Mycotoxin Contamination in Hazelnut: Current Status, Analytical Strategies, and Future Prospects
Abstract
1. Introduction
2. Occurrence of Mycotoxins in Hazelnuts



3. Analytical Methods for the Determination of Mycotoxins in Hazelnuts
4. Mycotoxins in Hazelnuts and Fungal Infections
5. Control Strategies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Król, K.; Gantner, M. Morphological traits and chemical composition of hazelnut from different geographical. Agriculture 2020, 10, 375. [Google Scholar] [CrossRef]
- Squara, S.; Stilo, F.; Cialiè Rosso, M.; Liberto, E.; Spigolon, N.; Genova, G.; Castello, G.; Bicchi, C.; Cordero, C. Corylus avellana L. aroma blueprint: Potent odorants signatures in the volatilome of high quality hazelnuts. Front. Plant Sci. 2022, 13, 840028. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Fereidoon, S. Compositional characteristics and health effects of hazelnut (Corylus avellana L.): An overview. In Tree Nuts; CRC Press: Boca Raton, FL, USA, 2020; Volume 5, pp. 248–253. ISBN 9781119130536. [Google Scholar]
- Ukwuru, M.U.; Ohaegbu, C.G.; Muritala, A. A critical overview of mycotoxin contamination of foods and feeds. Curr. Res. Agric. Food Sci. 2021, 4, 77–98. [Google Scholar]
- Molyneux, R.J.; Mahoney, N.; Kim, J.H.; Campbell, B.C. Mycotoxins in edible tree nuts. Int. J. Food Microbiol. 2007, 119, 72–78. [Google Scholar] [CrossRef]
- Colović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Ðuragić, O.; Kos, J.; Pinotti, L. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- European Union. Commission Regulation (EC) No 165/2010 of 26 February 2010, amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off. J. Eur. Union. 2010, 50, 8–12. [Google Scholar]
- Bacaloni, A.; Cavaliere, C.; Cucci, F.; Foglia, P.; Samperi, R.; Laganà, A. Determination of aflatoxins in hazelnuts by various sample preparation methods and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2008, 1179, 182–189. [Google Scholar] [CrossRef]
- Imperato, R.; Campone, L.; Piccinelli, A.L.; Veneziano, A.; Rastrelli, L. Survey of aflatoxins and ochratoxin a contamination in food products imported in Italy. Food Control 2011, 22, 1905–1910. [Google Scholar] [CrossRef]
- Abdulla, N.Q.F. Evaluation of fungal flora and mycotoxin in some important nut products in erbil local markets. Res. J. Environ. Earth Sci. 2013, 5, 330–336. [Google Scholar] [CrossRef]
- Ekinci, R.; Otaǧ, M.; Kadakal, Ç. Patulin & ergosterol: New quality parameters together with aflatoxins in hazelnuts. Food Chem. 2014, 150, 17–21. [Google Scholar]
- Varga, E.; Glauner, T.; Berthiller, F.; Krska, R.; Schuhmacher, R.; Sulyok, M. Development and validation of a (semi-)quantitative UHPLC-MS/MS method for the determination of 191 mycotoxins and other fungal metabolites in almonds, hazelnuts, peanuts and pistachios. Anal. Bioanal. Chem. 2013, 405, 5087–5104. [Google Scholar] [CrossRef]
- Reinhold, L.; Reinhardt, K. Mycotoxins in foods in Lower Saxony (Germany): Results of official control analyses performed in 2009. Mycotoxin Res. 2011, 27, 137–143. [Google Scholar] [CrossRef]
- Abdel-Hafez, A.I.I.; Saber, S.M. Mycoflora and mycotoxin of hazelnut (Corylus avellana L.) and walnut (Juglans regia L.) seeds in Egypt. Zentralbl. Mikrobiol. 1993, 148, 137–147. [Google Scholar] [CrossRef]
- Gürses, M. Mycoflora and aflatoxin content of hazelnuts, walnuts, peanuts, almonds and roasted chickpeas (LEBLEBI) sold in Turkey. Int. J. Food Prop. 2007, 9, 395–399. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Yan, Z.; Li, Z.; Cheng, Y.; Chang, W. Occurrence and co-occurrence of mycotoxins in nuts and dried fruits from China. Food Control 2018, 88, 181–189. [Google Scholar] [CrossRef]
- Keskin, Z.S.; Gürsoy, N. Investigation of natural mycoflora and aflatoxin formation in hazelnuts and products. Cumhur. Sci. J. 2019, 40, 967–977. [Google Scholar]
- Zvizdic, S.; Hamzic, S.; Rodinis-Pejic, I.; Avdic-Kamberovic, F.; Bektas, S.; Sacic, E. Detection of mycotoxins in selected foods sample. Mater. Sociomed. 1988, 21, 47–76. [Google Scholar]
- Cunha, S.C.; Sá, S.V.M.; Fernandes, J.O. Multiple mycotoxin analysis in nut products: Occurrence and risk characterization. Food Chem. Toxicol. 2018, 114, 260–269. [Google Scholar] [CrossRef]
- Spadaro, D.; Meloni, G.R.; Siciliano, I.; Prencipe, S.; Gullino, M.L. HPLC-MS/MS method for the detection of selected toxic metabolites produced by Penicillium spp. in nuts. Toxins 2020, 12, 307. [Google Scholar] [CrossRef]
- Tolosa, J.; Font, G.; Mañes, J.; Ferrer, E. Nuts and dried fruits: Natural occurrence of emerging Fusarium mycotoxins. Food Control 2013, 33, 215–220. [Google Scholar] [CrossRef]
- Salem, N.M.; Ahmad, R. Mycotoxins in food from Jordan: Preliminary survey. Food Control 2010, 21, 1099–1103. [Google Scholar] [CrossRef]
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods 2020, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Naeem, I.; Gong, Y.Y.; Routledge, M.N.; Akhtar, S.; Riaz, M.; Ramalho, L.N.Z.; de Oliveira, C.A.F.; Ismail, Z. Early life exposure to dietary aflatoxins, health impact and control perspectives: A review. Trends Food Sci. Technol. 2021, 112, 212–224. [Google Scholar] [CrossRef]
- IARC; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer; WHO. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. In Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; IARC: Lyon, France, 2002. [Google Scholar]
- Heperkan, D. The importance of mycotoxins and a brief history of mycotoxin studies in Turkey. ARI Bull. Istanbul Tech. Univ. 2015, 54, 18–27. [Google Scholar]
- Van de Perre, E.; Jacxsens, L.; Lachat, C.; El Tahan, F.; De Meulenaer, B. Impact of maximum levels in European legislation on exposure of mycotoxins in dried products: Case of aflatoxin B1 and ochratoxin A in nuts and dried fruits. Food Chem. Toxicol. 2015, 75, 112–117. [Google Scholar] [CrossRef]
- Demirhan, B.E.; Demirhan, B. Investigation of twelve significant mycotoxin contamination in nut-based products by the LC—MS/MS method. Metabolites 2022, 12, 120. [Google Scholar] [CrossRef]
- Díaz Nieto, C.H.; Granero, A.M.; Zon, M.A.; Fernández, H. Sterigmatocystin: A mycotoxin to be seriously considered. Food Chem. Toxicol. 2018, 118, 460–470. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Huang, J.; Ma, L.; Chen, Z.; Wang, F. Aflatoxin B1 and sterigmatocystin in wheat and wheat products from supermarkets in China. Food Addit. Contam. Part B Surveill. 2018, 11, 9–14. [Google Scholar] [CrossRef]
- Zheng, R.; Xu, H.; Wang, W.; Zhan, R.; Chen, W. Simultaneous determination of aflatoxin B1, B2, G1, G2, ochratoxin A, and sterigmatocystin in traditional Chinese medicines by LC-MS-MS. Anal. Bioanal. Chem. 2014, 406, 3031–3039. [Google Scholar] [CrossRef]
- Zingales, V.; Fernández-Franzón, M.; Ruiz, M.J. Sterigmatocystin: Occurrence, toxicity and molecular mechanisms of action—A review. Food Chem. Toxicol. 2020, 146, 111802. [Google Scholar] [CrossRef]
- Škrbić, B.; Živančev, J.; Godula, M. Multimycotoxin analysis of crude extracts of nuts with ultra-high performance liquid chromatography/tandem mass spectrometry. J. Food Compos. Anal. 2014, 34, 171–177. [Google Scholar] [CrossRef]
- Heussner, A.H.; Bingle, L.E.H. Comparative ochratoxin toxicity: A review of the available data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- Solhaug, A.; Eriksen, G.S.; Holme, J.A. Mechanisms of action and toxicity of the mycotoxin alternariol: A review. Basic Clin. Pharmacol. Toxicol. 2016, 119, 533–539. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, D.; Chen, Y.; Zhu, X.; Tang, X.; Zhang, J.; Zhang, L.; Chen, J. General toxicity and genotoxicity of alternariol: A novel 28-day multi-endpoint assessment in male Sprague–Dawley rats. Mycotoxin Res. 2022, 38, 231–241. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Ropejko, K.; Twaru, M. Zearalenone and its metabolites—General overview, occurrence, and toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Degenkolb, T.; Kirschbaum, J.; Brückner, H. New sequences, constituents, and producers of peptaibiotics: An updated review. Chem. Biodivers. 2007, 4, 1052–1067. [Google Scholar] [CrossRef]
- Caloni, F.; Fossati, P.; Anadón, A.; Bertero, A. Beauvericin: The beauty and the beast. Environ. Toxicol. Pharmacol. 2020, 75, 103349. [Google Scholar] [CrossRef]
- Liuzzi, V.C.; Mirabelli, V.; Cimmarusti, M.T.; Haidukowski, M.; Leslie, J.F.; Logrieco, A.F.; Caliandro, R.; Fanelli, F.; Mulè, G. Enniatin and beauvericin biosynthesis in Fusarium species: Production profiles and structural determinant prediction. Toxins 2017, 9, 45. [Google Scholar] [CrossRef]
- Ivanova, L.; Skjerve, E.; Eriksen, G.S.; Uhlig, S. Cytotoxicity of enniatins A, A1, B, B1, B2 and B3 from Fusarium avenaceum. Toxicon 2006, 47, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Gessler, N.N.; Egorova, A.S.; Belozerskaya, T.A. Fungal anthraquinones. Appl. Biochem. Microbiol. 2013, 49, 85–99. [Google Scholar] [CrossRef]
- Mendili, M.; Khadhri, A.; Mediouni-Ben Jemâa, J.; Andolfi, A.; Tufano, I.; Aschi-Smiti, S.; DellaGreca, M. Anti-inflammatory potential of compounds isolated from tunisian lichens species. Chem. Biodivers. 2022, 19, e202200134. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chu, S.; Yang, S.; Peng, Y.; Ren, S.; Wen, B.; Chen, N. Physcion and physcion 8-O-β-glucopyranoside: A review of their pharmacology, toxicities and pharmacokinetics. Chem. Biol. Interact. 2019, 310, 108722. [Google Scholar] [CrossRef]
- Arunachalam, C.; Doohan, F.M. Trichothecene toxicity in eukaryotes: Cellular and molecular mechanisms in plants and animals. Toxicol. Lett. 2013, 217, 149–158. [Google Scholar] [CrossRef]
- Gutiérrez, S.; McCormick, S.P.; Cardoza, R.E.; Lindo, L.; Alexander, N.J.; Proctor, R.H. Trichoderma trichothecenes: Beyond their toxic effect. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 281–301. ISBN 978-0-12-819453-9. [Google Scholar]
- Sliwi, M.P. Trichothecenes in food and feed, relevance to human and animal health and methods of detection: A systematic review. Molecules 2021, 26, 454. [Google Scholar]
- Nover, L.; Luckner, M. On the biosynthesis of cyclopenin and cyclopenol, benzodiazepine alkaloids from Penicillium cyclopium westling. Eur. J. Biochem. 1969, 10, 268–273. [Google Scholar] [CrossRef]
- Mahato, D.K.; Kamle, M.; Sharma, B.; Pandhi, S.; Devi, S.; Dhawan, K.; Selvakumar, R.; Mishra, D.; Kumar, A.; Arora, S.; et al. Patulin in food: A mycotoxin concern for human health and its management strategies. Toxicon 2021, 198, 12–23. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Smedsgaard, J.; Larsen, T.O.; Samson, R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004, 2004, 201–241. [Google Scholar]
- Bentley, R. Mycophenolic acid: A one hundred year odyssey from antibiotic to immunosuppressant. Chem. Rev. 2000, 100, 3801–3825. [Google Scholar] [CrossRef]
- Krska, R.; Schubert-Ullrich, P.; Molinelli, A.; Sulyok, M.; MacDonald, S.; Crews, C. Mycotoxin analysis: An update. Food Addit. Contam.—Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 152–163. [Google Scholar] [CrossRef]
- Şengül, Ü. Comparing determination methods of detection and quantification limits for aflatoxin analysis in hazelnut. J. Food Drug Anal. 2016, 24, 56–62. [Google Scholar] [CrossRef]
- Jeurissen, S.M.F.; Seyhan, F.; Kandhai, M.C.; Dekkers, S.; Booij, C.J.H.; Bos, P.M.J.; Van Der Fels-Klerx, H.J. An indicator based “traffic light” model to pro-actively assess the occurrence of mycotoxins in tree nuts. World Mycotoxin J. 2011, 4, 405–412. [Google Scholar] [CrossRef]
- Mahdjoubi, C.K.; Arroyo-manzanares, N.; Hamini-kadar, N. Multi-mycotoxin occurrence and exposure assessment approach in foodstuffs from Algeria. Toxins 2020, 12, 194. [Google Scholar] [CrossRef]
- EU European Union. Commission Regulation (EU), No.1058/2012 of 12 November 2012 amending Regulation (EC) No. 1881/2006 as regards maximum levels for aflatoxins in dried figs. Off. J. Eur. Union 2012, 313, 14–15. [Google Scholar]
- Malachová, A.; Sulyok, M.; Beltrán, E.; Berthiller, F.; Krska, R. Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. A 2014, 1362, 145–156. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Huertas-Pérez, J.F.; Gámiz-Gracia, L.; García-Campaña, A.M. A new approach in sample treatment combined with UHPLC-MS/MS for the determination of multiclass mycotoxins in edible nuts and seeds. Talanta 2013, 115, 61–67. [Google Scholar] [CrossRef]
- Dreolin, N.; Stead, S.; Hird, S.; Jenkins, T. Determination of regulated and emerging mycotoxins in cereals, nuts, figs, and animal feeds using Pass-Through SPE and UPLC- MS/MS. Waters Corporation Application Note (720007377EN). Available online: https://www.waters.com (accessed on 12 December 2022).
- Lacina, O.; Zachariasova, M.; Urbanova, J.; Vaclavikova, M.; Cajka, T.; Hajslova, J. Critical assessment of extraction methods for the simultaneous determination of pesticide residues and mycotoxins in fruits, cereals, spices and oil seeds employing ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1262, 8–18. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. Multiclass mycotoxin analysis in Silybum marianum by ultra high performance liquid chromatography-tandem mass spectrometry using a procedure based on QuEChERS and dispersive liquid-liquid microextraction. J. Chromatogr. A 2013, 1282, 11–19. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Celano, R.; Rastrelli, L. Application of dispersive liquid-liquid microextraction for the determination of aflatoxins B 1, B 2, G 1 and G 2 in cereal products. J. Chromatogr. A 2011, 1218, 7648–7654. [Google Scholar] [CrossRef]
- Mousavi, L.; Tamiji, Z.; Khoshayand, M.R. Applications and opportunities of experimental design for the dispersive liquid–liquid microextraction method—A review. Talanta 2018, 190, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Arciuolo, R.; Chiusa, G.; Castello, G.; Camardo Leggieri, M.; Spigolon, N.; Battilani, P. Diaporthe spp. is confirmed as the main fungus associated with defective Turkish hazelnuts. Plant Health Prog. 2022, 25. [Google Scholar] [CrossRef]
- Ozay, G.; Seyhan, F.; Pembeci, C.; Saklar, S.; Yilmaz, A. Factors influencing fungal and aflatoxin levels in Turkish hazelnuts (Corylus avellana L.) during growth, harvest, drying and storage: A 3-year study. Food Addit. Contam. 2008, 25, 209–218. [Google Scholar] [CrossRef]
- Duran, P.; Barra, P.J.; de la Luz Mora, M.; Morina, F.; Viscardi, S.; Meriño-Gergichevich, C. First report of fungal complex causing grey necrosis of hazelnut in Chile. New Dis. Rep. 2020, 42, 7. [Google Scholar] [CrossRef]
- Belisario, A.; Maccaroni, M.; Coramusi, A.; Corazza, L.; Pryor, B.M.; Figuli, P. First report of Alternaria species groups involved in disease complexes of hazelnut and walnut fruit. Plant Dis. 2004, 88, 426. [Google Scholar] [CrossRef]
- Battilani, P.; Chiusa, G.; Arciuolo, R.; Somenzi, M.; Fontana, M.; Castello, G.; Spigolon, N. Diaporthe as the main cause of hazelnut defects in the Caucasus region. Phytopathol. Mediterr. 2018, 54, 241–252. [Google Scholar]
- Xu, Y.; Bianchini, A.; Hanna, M.A. Evaluation of mold and mycotoxin contaminations in hybrid hazelnuts grown in Nebraska. Food Process. Technol. 2011, 2, 119. [Google Scholar] [CrossRef]
- Arciuolo, R.; Santos, C.; Soares, C.; Castello, G.; Spigolon, N.; Chiusa, G.; Lima, N.; Battilani, P. Molecular characterization of Diaporthe species associated with hazelnut defects. Front. Plant Sci. 2020, 11, 611655. [Google Scholar] [CrossRef]
- Pscheidt, J.W.; Heckert, S.; Wiseman, M.; Jones, L. Fungi associated with and influence of moisture on development of kernel mold of hazelnut. Plant Dis. 2019, 103, 922–928. [Google Scholar] [CrossRef]
- Sezer, A.; Dolar, F. Hazelnut kernel defects and associated fungi in three provinces in Turkey. In Proceedings of the VII International Scientific Agriculture Symposium,” Agrosym 2016”, Jahorina, Bosnia and Herzegovina, 6–9 October 2016; pp. 1312–1318. [Google Scholar]
- Sezer, A.; Dolar, F.S. Colletotrichum acutatum, a new pathogen of hazelnut. J. Phytopathol. 2012, 160, 428–430. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Piotrowska, A. Morphological traits, kernel composition and sensory evaluation of hazelnut (Corylus avellana L.) cultivars grown in Poland. Agronomy 2019, 9, 703. [Google Scholar] [CrossRef]
- Sezer, A.; Dolar, F.S.; Ünal, F. First report of Colletotrichum fioriniae infection of hazelnut. Mycotaxon 2017, 132, 495–502. [Google Scholar] [CrossRef]
- Guerrero Contreras, J.; Galdames Gutierrez, R.; Ogass Contreras, K.; Pérez Fuentealba, S. First report of Diaporthe foeniculina causing black tip and necrotic spot on hazelnut kernel in Chile. Plant Dis. 2020, 104, 975. [Google Scholar] [CrossRef]
- Scarpari, M.; Vitale, S.; Di Giambattista, G.; Luongo, L.; De Gregorio, T.; Schreiber, G.; Petrucci, M.; Belisario, A.; Voglmayr, H. Didymella corylicola sp. nov., a new fungus associated with hazelnut fruit development in Italy. Mycol. Prog. 2020, 19, 317–328. [Google Scholar] [CrossRef]
- Bobev, S.G.; Angelov, L.T.; Van Poucke, K.; Maes, M. First report of kernel spot caused by Eremothecium coryli on hazelnut in Bulgaria. Plant Dis. 2018, 102, 243. [Google Scholar] [CrossRef]
- Halliwell, G. The breakdown of cellulose and its derivatives by enzymes from Myrothecium verrucaria. Biochem. J. 1962, 85, 67–72. [Google Scholar] [CrossRef]
- Bobev, S.G.; Angelov, L.T.; Van Poucke, K.; Maes, M. First report of hazelnut kernel rot caused by Eremothecium cymbalariae in Bulgaria. Plant Dis. 2018, 102, 818. [Google Scholar] [CrossRef]
- Santori, A.; Vitale, S.; Luongo, L.; Belisario, A. First report of Fusarium lateritium as the agent of nut gray necrosis on hazelnut in Italy. Plant Dis. 2010, 94, 484. [Google Scholar] [CrossRef]
- Turco, S.; Grottoli, A.; Drais, M.I.; De Spirito, C.; Faino, L.; Reverberi, M.; Cristofori, V.; Mazzaglia, A. Draft genome sequence of a new Fusarium isolate belonging to Fusarium tricinctum species complex collected from hazelnut in central italy. Front. Plant Sci. 2021, 12, 2835. [Google Scholar] [CrossRef]
- Sezer, A.; Dolar, F.S. Determination of Pestalotiopsis sp. causing disease on fruit clusters in hazelnut growing areas of Ordu, Giresun and Trabzon provinces in Turkey. J. Agric. For. 2015, 61, 183. [Google Scholar]
- Ebrahimi, K.S.; Ansari, M.; Hosseyni Moghaddam, M.S.; Ebrahimi, Z.; Salehi, Z.; Shahlaei, M.; Moradi, S. In silico investigation on the inhibitory effect of fungal secondary metabolites on RNA dependent RNA polymerase of SARS-CoV-II: A docking and molecular dynamic simulation study. Comput. Biol. Med. 2021, 135, 104613. [Google Scholar] [CrossRef] [PubMed]
- Abdel-gawad, K.M.; Zohri, A.A. Fungal flora and mycotoxins of six kinds of nut seeds for human consumption in Saudi Arabia. Mycopathologia 1993, 124, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Simsek, O.; Arici, M.; Demir, C. Mycoflora of hazelnut (Corylus avellana L.) and aflatoxin content in hazelnut kernels artificially infected with Aspergillus parasiticus. Food/Nahrung 2002, 46, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.R.; Shokri, H.; Ziglarí, T. Evaluation of fungal flora in some important nut products (pistachio, peanut, hazelnut and almond) in Tehran, Iran. Pak. J. Nutr. 2007, 6, 460–462. [Google Scholar] [CrossRef]
- Saffari, E.; Madani, M.; Karbasizade, V.; Shakib, P. Detection of fungal and bacterial contamination of hazelnut and determination of aflatoxin B by HPLC method in Isfahan, Iran. Curr. Med. Mycol. 2021, 7, 1–5. [Google Scholar] [CrossRef]
- Mir Hosseini Moghaddam, S.A.; Taherzadeh, M. Isolated fungi from hazelnut, their damage and economic importance in Guilan province. Iran. J. For. Range Prot. Res. 2007, 5, 96–98. [Google Scholar]
- Krasauskas, A.; Paulauskienė, A.; Tarasevičienė, Ž. Micromycetes contaminating nuts used for food. Biologija 2015, 61, 109–115. [Google Scholar] [CrossRef]
- Jiménez, M.; Mateo, R.; Querol, A.; Huerta, T.; Hernández, E. Mycotoxins and mycotoxigenic moulds in nuts and sunflower seeds for human consumption. Mycopathologia 1991, 115, 121–127. [Google Scholar] [CrossRef]
- Amar, R.; Amina, M.; Salim, M.; Florence, M.; Nasserdine, S. Investigations on aflatoxigenic fungi and aflatoxins contamination in some nuts sampled in Algeria. Afr. J. Microbiol. Res. 2013, 7, 4974–4980. [Google Scholar] [CrossRef]
- Wang, Y.-j.; Nie, J.-y.; Yan, Z.; Li, Z.-x.; Cheng, Y.; Farooq, S. Multi-mycotoxin exposure and risk assessments for Chinese consumption of nuts and dried fruits. J. Integr. Agric. 2018, 17, 1676–1690. [Google Scholar] [CrossRef]
- Alhussaini, M.S.R. Mycobiota and mycotoxins of nuts and some dried fruits from Saudi Arabia. J. Am. Sci. 2012, 8, 525–534. [Google Scholar]
- Habibi, A.; Afzali, D. Aspergillus section Flavi from four agricultural products and association of mycotoxin and sclerotia production with isolation source. Curr. Microbiol. 2021, 78, 3674–3685. [Google Scholar] [CrossRef]
- Habibi, A. Aspergillus species in retail samples of pistachio, walnut and hazelnut in Kerman, Iran. Mycol. Iran. 2022, 8, 77–94. [Google Scholar]
- Greco, M.; Kemppainen, M.; Pose, G.; Pardo, A. Taxonomic characterization and secondary metabolite profiling of Aspergillus section Aspergillus contaminating feeds and feedstuffs. Toxins 2015, 7, 3512–3537. [Google Scholar] [CrossRef]
- Perrone, G.; Gallo, A. Aspergillus species and their associated mycotoxins. In Mycotoxigenic Fungi; Springer: Berlin/Heidelberg, Germany, 2017; pp. 33–49. ISBN 978-1-4939-6705-6. [Google Scholar]
- Perrone, G.; Susca, A. Penicillium species and their associated mycotoxins. In Mycotoxigenic Fungi; Springer: Berlin/Heidelberg, Germany, 2017; pp. 107–119. ISBN 978-1-4939-6705-6. [Google Scholar]
- Chepkirui, C.; Stadler, M. The genus Diaporthe: A rich source of diverse and bioactive metabolites. Mycol. Prog. 2017, 16, 477–494. [Google Scholar] [CrossRef]
- Xu, T.C.; Lu, Y.H.; Wang, J.F.; Song, Z.Q.; Hou, Y.G.; Liu, S.S.; Liu, C.S.; Wu, S.H. Bioactive secondary metabolites of the genus Diaporthe and anamorph Phomopsis from terrestrial and marine habitats and endophytes: 2010–2019. Microorganisms 2021, 9, 217. [Google Scholar] [CrossRef]
- Andolfi, A.; Boari, A.; Evidente, M.; Cimmino, A.; Vurro, M.; Ash, G.; Evidente, A. Gulypyrones A and B and phomentrioloxins B and C produced by Diaporthe gulyae, a potential mycoherbicide for saffron thistle (Carthamus lanatus). J. Nat. Prod. 2015, 78, 623–629. [Google Scholar] [CrossRef]
- Talontsi, F.M.; Islam, M.T.; Facey, P.; Douanla-Meli, C.; Von Tiedemann, A.; Laatsch, H. Depsidones and other constituents from Phomopsis sp. CAFT69 and its host plant Endodesmia calophylloides with potent inhibitory effect on motility of zoospores of grapevine pathogen Plasmopara viticola. Phytochem. Lett. 2012, 5, 657–664. [Google Scholar] [CrossRef]
- Riga, R.; Happyana, N.; Quentmeier, A.; Zammarelli, C.; Kayser, O.; Hakim, E.H. Secondary metabolites from Diaporthe lithocarpus isolated from Artocarpus heterophyllus. Nat. Prod. Res. 2021, 35, 2324–2328. [Google Scholar] [CrossRef]
- Hu, Q.; Yang, Y.; Yang, S.; Cao, H.; Chunyang, M.; Yang, H.; Gao, X.; Du, G. Xanthones from the fermentation products of the endophytic fungus of Phomopsis amygdali. Chem. Nat. Compd. 2015, 51, 456–459. [Google Scholar] [CrossRef]
- Peng, W.X.; Marchal, J.L.M.; van der Poel, A.F.B. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim. Feed Sci. Technol. 2018, 237, 129–153. [Google Scholar] [CrossRef]
- Wan, J.; Chen, B.; Rao, J. Occurrence and preventive strategies to control mycotoxins in cereal-based food. Compr. Rev. Food Sci. Food Saf. 2020, 19, 928–953. [Google Scholar] [CrossRef] [PubMed]
- Valente, S.; Meloni, G.R.; Prencipe, S.; Spigolon, N.; Somenzi, M.; Fontana, M.; Gullino, M.L.; Spadaro, D. Effect of drying temperatures and exposure times on Aspergillus flavus growth and aflatoxin production on artificially inoculated hazelnuts. J. Food Prot. 2020, 83, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Vrtodušić, R.; Ivić, D.; Jemrić, T.; Vuković, M. Hazelnut postharvest technology: A review. J. Cent. Eur. Agric. 2022, 23, 423–454. [Google Scholar] [CrossRef]
- Misra, N.N.; Yadav, B.; Roopesh, M.S.; Jo, C. Cold plasma for effective fungal and mycotoxin control in foods: Mechanisms, inactivation effects, and applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 106–120. [Google Scholar] [CrossRef]
- Yousefi, M.; Mohammadi, M.A.; Khajavi, M.Z.; Ehsani, A. Application of novel non-thermal physical technologies to degrade mycotoxins. J. Fungi 2021, 7, 395. [Google Scholar] [CrossRef]
- Dasan, B.G.; Boyaci, I.H.; Mutlu, M. Nonthermal plasma treatment of Aspergillus spp. spores on hazelnuts in an atmospheric pressure fluidized bed plasma system: Impact of process parameters and surveillance of the residual viability of spores. J. Food Eng. 2017, 196, 139–149. [Google Scholar] [CrossRef]
- Dasan, B.G.; Mutlu, M.; Boyaci, I.H. Decontamination of Aspergillus flavus and Aspergillus parasiticus spores on hazelnuts via atmospheric pressure fluidized bed plasma reactor. Int. J. Food Microbiol. 2016, 216, 50–59. [Google Scholar] [CrossRef]
- Siciliano, I.; Spadaro, D.; Prelle, A.; Vallauri, D.; Cavallero, M.C.; Garibaldi, A.; Gullino, M.L. Use of cold atmospheric plasma to detoxify hazelnuts from aflatoxins. Toxins 2016, 8, 125. [Google Scholar] [CrossRef]
- Sen, Y.; Onal-Ulusoy, B.; Mutlu, M. Detoxification of hazelnuts by different cold plasmas and gamma irradiation treatments. Innov. Food Sci. Emerg. Technol. 2019, 54, 252–259. [Google Scholar] [CrossRef]
- Jalili, M. A review on aflatoxins reduction in food. Iran. J. Health Saf. Environ. 2015, 3, 445–459. [Google Scholar]
- Kashanian, A.; Panjekeh, N.; Fakhtak, T. Investigating diversity and spatial distribution of endophytic fungi in hazelnut (Corylus avellana) in its different habitats of Iran. Biol. J. Microorg. 2021, 10, 53–69. [Google Scholar]
- Nicoletti, R.; Petriccione, M.; Curci, M.; Scortichini, M. Hazelnut-associated bacteria and their implications in crop management. Horticulturae 2022, 8, 1195. [Google Scholar] [CrossRef]
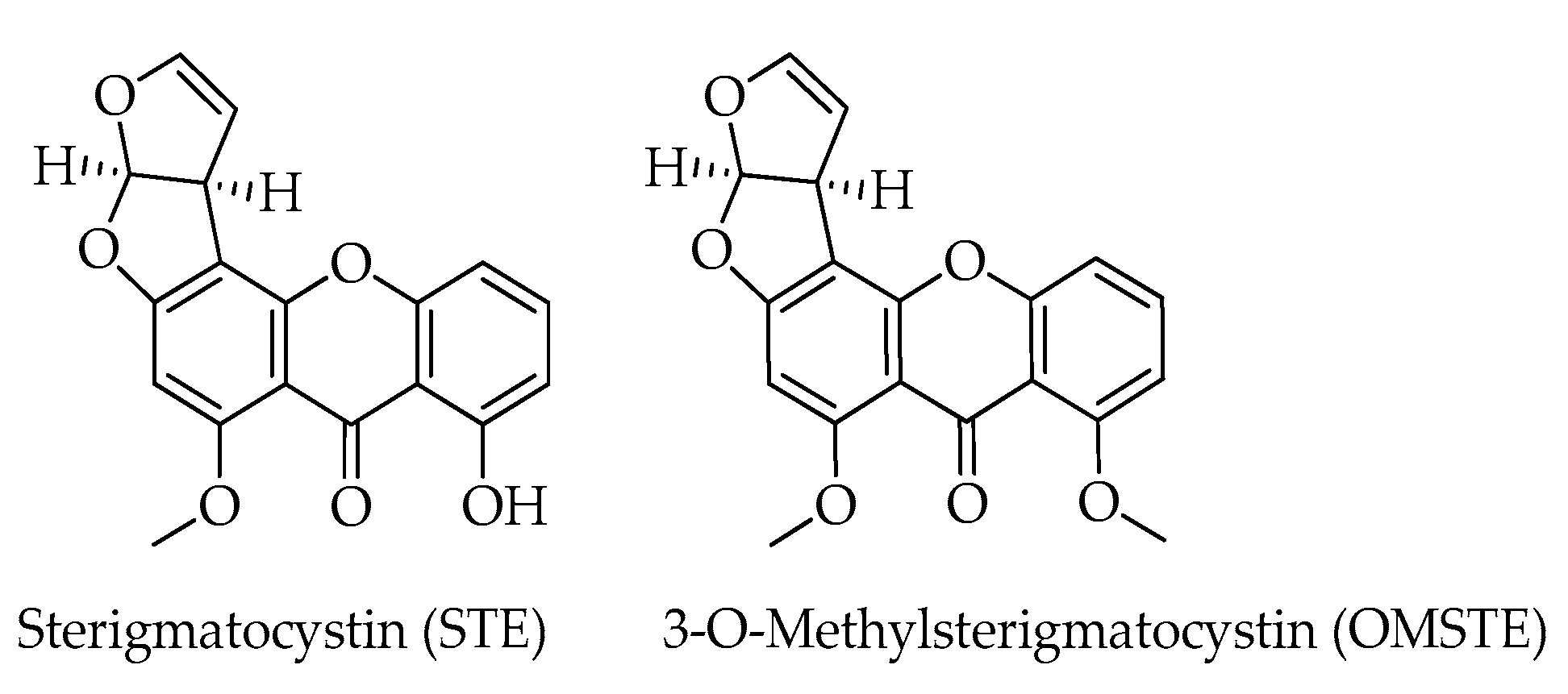

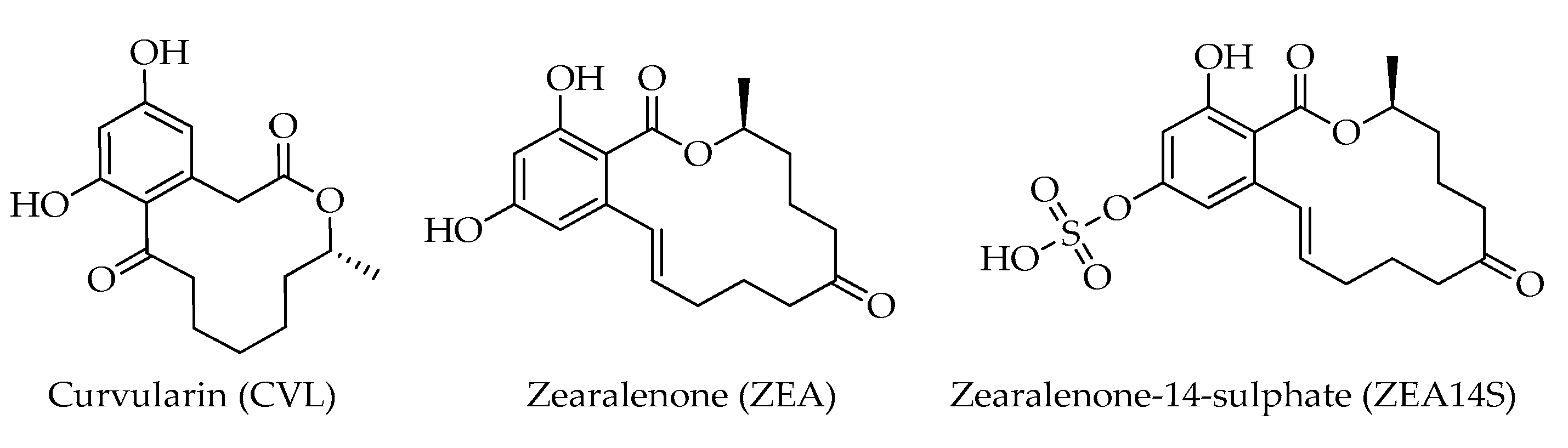

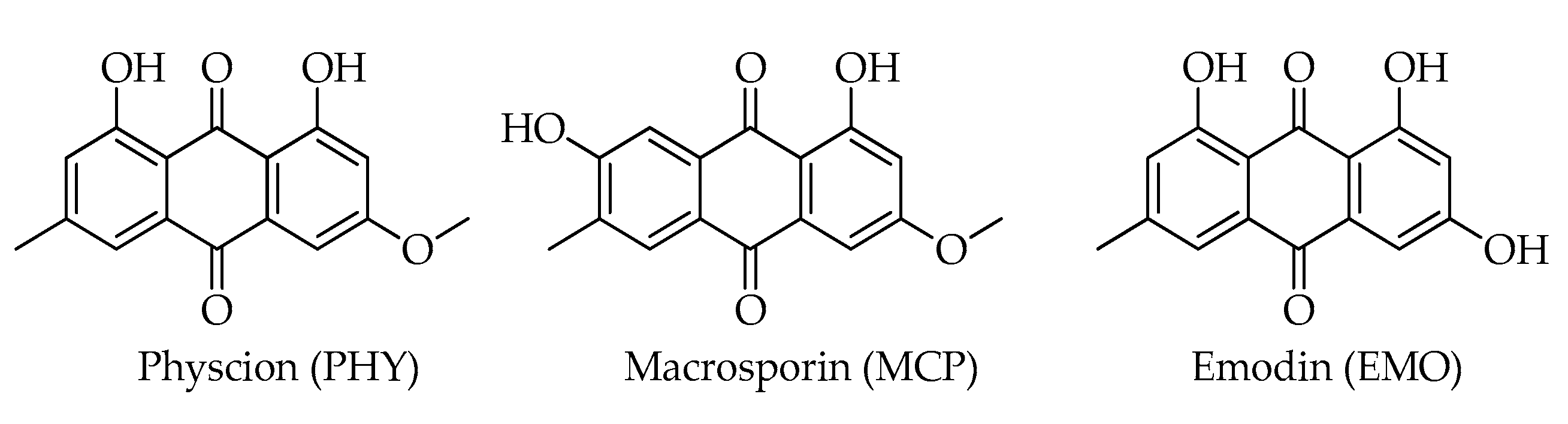
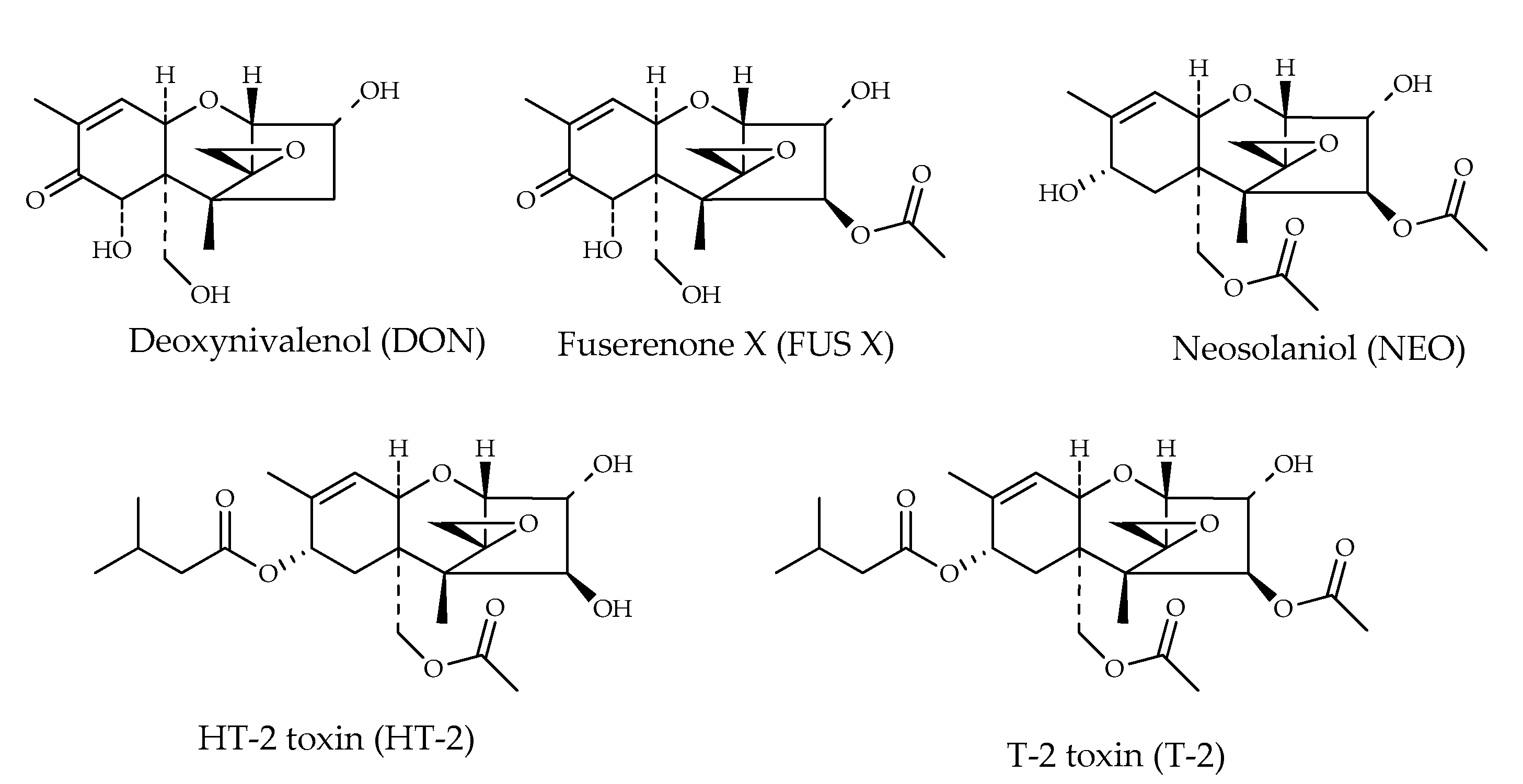

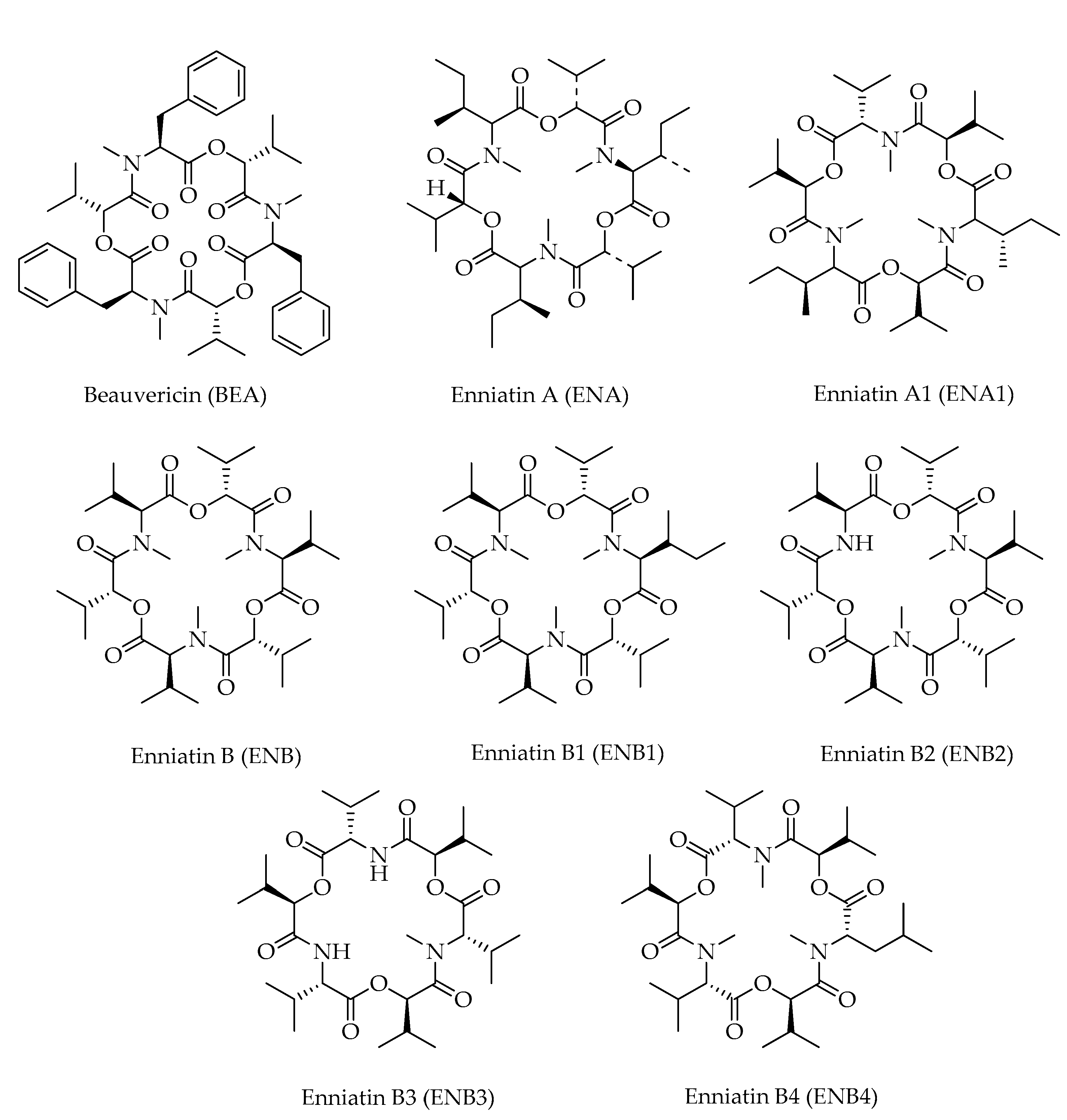
| Mycotoxin | Formula | Nominal Mass (U) | Reference |
|---|---|---|---|
| Aflatoxins | |||
| Aflatoxin B1 (AFB1) | C17H12O6 | 312 | [8,9,10,11,12,13,14,15,16,17,18] |
| Aflatoxin B2 (AFB2) | C17H14O6 | 314 | [8,9,10,11,12,13,14,16,17,18] |
| Aflatoxin G1 (AFG1) | C17H12O7 | 328 | [8,9,10,11,12,13,14,17,18] |
| Aflatoxin G2 (AFG2) | C17H14O7 | 330 | [9,10,11,12,13,14,17,18,19] |
| Amino acid derivatives | |||
| Alamethicin F30 (ALMF30) | C92H150N22O25 | 1964 | [12] |
| Apicidin (APC) | C34H49N5O6 | 624 | [12] |
| Tentoxin (TEN) | C22H30N4O4 | 414 | [12,16] |
| Anthraquinones | |||
| Emodin (EMO) | C15H10O5 | 270 | [12] |
| Macrosporin (MCP) | C16H12O5 | 284 | [12] |
| Physcion (= parietin) (PHY) | C16H12O5 | 284 | [12] |
| Benzodiazepine alkaloids | |||
| Cyclopenin (CPN) | C17H14N2O3 | 294 | [20] |
| Cyclopenol (CPL) | C17H14N2O4 | 310 | [20] |
| Cyclodepsipeptides | |||
| Beauvericin (BEA) | C45H57N3O9 | 784 | [12,21] |
| Enniatin A (ENA) | C36H63N3O9 | 682 | [12,16,21] |
| Enniatin A1 (ENA1) | C35H61N3O9 | 668 | [12,16,21] |
| Enniatin B (ENB) | C33H57N3O9 | 640 | [12,16,21] |
| Enniatin B1 (ENB1) | C34H59N3O9 | 654 | [16,21] |
| Enniatin B2 (ENB2) | C32H55N3O9 | 626 | [12] |
| Enniatin B3 (ENB3) | C31H53N3O9 | 612 | [12] |
| Enniatin B4 (ENB4) | C34H59N3O9 | 654 | [12] |
| Macrolides | |||
| Curvularin (CVL) | C16H20O5 | 292 | [12] |
| Zearalenone (ZEA) | C18H22O5 | 318 | [12] |
| Zearalenone-14-sulphate (ZEA14S) | C18H22O8S | 398 | [12] |
| Ochratoxins | |||
| Ochratoxin A (OTA) | C20H18ClNO6 | 404 | [10,12,22] |
| Ochratoxin B (OTB) | C20H19NO6 | 369 | [12,16] |
| Resorcylic acid lactones | |||
| Alternariol (AOH) | C14H10O5 | 258 | [12,16] |
| Alternariol methyl ether (AME) | C15H12O5 | 272 | [12,16] |
| Sterigmatocystins | |||
| 3-O-Methylsterigmatocystin (OMST) | C19H14O6 | 338 | [12] |
| Sterigmatocystin (STE) | C18H12O6 | 324 | [12] |
| Trichothecenes | |||
| Deoxynivalenol (DON) | C15H20O6 | 296 | [19] |
| Fuserenone X (FUS X) | C17H22O8 | 354 | [19] |
| HT-2 toxin (HT-2) | C22H32O8 | 424 | [12] |
| Neosolaniol (NEO) | C19H26O8 | 382 | [19] |
| T-2 toxin (T-2) | C24H34O9 | 467 | [12] |
| Miscellaneous | |||
| Altertoxin I (ALI) | C20H16O6 | 352 | [12] |
| Chaetoglobosin A (CHA) | C32H36N2O5 | 529 | [20] |
| Equisetin (EQS) | C22H31NO4 | 373 | [12] |
| Kojic acid (KA) | C6H6O4 | 142 | [12] |
| Moniliformin (MON) | C4H2O3 | 98 | [12] |
| Mycophenolic acid (MPA) | C17H20O6 | 320 | [12,20] |
| 3-Nitropropionic acid (BNP) | C3H5NO4 | 119 | [12] |
| Patulin (PA) | C7H6O4 | 154 | [11] |
| Pestalotin (PE) | C11H18O4 | 214 | [12] |
| Roquefortine C (ROQC) | C22H23N5O2 | 389 | [20] |
| Viridicatin (VRD) | C15H11NO2 | 237 | [12] |
| Type of Sample | Mycotoxins | Samples | Sample Preparation | Detection | Levels (µg kg−1) | Ref. |
|---|---|---|---|---|---|---|
| Hazelnuts | AFB1 | 35 | Ultrasound extraction with ACN:H2O (8:2, v/v), cleaning up with a Carbograph-4 SPE cartridge eluted with CH2Cl2:MeOH:acetic acid (88:10:2, v/v/v) | LC/ESI-MS/MS Mobile phase: (A) ACN:H2O (95:5, v/v); (B) H2O | not detected (n.d.)–0.9 | [8] |
| AFB2 | n.d.–<LOQ | |||||
| AFG1 | n.d.–0.1 | |||||
| Hazelnut paste | AFB1 | 5 | Extraction with MeOH:H2O (8:2, v/v) and n-hexane, cleaning up with immunoaffinity columns (IAC) eluted with MeOH | HPLC-FLD Mobile phase: ACN:MeOH:H2O (20:20:60, v/v/v) | 0.45–3.61 | [9] |
| AFB2 | <LOQ–0.55 | |||||
| AFG1 | n.d.–1.84 | |||||
| AFG2 | <LOQ–0.30 | |||||
| Hazelnuts without shell | AFB1 | 32 | 0.20 | |||
| AFG1 | 031 | |||||
| Roasted hazelnuts | AFB1 | 9 | 3.45 | |||
| AFB2 | 1.16 | |||||
| AFG1 | 0.16 | |||||
| AFG2 | 1.82 | |||||
| Hazelnuts | Total AFs | - | Extraction with 70% MeOH and filtration | Commercially available kit based on CD-ELISA | 10.3 | [10] |
| OTA | 1.5 | |||||
| Sound hazelnuts | AFB1 | 5 | AFs determination: extraction in MeOH:H2O (8:2, v/v), cleaning up with immunoaffinity assays. PA determination: extraction with ethyl acetate and filtration, subsequent extraction with 3% sodium carbonate solution, acidification of the organic phase | AFs determination: HPLC-FLD Mobile phase: H2O:ACN:MeOH (6:2:3, v/v/v). PA determination: HPLC-DAD: Mobile phase: H2O:ACN (1:9, v/v) | 0.4–0.9 | [11] |
| Moldy hazelnuts | AFB1 | 510–246 | ||||
| AFB2 | 4.4–1.6 | |||||
| AFG1 | 205–98.7 | |||||
| AFG2 | 1.3–4.0 | |||||
| PA | 65.8–25.6 | |||||
| Hidden moldy hazelnuts | AFB1 | 422–141 | ||||
| AFB2 | 0.8–2.0 | |||||
| AFG1 | 78.6–96.4 | |||||
| AFG2 | 0.5–2.1 | |||||
| PA | 67.6–16.6 | |||||
| Hazelnuts | AFB1 | 22 | Extraction with ACN:H2O:Acetic acid (79:20:1, v/v/v), dilution of the extract with ACN:H2O:acetic acid (79:20:1, v/v/v) | UHPLC-MS/MS Mobile phase: (A) MeOH:H2O:Acetic acid (10:89:1, v/v/v) (B) MeOH:H2O:Acetic acid (97:2:1, v/v/v) | 7.4 | [12] |
| AFB2 | 5.5 | |||||
| AFG1 | 16 | |||||
| AFG2 | 5.5 | |||||
| ALMF30 | 110 | |||||
| AOH | 78 | |||||
| AME | 59 | |||||
| ALI | 7.0 | |||||
| APC | 3.4 | |||||
| BEA | 2.4 | |||||
| CVL | 19 | |||||
| EMO | 5.5 | |||||
| ENA | 28 | |||||
| ENA1 | 140 | |||||
| ENB | 37 | |||||
| ENB2 | 3.0 | |||||
| ENB3 | 0.06 | |||||
| ENB4 | 22 | |||||
| EQS | 110 | |||||
| HT-2 | 39 | |||||
| KA | 1100 | |||||
| MCP | 280 | |||||
| OMST | 1.7 | |||||
| MPA | 700 | |||||
| BNP | 440 | |||||
| OTA | 220 | |||||
| OTB | 6.9 | |||||
| PE | 3.1 | |||||
| PHY | 700 | |||||
| STE | 2.3 | |||||
| T-2 | 32 | |||||
| TEN | 5.4 | |||||
| VRD | 5.7 | |||||
| ZEA | 7.6 | |||||
| ZEA14S | 3.9 | |||||
| Hazelnuts | AFB1 | 42 | Extraction with MeOH:H2O, cleaning up with immunoaffinity columns | HPLC-FLD | 1.37 | [13] |
| Total AFs | 4.11 | |||||
| Hazelnuts | AFB1 | 20 | Soxhlet extraction with n-hexane, subsequent extraction with CHCl3, cleaning up with silica gel columns | TLC Mobile phase: MeOH:CHCl3 (3:97, v/v) | 25–175 | [14] |
| AFB2 | 25–175 | |||||
| AFG1 | 25–175 | |||||
| AFG2 | 25–175 | |||||
| Hazelnuts | AFB1 | 28 | Extraction with CH2Cl2, cleaning up with columns eluted with CHCl3:acetone (90:10, v/v) | TLC Mobile phase: diethyl ether | 34.4 ppb | [15] |
| Edible part of hazelnuts | AFB1 | 20 | Extraction with acidified ACN, cleaning up with C18 sorbent | UPLC-MS/MS Mobile phase: (A) ACN (B) 0.5% formic acid in water with 10 mMol/L citric acid | - | [16] |
| AFB2 | - | |||||
| ENA | 1.00 | |||||
| ENA1 | 4.48 | |||||
| ENB | 1.58 | |||||
| ENB1 | 1.04 | |||||
| Total Afs | < LOQ–2.10 | |||||
| Raw hazelnuts | Total AFs | 30 | Neogen Veratox® | CD-ELISA | 2.11–10.03 | [17] |
| Roasted hazelnuts | Total AFs | 50 | 0.1–4.04 | |||
| Inner membrane of hazelnuts | Total AFs | 50 | 0.7–38.2 | |||
| Hazelnuts | AFs | 43 | Immunocompetition assay | ELISA | - | [18] |
| Hazelnuts | AFG2 | 7 | QuEChERS extraction with acidified ACN, d-SPE cleaning up with C18 and Z-Sep+ | HPLC-MS/MS Mobile phase: (A) H2O:MeOH:Acetic acid (94:5:1, v/v/v) (B) H2O:MeOH:Acetic acid (97:2:1, v/v/v) | 2.6 | [19] |
| DON | 56.01 | |||||
| FUS X | 45.09 | |||||
| NEO | <LOQ | |||||
| Hazelnuts | CHA | 13 | Sequential extractions with solvents of different polarity | HPLC-MS/MS Mobile phase: (A) acidified H2O (B) ACN. | 7.6–29.2 | [20] |
| CPN | 1.32–1.37 | |||||
| CPL | 11.02–21.45 | |||||
| MPA | 2.7 | |||||
| ROQC | <LOQ | |||||
| Hazelnut fruit | ENA | 4 | Ultrasonic extraction with CAN, cleaning up with C18 columns. Ultrasonic extraction of the residues dissolved in ACN:MeOH (5:5, v/v) | LC-MS/MS Mobile phase (gradient elution): (A) MeOH (B) ACN | 0.263 | [21] |
| ENA1 | 0.007 | |||||
| ENB | 0.146 | |||||
| Hazelnut shell | BEA | 0.03 | ||||
| ENA | 0.732 | |||||
| ENB | 0.076 | |||||
| ENB1 | 0.417 | |||||
| Hazelnuts | OTA | 1 | Extraction with MeOH:H2O (7:3, v/v) | Commercially available kit based on ELISA | - | [22] |
| Species | Country | References |
|---|---|---|
| Alternaria alternata | Chile | [68] |
| Italy | [69] | |
| Alternaria arborescens | Italy | [69] |
| Alternaria sp. | Georgia | [70] |
| Nebraska (USA) | [71] | |
| Turkey | [72] | |
| Alternaria tenuissima | Italy | [69] |
| Aspergillus sp. | Oregon (USA) | [73] |
| Georgia | [70] | |
| Turkey | [66,72,74] | |
| Botryosphaeria sp. | Turkey | [66,72] |
| Botrytis cinerea | Turkey | [75] |
| Botrytis sp. | Georgia | [70] |
| Turkey | [72] | |
| Chrysonilia sp. | Nebraska (USA) | [71] |
| Ciboria (Monilia) coryli | Poland | [76] |
| Cladosporium sp. | Georgia | [70] |
| Nebraska, Oregon (USA) | [71,73] | |
| Turkey | [72,74] | |
| Colletotrichum acutatum | Turkey | [75] |
| Colletotrichum fioriniae | Turkey | [77] |
| Colletotrichum sp. | Georgia | [70] |
| Diaporthe arecae | Turkey | [72] |
| Diaporthe eres | Georgia | [70] |
| Turkey | [72] | |
| Diaporthe foeniculina | Chile | [78] |
| Diaporthe hongkongensis | Turkey | [72] |
| Diaporthe oculi | Turkey | [72] |
| Diaporthe pseudoculi | Turkey | [72] |
| Diaporthe rudis | Oregon (USA) | [73] |
| Diaporthe sojae | Turkey | [72] |
| Diaporthe sp. | Chile | [68] |
| Georgia | [70] | |
| Turkey | [66,72] | |
| Diaporthe unshiuensis | Turkey | [72] |
| Didymella corylicola | Italy | [79] |
| Diplodia sp. | Oregon (USA) | [73] |
| Eremothecium coryli | Bulgaria | [80] |
| Oregon (USA) | [81] | |
| Eremothecium cymbalariae | Bulgaria | [82] |
| Fusarium chlamydosporum (= F. sporotrichioides) | Chile | [68] |
| Fusarium culmorum | Oregon (USA) | [73] |
| Fusarium lateritium | Italy | [83] |
| Oregon (USA) | [73] | |
| Fusarium sp. | Georgia | [70] |
| Nebraska (USA) | [71] | |
| Turkey | [66,72] | |
| Fusarium tricinctum | Italy | [84] |
| Gnomoniopsis idaeicola | Oregon (USA) | [73] |
| Mucor sp. | Turkey | [72] |
| Neofusicoccum sp. | Chile | [68] |
| Paecilomyces sp. | Nebraska (USA) | [71] |
| Penicillium sp. | Georgia | [70] |
| Nebraska, Oregon (USA) | [71,73] | |
| Turkey | [66,72,74] | |
| Pestalotiopsis sp. | Georgia | [70] |
| Turkey | [72,85] | |
| Phoma sp. | Georgia | [70] |
| Ramularia sp. | Oregon (USA) | [73,86] |
| Rhizopus sp. | Georgia | [70] |
| Turkey | [72] | |
| Septoria sp. | Georgia | [70] |
| Sphaceloma sp. | Georgia | [70] |
| Trichoderma sp. | Turkey | [72] |
| Trichothecium roseum | Turkey | [74] |
| Trichothecium sp. | Georgia | [70] |
| Turkey | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatore, M.M.; Andolfi, A.; Nicoletti, R. Mycotoxin Contamination in Hazelnut: Current Status, Analytical Strategies, and Future Prospects. Toxins 2023, 15, 99. https://doi.org/10.3390/toxins15020099
Salvatore MM, Andolfi A, Nicoletti R. Mycotoxin Contamination in Hazelnut: Current Status, Analytical Strategies, and Future Prospects. Toxins. 2023; 15(2):99. https://doi.org/10.3390/toxins15020099
Chicago/Turabian StyleSalvatore, Maria Michela, Anna Andolfi, and Rosario Nicoletti. 2023. "Mycotoxin Contamination in Hazelnut: Current Status, Analytical Strategies, and Future Prospects" Toxins 15, no. 2: 99. https://doi.org/10.3390/toxins15020099
APA StyleSalvatore, M. M., Andolfi, A., & Nicoletti, R. (2023). Mycotoxin Contamination in Hazelnut: Current Status, Analytical Strategies, and Future Prospects. Toxins, 15(2), 99. https://doi.org/10.3390/toxins15020099







