wang, A.H.-J. A Modified Protein Precipitation Procedure for Efficient Removal of Albumin from Serum. Electrophoresis 2005, 26, 2117–2127. [Google Scholar] [CrossRef]
- Govorukhina, N.I.; Keizer-Gunnink, A.; van der Zee, A.G.J.; de Jong, S.; de Bruijn, H.W.A.; Bischoff, R. Sample Preparation of Human Serum for the Analysis of Tumor Markers. J. Chromatogr. A 2003, 1009, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Quero, C.; Colomé, N.; Prieto, M.R.; Carrascal, M.; Posada, M.; Gelpí, E.; Abian, J. Determination of Protein Markers in Human Serum: Analysis of Protein Expression in Toxic Oil Syndrome Studies. Proteomics 2004, 4, 303–315. [Google Scholar] [CrossRef]
- Millioni, R.; Tolin, S.; Puricelli, L.; Sbrignadello, S.; Fadini, G.P.; Tessari, P.; Arrigoni, G. High Abundance Proteins Depletion vs Low Abundance Proteins Enrichment: Comparison of Methods to Reduce the Plasma Proteome Complexity. PLoS ONE 2011, 6, e19603. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake Envenoming: A Disease of Poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef]
- Ramsey, J.M.; Cooper, J.D.; Penninx, B.W.J.H.; Bahn, S. Variation in Serum Biomarkers with Sex and Female Hormonal Status: Implications for Clinical Tests. Sci. Rep. 2016, 6, 26947. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.J.; Vitzthum, F. Effects of Preanalytical Variables on Peptide and Protein Measurements in Human Serum and Plasma: Implications for Clinical Proteomics. Expert Rev. Proteom. 2006, 3, 409–426. [Google Scholar] [CrossRef]
- Bahk, Y.Y.; Kim, S.A.; Kim, J.-S.; Euh, H.-J.; Bai, G.-H.; Cho, S.-N.; Kim, Y.S. Antigens Secreted FromMycobacterium Tuberculosis: Identification by Proteomics Approach and Test for Diagnostic Marker. Proteomics 2004, 4, 3299–3307. [Google Scholar] [CrossRef]
- Garg, N.J.; Soman, K.V.; Zago, M.P.; Koo, S.-J.; Spratt, H.; Stafford, S.; Blell, Z.N.; Gupta, S.; Nuñez Burgos, J.; Barrientos, N.; et al. Changes in Proteome Profile of Peripheral Blood Mononuclear Cells in Chronic Chagas Disease. PLoS Negl. Trop. Dis. 2016, 10, e0004490. [Google Scholar] [CrossRef]
- Bexkens, M.L.; van Gestel, R.A.; van Breukelen, B.; Urbanus, R.T.; Brouwers, J.F.; Nieuwland, R.; Tielens, A.G.M.; van Hellemond, J.J. Schistosoma Mansoni Infection Affects the Proteome and Lipidome of Circulating Extracellular Vesicles in the Host. Mol. Biochem. Parasitol. 2020, 238, 111296. [Google Scholar] [CrossRef]
- Marques, M.A.M.; Neves-Ferreira, A.G.C.; da Silveira, E.K.X.; Valente, R.H.; Chapeaurouge, A.; Perales, J.; da Silva Bernardes, R.; Dobos, K.M.; Spencer, J.S.; Brennan, P.J.; et al. Deciphering the Proteomic Profile of Mycobacterium Leprae Cell Envelope. Proteomics 2008, 8, 2477–2491. [Google Scholar] [CrossRef]
- Thadikkaran, L.; Siegenthaler, M.A.; Crettaz, D.; Queloz, P.-A.; Schneider, P.; Tissot, J.-D. Recent Advances in Blood-Related Proteomics. Proteomics 2005, 5, 3019–3034. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, S.A.; Torres, V.M.; Louro, H.; Gomes, F.; Lopes, C.; Marçal, N.; Fragoso, E.; Martins, C.; Oliveira, C.L.; Hagenfeldt, M.; et al. Effects of Occupational Exposure to Tobacco Smoke: Is There a Link Between Environmental Exposure and Disease? J. Toxicol. Environ. Health A 2013, 76, 311–327. [Google Scholar] [CrossRef]
- Sgaier, S.K.; Jha, P.; Mony, P.; Kurpad, A.; Lakshmi, V.; Kumar, R.; Ganguly, N.K. Biobanks in Developing Countries: Needs and Feasibility. Science 2007, 318, 1074–1075. [Google Scholar] [CrossRef]
- Ibiapina, H.N.S.; Costa, A.G.; Sachett, J.A.G.; Silva, I.M.; Tarragô, A.M.; Neves, J.C.F.; Kerr, M.W.A.; Santana, M.F.; Martins-Filho, O.A.; Lacerda, M.V.G.; et al. An Immunological Stairway to Severe Tissue Complication Assembly in Bothrops Atrox Snakebites. Front. Immunol. 2019, 10, 1882. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.C.F.; Ibiapina, H.N.S.; Magalhães-Gama, F.; Sachett, J.A.G.; Silva, I.M.; Coelho, K.F.; Alves, E.C.; Tarragô, A.M.; de Lima Ferreira, L.C.; Malheiro, A.; et al. CCL-2 and CXCL-8: Potential Prognostic Biomarkers of Acute Kidney Injury after a Bothrops Atrox Snakebite. Mediat. Inflamm. 2022, 2022, 8285084. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, S.N.C.; Sachett, J.A.G.; Colombini, M.; Freitas-de-Sousa, L.A.; Ibiapina, H.N.S.; Costa, A.G.; Santana, M.F.; Park, J.-J.; Sherman, N.E.; Ferreira, L.C.L.; et al. Observation of Bothrops Atrox Snake Envenoming Blister Formation from Five Patients: Pathophysiological Insights. Toxins 2021, 13, 800. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalcante, J.d.S.; de Almeida, D.E.G.; Moraes, M.S.; Santos, S.R.; Pincinato, P.M.; Riciopo, P.M.; de Oliveira, L.L.B.; Monteiro, W.M.; Ferreira-Junior, R.S. Challenges and Opportunities in Clinical Diagnostic Routine of Envenomation Using Blood Plasma Proteomics. Toxins 2023, 15, 180. https://doi.org/10.3390/toxins15030180
Cavalcante JdS, de Almeida DEG, Moraes MS, Santos SR, Pincinato PM, Riciopo PM, de Oliveira LLB, Monteiro WM, Ferreira-Junior RS. Challenges and Opportunities in Clinical Diagnostic Routine of Envenomation Using Blood Plasma Proteomics. Toxins. 2023; 15(3):180. https://doi.org/10.3390/toxins15030180
Chicago/Turabian StyleCavalcante, Joeliton dos Santos, Denis Emanuel Garcia de Almeida, Micael Saggion Moraes, Sophia Ribeiro Santos, Pedro Moriel Pincinato, Pedro Marques Riciopo, Laís Lacerda B. de Oliveira, Wuelton Marcelo Monteiro, and Rui Seabra Ferreira-Junior. 2023. "Challenges and Opportunities in Clinical Diagnostic Routine of Envenomation Using Blood Plasma Proteomics" Toxins 15, no. 3: 180. https://doi.org/10.3390/toxins15030180
APA StyleCavalcante, J. d. S., de Almeida, D. E. G., Moraes, M. S., Santos, S. R., Pincinato, P. M., Riciopo, P. M., de Oliveira, L. L. B., Monteiro, W. M., & Ferreira-Junior, R. S. (2023). Challenges and Opportunities in Clinical Diagnostic Routine of Envenomation Using Blood Plasma Proteomics. Toxins, 15(3), 180. https://doi.org/10.3390/toxins15030180