Abstract
Type B trichothecenes (deoxynivalenol, nivalenol, 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol) and deoxynivalenol-3-glucoside (DON-3G) are secondary toxic metabolites produced mainly by mycotoxigenic Fusarium fungi and have been recognized as natural contaminants in cereals and cereal-based foods. The latest studies have proven the various negative effects of type B trichothecenes on human health. Due to the widespread occurrence of Fusarium species, contamination by these mycotoxins has become an important aspect for public health and agro-food systems worldwide. Hence, their monitoring and surveillance in various foods have received a significant deal of attention in recent years. In this review, an up-to-date overview of the occurrence profile of major type B trichothecenes and DON-3G in cereal grains and their toxicological implications are outlined. Furthermore, current trends in analytical methodologies for their determination are overviewed. This review also covers the factors affecting the production of these mycotoxins, as well as the management strategies currently employed to mitigate their contamination in foods. Information presented in this review provides good insight into the progress that has been achieved in the last years for monitoring type B trichothecenes and DON-3G, and also would help the researchers in their further investigations on metabolic pathway analysis and toxicological studies of these Fusarium mycotoxins.
Keywords:
type B trichothecenes; deoxynivalenol-3-glucoside; Fusarium species; cereals; cereal-based foods; occurrence; toxicology; analytical methods; mitigation strategies Key Contribution:
Contamination of food with type B trichothecenes has gained growing concern worldwide due to their adverse health effects and substantial economic losses. This review briefly highlights the occurrence profile and toxicological effects of these mycotoxins. Current trends in analytical methodologies for their determination as well as management strategies to control their contamination in foods are also overviewed.
1. Introduction
Mycotoxins are a diverse group of secondary metabolites produced mainly by toxigenic microscopic fungi, such as Penicillium, Aspergillus, Alternaria, and Fusarium species, which can colonize various agricultural commodities in the field site or during storage [1]. The growth of fungal species and subsequent production of mycotoxins are influenced by a complex interaction of biotic and abiotic factors (i.e., fungal interaction, fungal host characteristics, and environmental conditions). Mycotoxins are frequently occurring in both tropical and temperate regions of the world, contaminating food, particularly grains (e.g., wheat, maize, oats, rice, barley, sorghum, and rye), which account for a sizeable proportion of agricultural production. Spices, nuts, coffee, oilseeds, dried peas, and various fruits have been also contaminated with different mycotoxins [2]. Worryingly, mycotoxins are not entirely eliminated throughout food processing operations and can also be detected in processed foodstuffs [3,4]. As reported by the Food and Agriculture Organization (FAO) of the United Nations, over 25% of globally produced cereal crops have been estimated to be contaminated with mycotoxins, thereby resulting in a loss of approximately one billion tons of foodstuffs annually [5,6,7,8]. Mycotoxin-contaminated food and feed can provoke serious acute and chronic health effects conjointly referred to as mycotoxicoses and these effects depend on various factors, such as toxicity level of contaminating mycotoxin, degree of exposure as well as the nutritional status of the individuals [8,9].
Over 500 compounds have been identified as mycotoxins up to now, but the most commonly studied mycotoxins with the greatest concern to human and animal health are aflatoxins, zearalenone, trichothecenes, patulin, ochratoxins, and fumonisins [10]. Trichothecenes, a broad spectrum of structurally related compounds, are of significant importance since they are produced by the mycotoxigenic Fusarium fungi, which colonize crops at the pre-harvest stages of production [11,12]. Thus, it has become difficult to avoid trichothecene contamination due to the considerable influence of abiotic conditions [13]. These mycotoxins belong to a unique family of cyclic sesquiterpenoids that consist of over 200 analogs with variable toxicological activities [14,15]. Chemically, this family of compounds is characterized by having an olefinic bond with various hydroxyl/acetoxy substitutions and a tetracyclic epoxytrichothene skeleton with a stable C-12, C-13 epoxy group that is responsible for their toxicity [15,16]. They are generally categorized into four subgroups (types A, B, C, and D) on the basis of the characteristics of substituted groups and respective fungal producers [17]. Among the currently available trichothecenes mycotoxins, particular attention has been paid to type B trichothecenes. This group of mycotoxins has been widely researched as they could pose a significant threat to both public health and agro-food systems due to their widespread geographical occurrence [18]. The major forms of type B trichothecenes are deoxynivalenol (DON or vomitoxin), nivalenol (NIV), 3-acetyldeoxynivalenol (3-ADON), 15-acetyldeoxynivalenol (15-ADON), and fusarenon-X (FUS-X; 4-acetylnivalenol) [18,19]. As can be noted from Figure 1, these mycotoxins share a common non-macrocyclic structure and a keto (carbonyl) function at position C-8. They are generally produced by F. culmorum and F. graminearum fungi species, which commonly contaminate a wide range of cereal grains including wheat, corn, oats, barley, and rice [18,19].
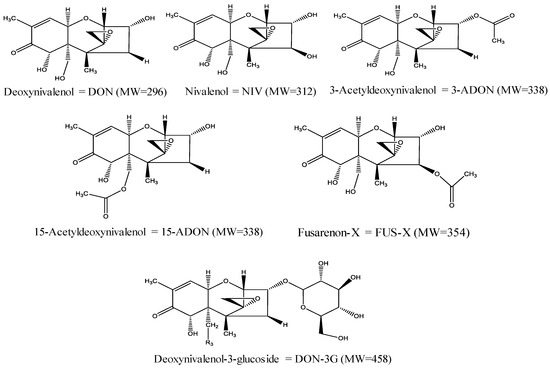
Figure 1.
Chemical structures, names, acronyms, and molecular weights of the major type B trichothecenes and deoxynivalenol-3-glucoside.
Interestingly, living plants can bind certain mycotoxins with different polar moieties, such as glucose, sugar, amino acid, sulfate, etc., via an enzymatic reaction in plant phase II metabolism to transform mycotoxins into less toxic metabolites (i.e., masked or conjugated mycotoxins) [15,19,20]. In recent times, biologically modified mycotoxins have raised substantial concerns relating to the safety of contaminated food commodities as these compounds are more polar than the precursor mycotoxins and normally elude routine determination due to unusual physicochemical properties [1,20]. The most prevalent masked mycotoxin of DON is DON-3G, which was originally isolated and identified from Zea mays cell suspension cultures treated with DON [20]. In the 1980s, DON-3G was first described as a plant conjugate of DON. A potential health hazard of DON-3G for consumers is arising from the possible hydrolysis and reactivation of its toxic-free form (DON) at specific conditions in mammal metabolism [19,20,21]. So far, the natural occurrence of DON-3G was first reported in naturally incurred maize and wheat samples in 2005 [20]. Since then, it has been detected in different cereal grains, animal feeds, and cereal-based products, such as beer and malt [19,22,23,24,25]. The chemical structure of DON-3G is depicted in Figure 1.
Given the widespread distribution of DON and other type B trichothecenes, the ingestion of contaminated cereal products appears to constitute a major source of their exposure in humans [18,26]. Furthermore, DON in feeds is one of the major mycotoxins that has been associated with significant economic losses due to its contribution to the reduced performance in livestock productivity [27]. To actively implement efficient scientific control strategies for reducing the exposure of humans and animals to type B trichothecenes, surveillance and monitoring studies using reliable analytical methodologies are of paramount significance to investigate the real incidence and dietary intakes of these mycotoxins in raw food materials and their final products. This review provides an overview of the toxicology-related aspects of major type B trichothecenes and their occurrence in a variety of foods. Additionally, different methodological approaches and detection techniques proposed for their determination, as well as management and decontamination strategies for limiting their presence in foods are described.
2. Factors Affecting Type B Trichothecenes Production
The growth of fungal species and subsequent production of mycotoxins at different stages of crop production are influenced by a complex interaction of biotic and abiotic conditions. Figure 2 shows the conditions that influence mycotoxins production in food and feed chains [10]. These conditions are mainly related to environmental/physical, biological, and chemical factors. The environmental factors involve temperature (0–50 °C), relative humidity of surroundings (70%), precipitation patterns, water activity (>0.88) “the amount of free water available in food that can be utilized by microorganisms”, and mechanical injury [10,28]. These climatic conditions, especially moisture content and ambient temperature, are extremely important factors in determining the occurrence of mycotoxigenic fungi, their level of colonization, and subsequent accumulation of relevant mycotoxins in the field [29,30,31]. Thus, variations in fungal development and mycotoxin generation are evident across geographical locations owing to variances in climatic conditions and fungal growth requirements [32].
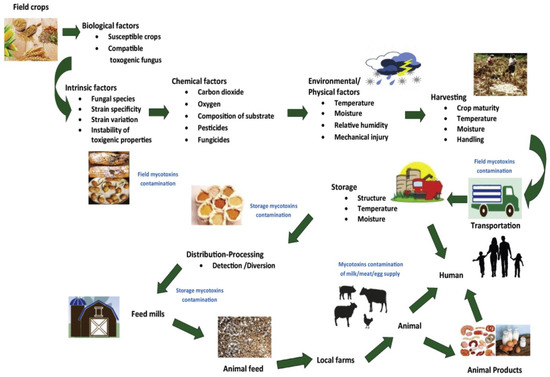
Figure 2.
Factors influencing mycotoxin production in the food chain. Reproduced with permission from Haque et al. [10]. Copyright 2020, Elsevier.
Mycotoxigenic fungi are conventionally classified as “field” (also known as plant-pathogenic) and “storage” (also known as saprophytic/spoilage) species [33,34]. Field fungi attack seeds while the plant crops are growing in the field and require high moisture levels (≥20%) to thrive. These fungi species involve Fusarium, Cladosporium, Claviceps, Helminthosporium, Neoitphodium, Gibberella, Cladosporium, and Alternaria [34]. Storage fungi attack seeds or grains while they are being stored and require lesser moisture content (13–18%) than field fungi. Thus, they are not associated with serious food safety issues at the pre-harvest stages. Moreover, they can grow at equilibrium moisture contents with relative humidity levels of 70–90% in the absence of free water [34]. Aspergillus and Penicillium are among the fungal species that belong to this group. However, it is widely accepted that the majority of contaminations in storage facilities are caused by infections that previously originated in the field [34]. Generally, no further deterioration in grains will take place if the product is dry and stored in a dry location. Nonetheless, if there is water leakage, condensation, or insect/rodent activity, mycotoxin-causing fungus growth will develop.
Chemical factors that influence mold growth and mycotoxin synthesis include fungicides/pesticides application, oxygen, and carbon dioxide concentrations, as well as the composition of the substrate. Meanwhile, other biological factors are related to susceptible crops (substrate), compatible toxigenic fungi (fungal strains), strain specificity, variation, and instability [8,10]. Under field conditions, a plant’s susceptibility to infestation and colonization by toxigenic fungi is often enhanced by stress and subsequent reduction in vitality. Certain fungal strains are capable of creating many mycotoxins, while a single mycotoxin may be generated by multiple fungi [35]. Molds may be found on a variety of substrates, i.e., nearly every kind of food could be infected by molds since the nutrients (carbon and nitrogen) necessary for their development are available in food, particularly those that are high in carbohydrates [36]. However, the exact reason why fungi prevail on a particular food item remains unknown. During storage of grains, the growth of fungal species and subsequent production of mycotoxins arise generally from multiple factors interacting with one another, including moisture content, temperature, fungal abundance, oxygen (O2), and carbon dioxide (CO2) concentration, pH of the substrate and its composition, mechanical damage, and microbial interactions [34,37].
3. Toxicity of Type B Trichothecenes
Type B trichothecenes are considered the most widespread group of Fusarium-generated mycotoxins, which are typically characterized by containing a tetracyclic 12, 13-epoxytrichothene skeleton, accounting for many of their toxicological effects [18]. Type B trichothecenes are readily absorbed via different routes. The main route for potential exposure to these mycotoxins is through dietary ingestion of contaminated foods and feeds, but inhalation of toxigenic spores and direct skin contact have also been identified as possible sources of infection [38]. Trichothecenes are very stable mycotoxins; they are not eliminated completely from cereal-based foods during milling processing and are not degraded entirely by high temperatures [39,40]. Therefore, they are commonly detected in grains as well as their processed products, such as flour, baking products, breakfast cereals, pasta, malt, and beer [14,23,24,25,40,41,42]. Trichothecenes are harmful to plants, humans, farm animals, fish, birds, and various eukaryotic species in general. The cytotoxic potency of these mycotoxins is variable depending on the certain contaminating toxin and the studied animal species. An acute intoxication via oral, dermal, or respiratory exposures induces a spectrum of adverse effects, including hemorrhage, leukopenia, hematological disorders, abdominal pain, growth retardation, circulatory shock, immunosuppressive effects, and toxicity of the central nervous system, leading to the loss of appetite, nausea, fatigue, fever, and vomiting (emesis) [9,14,18,24].
DON and other type B trichothecenes are seriously intoxicating in view of their further capability to be topically absorbed, and their metabolites affect the skin, gastrointestinal tract, liver, kidney, immune, and hematopoietic progenitor cellular systems [43]. Once these mycotoxins disseminate into the systemic circulation, they readily impact tissue proliferation. Exposure of farm animals to feed containing moderate levels of DON leads to temporary feed refusal and a reduction in weight gain. The susceptibility to DON was shown to be varying among animal species in the following decreasing order: “pigs > mice > rats > poultry ≈ ruminants” [44].
The main targets of type B trichothecenes are leucocytes; they have the potential to up- and downregulate immunological response by interrupting intracellular signaling inside leukocytes [45]. Based on their dose, incidence, and duration of exposure, they typically induce immunosuppressive effects or act as immunostimulatory. Type B trichothecenes demonstrate their chronic toxicity through binding to the 60S subunit of ribosomes as a consequence of interaction with peptidyl transferase enzyme, thereby rapidly activating mitogen-activated protein kinases (MAPKs). This typically causes inhibition of peptide bond formation and suppression of DNA biosynthesis in eukaryotes. Furthermore, these compounds can inhibit mitochondrial function and reduce cell proliferation [46,47].
DON has been shown to induce apoptosis and dysfunction in mouse kidneys and diverse immune cells, such as dendritic cells, macrophages, and lymphocytes via the response to ribotoxic stress in a process termed a “ribotoxic stress syndrome” [48]. Pharmacological studies have indicated that two important upstream transducers of DON-induced MAPKs, namely double-stranded RNA (dsRNA)-activated protein kinase (PKR) and hematopoietic cell kinase (Hck) contribute to the activation of MAPKs, thus increasing DON-induced gene expression and apoptosis [49]. DON can also increase the susceptibility of farm animals to porcine reproductive and respiratory syndrome virus (PRRSV) infections by influencing the specific humoral immune responses, thereby enhancing the synergistic effects of the toxin and viral diseases on weight gain, lung lesions and mortality [50,51].
Recently, intestinal epithelial cells have also been identified as one of the main sensitive targets for these mycotoxins [52]. DON and NIV were shown to induce cell necrosis in intestinal epithelial cells and alter their capacity to proliferate. In former studies, it was demonstrated that a DON dose of 1–7 mg/kg diet can dramatically reduce the absorption area of the villus surface and also can increase intestinal permeability, resulting in low viability and immunological function in poultry [53]. On the same track, it has been proven that DON can impact the intestinal epithelium in pigs. It also modulates the barrier function of the intestinal epithelial cells (IECs) by altering the tight junction proteins (TJP) and mRNA expression, thereby affecting absorption and nutrient intake by enterocytes [54]. Consequently, researchers believe that trichothecenes can promote a variety of chronic intestinal inflammatory disorders, such as inflammatory bowel disease, and also contribute to food-related allergies, especially in youngsters [52].
NIV and FUS-X are more hazardous to humans and domestic animals compared to DON [13,48]. Recently, the ingestion of NIV-contaminated food from certain locations in China has been linked to an increased incidence of esophageal and gastric carcinomas [55]. During in vitro microsomal studies, FUS-X was almost completely (>90%) converted to NIV in the liver and kidney. FUS-X mainly affects organs that have rapidly growing cells such as the gastrointestinal tract, spleen, hematopoietic tissues, and the thymus, thereby eliciting intestinal inflammation and apoptosis. Moreover, genetic material alteration, which typically causes chromosomal aberrations (CAs), cell cycle delays, and sister chromatid exchanges, are toxicological issues related to FUS-X. In mice, NIV and FUS-X induced much longer, persistent anorectic effects than DON and other acetylated derivatives in the rank order of NIV > FUS-X > DON ≈ 3-ADON ≈ 15-ADON for IP exposure and FUS-X > NIV > DON ≈ 3-ADON ≈ 15-ADON for oral exposure [56]. In another study on mice and mink, FUS-X and NIV demonstrated longer durations of emesis than DON, which is more likely due to their slower elimination rates [57]. They are also potent inhibitors of protein, RNA, and DNA synthesis in mammalian cells [58]. Their exposure to poultry has been associated with certain negative impacts, including decreased gizzard weight and erosions in chickens, along with paler and more fragile than normal livers and kidneys in egg-laying hens [59,60,61]. Other critical toxicological effects in mice are related to intrauterine growth retardation, immunotoxicity, myelotoxicity, and hematotoxicity [62]. Despite all the recent information on trichothecenes’ toxicity, evidence of carcinogenicity from human and animal studies is still inadequate. Therefore, the International Agency for Research on Cancer (IARC) has classified certain trichothecenes (DON, NIV, and FUS-X) as IARC Group 3 compounds, which means that they are not proven to be carcinogenic to humans [63].
Living plants and fungal metabolism can modify the structures of DON through a certain defense mechanism, resulting in the natural occurrence of DON acetylated derivatives, such as 3- and 15-acetyldeoxynivalenol (3-ADON and 15-ADON), and modified (i.e., masked) forms such as deoxynivalenol-3-glucoside (DON-3G), which are of particular relevance in contaminated food and feed [48,64]. Contamination levels of the acetylated forms of DON can reach 10–20% of the total DON concentration and their occurrence has been studied in Fusarium-contaminated cereals from different countries [18,19,24,26]. Toxicological effects posed by ADONs in humans and animals are similar or even greater than that of DON due to their rapid absorption into the intestine [65]. The toxicological significance of DON acetylated derivatives has been recently highlighted in various reports for mammals with respect to the immune system, intestinal issues, cell cycle, and oxidative stress [48,66,67,68,69]. Exposure of human U937 macrophages to 3-ADON and 15-ADON can induce inflammatory cytokines, similar to DON [70]. In human in vitro cells, 3-ADON induced inhibition to the proliferative response of peripheral lymphocytes and reduced their capacity to generate antibodies, without modulating their viability [71]. In mice, 15-ADON and DON showed comparable effects in respect of reduced feed consumption and body weight gain [72]. Pinton et al. [65] explored the toxicological effects of DON, 3-ADON, and 15-ADON on the intestine of piglets regarding cell proliferation, barrier function, and intestinal structure. A general finding was that these mycotoxins demonstrate distinct cytotoxicity on the piglet intestine with respect to histological lesions, paracellular permeability, and cell proliferation ranked in the order as follows: 15-ADON ≫ DON > 3-ADON [65]. Even at a lower dosage than 3-ADON and DON, 15-ADON has a greater effect on decreasing the expression of the tight junction proteins, and activation of the MAPK (ERK1/2, p38, and JNK) in differentiated intestinal epithelial cells, explants, and jejunum of exposed piglets [65]. Similar findings were observed in human Caco-2 cells [73,74]. In other reports on human and porcine intestinal cells, 3-ADON was twofold less toxic in inducing cell proliferation than 15-ADON [75].
The main concern over the presence of conjugated or masked mycotoxins (i.e., DON-3G) in contaminated food and feed stems from the fact that their unusual physicochemical properties can present challenges for routine determination while the structures are still maintaining toxic effects. As conjugation is a detoxification process in plants against the effects of xenobiotic compounds such as mycotoxins, DON-3G appears to be less harmful compared to its parent form [52,76]. According to in vitro data, DON-3G is stable against acidic conditions in the stomach and is improbable to be hydrolyzed by the action of digestive enzymes [21]. Due to its reduced potential to evoke gut satiety hormones and anorectic responses, DON-3G is less potent than DON to stimulate emesis in mice [77]. DON-3G is also ineffective in inducing pro-inflammatory cytokine after oral exposure in mice, and incapable to stimulate ribotoxic stress in porcine intestinal epithelial cells [78]. This may be attributed to the steric hindrance effect of the glucose molecule, which suppresses the interaction with the A-site binding pocket at the peptidyl transferase center (PTC) of the ribosome [48]. Furthermore, DON-3G exhibited substantially smaller cytotoxicity compared with DON in treated Caco-2 cells derived from the human colon, porcine intestinal epithelial cells (IPEC-J2), and intestinal explants [79].
Several previous reports indicated that DON-3G can be partly hydrolyzed in the body under the influence of bacterial metabolism in the intestines, thereby increasing the bioavailability of the precursor toxin (DON) [21,41,80]. Therefore, clinical observations suggested that the modified mycotoxins could be responsible for the unpredicted higher degree of mycotoxicosis in animals that is not correlated with mycotoxins levels identified in the corresponding diet [81]. In this sense, DON-3G was considered as a possible factor involved in the total DON-induced toxicity according to the Joint FAO/WHO Expert Committee on Food Additives (JECFA) [82].
Based on the scientific literature, type B trichothecenes and D3G have shown a high incidence of co-contamination in cereal grains since it has been commonly established that Fusarium fungi can produce more than one mycotoxin within the same commodity [26,83,84]. According to the abovementioned toxicological effects of type B trichothecenes and based on the food safety point of view, the co-occurrence of these mycotoxins across cereal grains raises a major concern in public health. This is mainly because the current guidelines and risk assessments were typically established based on the toxicity studies of each individual mycotoxin, whereas the interaction among multiple mycotoxins in foods or diet may result in various impacts on human health, and therefore would increase the toxicological hazard due to synergistic effects of possible combined exposure [24,85].
4. Legislation of Type B Trichothecenes in Foodstuffs
As stated earlier, type B trichothecenes are considered the most widespread group of Fusarium-generated mycotoxins, which commonly infect a wide range of food commodities [18,26]. Since these mycotoxins can be formed at the pre-harvest stages of crop production, their incidence in agricultural crops is highly dependent on environmental conditions and is sometimes inevitable [18]. Due to the potential health hazards of these mycotoxins and the tremendous economic impacts arising from the losses in food quality, comprehensive food safety regulations regarding the maximum levels of these mycotoxins are essential to actively avoid their serious effects and to protect consumer health. Among the currently available type B trichothecenes, DON is the only regulated mycotoxin, while the current regulations do not specify maximum levels of other type B trichothecenes in foods. Although many developing countries still have no specific DON regulations for food commodities, they have recognized that minimizing mycotoxin contamination will not only reduce the financial burden on health care but will also provide international economic benefits such as increased exports to the lucrative European markets. Table 1 lists the maximum regulatory limits of DON in different foodstuffs fixed by various regulatory authorities, including the US Food and Drug Administration, European Union, and Food and Agricultural Organization of the United States/Codex Alimentarius, as well as some Asian countries [86,87]. In a practical situation of the ecological system, DON is more likely to be found in food commodities with other mycotoxins including its modified and masked forms. This could explain the variation in the maximum regulatory limits based on geographical origins and types of food commodities which creates new challenges for regulatory agencies responsible for establishing regulations, scientific legislation, and standards [88].
It is also important to note that the Scientific Committee on Food (SCF) in 2002 proposed tolerable daily intakes (TDIs) for NIV and DON at 0.7 and 1 µg/kg body weight, respectively [89]. However, there is still increasing evidence that 3-ADON and 15-ADON can contribute to total DON-induced toxicity due to their deacetylation during mammalian digestion [66]. Therefore, in 2010 the Joint FAO/WHO (World Health Organization) Expert Committee on Food Additives (JECFA) extended the previous provisional maximum tolerable daily intake (PMTDI) for DON (1 μg/kg body weight) to a group of DON and its acetylated derivatives and also defined a group acute reference dose (ARfD) at 8 μg/kg body weight, based on investigations on mice that showed a reduced feed consumption and growth retardation [82,90]. However, DON-3G was not included in the group PMTDI since the aspect regarding toxicological relevance and occurrence was still being considered. In 2017, with increasing JECFA evaluation and available toxicological data from human intoxications from Asian countries, the European Food Safety Authority (EFSA) CONTAM Panel reported on the risks to human and animal health related to the presence of DON, its acetylated derivatives and modified forms in food and feed [91]. In this report, EFSA suggested that the risk assessment needs to account for DON, including its acetylated derivatives and modified form as additional contributing factors to dietary exposure to DON, and therefore used the same group PMTDI (1 µg/kg body weight per day), but for the sum of DON, 3-ADON, 15-ADON, and DON-3G in infants, toddlers, and other children, and applied it for the overall human risk assessment [43,91].

Table 1.
Maximum regulatory limits of DON in different foodstuffs established by various regulatory authorities.
Table 1.
Maximum regulatory limits of DON in different foodstuffs established by various regulatory authorities.
| Regulation Authority | Foodstuff | Maximum Level μg/kg | Reference |
|---|---|---|---|
| European Commission | Unprocessed durum wheat, maize, and oats | 1750 | [86] |
| Unprocessed cereals | 1250 | ||
| Cereal flour for human consumption and dry pasta | 750 | ||
| Bread, pastries, breakfast cereals, and cereal snacks | 500 | ||
| Cereal-based foods and baby foods for infants and young children | 200 | ||
| United States Food and Drug Administration | Flour, germ, and bran derived from wheat cereal intended for human consumption | 1000 | [87] |
| Food and Agricultural Organization of the United States/Codex Alimentarius | Raw grains such as maize, wheat, and barley | 2000 | [87] |
| Maize, wheat, and barley-derived foods (flour, meal, semolina, and flakes) | 1000 | ||
| Cereal-based foods and baby foods for infants and young children | 500 | ||
| Republic of Korea | Grain and their processed foods | 1000 | [92] |
| Corn and their processed foods | 2000 | ||
| Cereals | 500 | ||
| China | Corn, corn flour (grits, flake) | 1000 | [93] |
| Barley, wheat, cereal, wheat flour | 1000 |
5. Occurrence of Type B Trichothecenes and DON-3G in Cereal Grains and Their Products
As previously mentioned, Fusarium fungi can colonize crops at the pre-harvest stages of production. Thus, it has become difficult to avoid trichothecenes contamination due to the considerable influence of abiotic conditions [11,12]. In this respect, type B trichothecenes are considered the most widespread group of Fusarium-generated mycotoxins, which commonly infect a wide range of cereal grains including wheat, corn, oats, barley, rice, rye, sorghum, and their products [19,94]. It is generally accepted that the geographic distribution of type B trichothecenes-producing fungi (F. graminearum and F. culmorum) can be influenced by climatic factors. For instance, F. culmorum is mostly found in cooler areas, including Western Europe, while F. graminearum is typically present in hot, tropical climates, including Africa, eastern Asia, eastern Australia, eastern Europe, and North America [37].
5.1. Wheat
Wheat crops are readily susceptible to infestation with Fusarium culmorum species during the heading-to-flowering phases when climatic conditions are favorable for their growth, thereby causing the destructive Fusarium head blight (FHB) disease in wheat [93]. Many countries monitor the presence of type B trichothecenes, particularly DON in wheat and their products, and recent occurrence studies have shown their common prevalence in these food commodities (Table 2). Liu et al. [95] surveyed the prevalence of DON, 15-ADON, and 3-ADON in 672 wheat samples from China during 2012–2013. DON was identified as the most prevalent mycotoxin (91.5%) at contamination levels ranging from 2.4 to 1130 μg/kg (mean value: 178 μg/kg). Meanwhile, the incidence rates and levels of 15-ADON and 3-ADON were considerably less than those of DON. 15-ADON and 3-ADON were detected in only 18.0% (mean value: 2.1 μg/kg, range: 0.62–6.0 μg/kg) and 1.6% (mean value: 1.3 μg/kg, range: 1.5–2.6 μg/kg), respectively. Only 0.3% of the total wheat samples contained DON at levels above the regulatory limit of China for DON in wheat (1000 μg/kg). In another recent research from China, extremely high values (up to 86,255 μg/kg) of DON were reported for wheat with a 100% frequency [96]. Bryła et al. [97] investigated the presence of DON, DON-3G, and NIV in 92 wheat samples collected from Polish markets. DON was the most dominant mycotoxin in the samples, while NIV showed the highest contamination levels. The incidence rates of DON, DON-3G, and NIV were 83%, 27%, and 70% at contamination ranges of 5.1–373 μg/kg, 15.8–138 μg/kg, and 10.5–1265 μg/kg, respectively. Moreover, the relative proportion of DON-3G compared to DON (glucosylation percentage) ranged from 4% to 37% in positive samples. In recent years, the relationship between the influence of agricultural practices (conventional and organic) and mycotoxin contamination have been discussed by several researchers [26,84]. Gab-Allah et al. [26] evaluated the contamination of type B trichothecenes and DON-3-G in 27 wheat flour samples from both organic and conventional production in Republic of Korea. The highest incidence rates were reported for DON (96%) followed by DON-3G (96%) and NIV (85%). Meanwhile, 3-ADON, 15-ADON, and FUS-X were less frequently occurring in wheat samples at incidence rates of 11%, 18.5%, and 37% of the total samples, respectively. The contamination ranges were recorded as 0.74–154 μg/kg (DON), 0.25–24.7 μg/kg (DON-3G), 0.45–126 μg/kg (NIV), 2.3–10.3 μg/kg (3-ADON), 6.0–30.6 μg/kg (15-ADON), and 0.80–4.6 μg/kg (FUS-X). According to the results, higher incidences and concentrations of all mycotoxins were found in organic wheat samples compared with conventional ones, except for 3-ADON. Palacios et al. [98] from Argentina investigated the presence and contamination levels of DON, DON-3G, and the sum of 3 and 15-ADON in 84 wheat samples during 2012-2014. All wheat samples were contaminated with DON (100%) at concentration ranges from <LOQ to 9480 μg/kg (mean value: 1762 μg/kg). DON-3G was found in 93% of the samples at concentration ranges from <LOQ to 850 μg/kg (mean value: 198 μg/kg), whereas the acetylated DON derivatives (3 and 15-ADON) occurred less frequently in the samples (49%) at noticeably lower contamination levels (<LOQ to 190 μg/kg). The glucosylation percentage across the samples varied between 6% to 22%, and the contamination with DON was found to be affected by the geographical location and year of harvest due to varying climatic conditions.

Table 2.
Representative occurrence studies on type B trichothecenes in different food commodities around the world.
According to the available results, type B trichothecenes and DON-3G were detected more frequently in wheat and its processed products. DON was the most prevalent mycotoxin in the samples found at exceedingly high contamination levels in some cases, thus posing a potential food safety problem. Furthermore, DON derivatives and other type B trichothecenes co-occurred with DON in the same wheat samples, which can maximize the toxicological hazard due to the synergistic effects of possible combined exposure the risk of their combined exposure, and mycotoxin interactions. Therefore, special attention should be paid to the constant monitoring of these compounds in wheat and its products in order to avoid or reduce their adverse health effects, especially those arising from human exposure to DON.
5.2. Corn
Representative data on the occurrence of type B trichothecenes and DON-3G in corn is listed in Table 2. Iqbal et al. [99] studied the contamination of DON in 142 winter corn and 128 summer corn, as well as their products from Pakistan. DON was detected in 61.2% and 44.5% of winter corn and summer corn samples, respectively, at contamination levels of <LOQ–2967 μg/kg (winter corn) and <LOQ–2490 μg/kg (summer corn). The results also showed that the incidence rates of DON in corn and its products from the winter and summer seasons were statistically significant except for cornbread samples. Moreover, a significant number of samples showed contamination levels of DON exceeding the regulatory limits of the EU. In Croatia, a higher incidence rate (84%) and exceedingly high contamination levels (up to 17,900 μg/kg, mean value: 2150 μg/kg) of DON were documented in maize samples [100]. Berthiller et al. [101] collected 54 maize samples from Austria, Germany, and Slovakia, and found that all the samples (100%) were contaminated with DON and DON-3G at contamination ranges of 238–3680 μg/kg (average level: 753 μg/kg) and 25.0–763 μg/kg (average level: 141 μg/kg), respectively. In a more recent study from Republic of Korea, Gab-Allah et al. [26] surveyed 25 organic and conventional corn samples for all major type B trichothecenes and DON-3-G. The incidences were recorded as 96% (DON), 96% (DON-3G), 80% (NIV), 72% (3-ADON), 60% (15-ADON), and 60% (FUS-X). The maximum contamination levels of these mycotoxins were 1223 μg/kg (DON), 419 μg/kg (DON-3G), 234 μg/kg (NIV), 11.8 μg/kg (3-ADON), 298 μg/kg (15-ADON), and 28.7 μg/kg (FUS-X). According to the results, higher incidences and concentrations of these trichothecenes were found in organic corn samples compared with conventional ones, and the glucosylation percentage reached up to 35% in contaminated corn samples. In another recent research, Gab-Allah et al. [102] investigated DON, NIV, and DON-3G in corn samples collected from Egypt. Among these mycotoxins, DON was the most common (83.3%) followed by NIV (74.1%) and DON-3G (40.7%), with maximum concentration levels of 853 μg/kg, 462 μg/kg, and 257 μg/kg, respectively. In Southwest Nigeria, DON was found in maize at an incidence level of 22.2% within the range of 9.6–745 μg/kg and its modified 3-ADON was detected at an incidence level of 17.2% (0.70–72.4 μg/ kg) [103], while DON was found within a noticeably very low range of 0.10–0.70 μg/kg in maize from Northeast Nigeria [104]. EFSA also reported a high frequency of DON occurrence (70 in 136 samples, 51.5%) in maize for human consumption, with an average value of 238 μg/kg [90]. Other studies revealed that maize samples were contaminated with DON at incidence rates of 32.4% (27.0 to 2210 μg/kg) in Serbia [105], 21.4% (3.0 to 428 μg/kg) in Italy [106], 63.0% (68.0 to 2196 μg/kg) in Tanzania [107], 50.8% (<LOQ to 90.0 μg/kg) in Poland [108], 86.0% (225 to 2963 μg/kg) in Hungary [109], 24% (10.0 to 1070 μg/kg) in India [110], and 71% (215 to 278 μg/kg) in Croatia [111].
Based on the available data on corn and its products, type B trichothecenes and DON-3G were detected in varying concentrations with the highest positive rates and concentrations being registered for DON. Still, the necessity for more studies that evaluate the co-contamination of corn and its products with DON and other type B trichothecenes is of prime importance to avoiding or reducing the risk arising from the intake of these toxins from contaminated corn and its processed products.
5.3. Oats
Table 2 summarizes representative data on the prevalence of type B trichothecenes and DON-3G in oats. Nathanail et al. [14] investigated the contamination of trichothecenes in 31 oat samples collected from Finland. All samples were contaminated with DON at high levels up to 23,800 μg/kg (mean value: 2690 μg/kg). The incidences of DON-3G, 3-ADON, and NIV were 87.1%, 77.4%, and 71.1%, respectively, with maximum contamination levels of 6600 μg/kg (DON-3G), 2700 μg/kg (3-ADON), and 4940 μg/kg (NIV). The frequency and contamination levels of these mycotoxins in oat samples were more extreme than those in wheat and barley, with 32% of the tested oat samples containing DON levels beyond the legislative MRL for unprocessed oats (1750 μg/kg). In more recent research, Tarazona et al. [112] from Spain stated that 22.0% and 3.0% of oat samples (n = 100) contained detectable DON and 3-ADON, with the maximum levels of 736 μg/kg and 42.6 μg/kg, respectively. In another study conducted on oat bran from Spanish markets, a total of 17% of the samples were contaminated with DON at the maximum level of 230 μg/kg. Schöneberg et al. [113] collected 325 oat samples from Switzerland during 2013–2015 and found that 49% and 64.3% of samples were contaminated with DON and NIV, respectively, at maximum contamination levels of 1328 μg/kg (DON) and 1653 μg/kg (NIV). They found that the average contamination with DON was the highest in 2013, while NIV showed the highest levels in 2015. DON was also detected in a total of 60% (15/25) of oat samples from China with a concentration range varying between 16.8 and 244 μg/kg [114]. Islam et al. [115] from Canada analyzed 168 oat samples during 2016–2018 and documented that NIV and DON were the predominant mycotoxins in the samples with incidences of 92.0% and 55.3% and maximum concentrations of 795 μg/kg and 4143 μg/kg, respectively. Juan et al. [83] detected DON, 3-ADON, FUS-X, and NIV in 57.0%, 14.2%, 42.8%, and 57.0%, respectively, in oat samples (n = 7) collected from Italy at levels ranging from 10.3 to 83.0 μg/kg (DON), <LOQ to 5.2 μg/kg (3-ADON), 26.0 to 75.0 μg/kg (FUS-X), and 45.5 to 50.4 μg/kg (NIV). Previous research from Republic of Korea showed the absence of DON and DON-3G in oat samples, while NIV was detected in only 9.1% of the samples with a mean value of 23.5 μg/kg [116]. In contrast, higher incidence rates and contamination levels were recorded for DON and NIV in oat samples collected from Sweden, where DON and NIV were presented in 95.0% and 91.5% of the samples with the ranges of 99.0–5544 μg/kg and 18.0–1743 μg/kg, respectively [117]. In another research, Edward at al. [118] surveyed the prevalence of NIV, FUS-X, and DON in 303 oat samples collected during 2006–2008 in the United Kingdom. NIV was the most abundant mycotoxin with an incidence rate of 73% and a maximum concentration of 741 μg/kg. DON was found in 32% of the samples at higher contamination levels with a maximum value of 1866 μg/kg. Only 1% of the samples contained FUS-X with a maximum concentration of 18 μg/kg. In Malaysia, Soleimany et al. [119] documented that 30% of oat samples were found to be positive for DON with concentrations ranging from 22.7 to 100 µg/kg. The same incidence rate (30%) was registered for DON in oat samples collected from Slovakia, but with relatively higher contamination levels (up to 490 µg/kg) [120].
In summary, DON and NIV were the most dominant type B trichothecenes in oat samples. Meanwhile, the occurrence of other type B trichothecenes (3-ADON, 15-ADON, FUS-X) needs to be studied more thoroughly.
5.4. Barley
Representative studies on the occurrence of type B trichothecenes and DON-3G in barley are summarized in Table 2. Nathanail et al. [14] surveyed the occurrence of some Fusarium mycotoxins in 34 barley samples collected from Finland. Among all mycotoxins, DON showed the highest incidence rate in the samples at concentrations up to 802 μg/kg (mean value: 234 μg/kg). The incidences of DON-3G, NIV, and 3-ADON were 73.5%, 73.5%, and 41.2%, respectively, with maximum contamination levels of 594 μg/kg (DON-3G), 262.0 μg/kg (NIV), and 18.3 μg/kg (3-ADON). The frequency and contamination levels of these mycotoxins in barley samples were the lowest among other tested cereal grains (wheat and oat), and no barley sample exceeded the maximum regulatory levels established for DON (1250 μg/kg). However, barley revealed the maximum DON glucosylation capacity among all tested cereals. In turn, Ok et al. [121] from Republic of Korea found DON in a total of 54% (38/70) of barley samples with a concentration range varying between 3.7 and 36.8 μg/kg (mean value: 9.4 μg/kg). In another study from Republic of Korea, Ok et al. [84] surveyed 39 barley samples for type B trichothecenes. NIV was the most frequently detected mycotoxin in the samples followed by DON and 15-ADON. The incidences were recorded as 59% (NIV), 56% (DON), 15% (FUS-X), 31% (15-ADON), and 26% (3-ADON), while the maximum contamination levels of these mycotoxins were 101 μg/kg (NIV), 40.1 μg/kg (DON), 9.9 μg/kg (FUS-X), 7.1 μg/kg (15-ADON), and 3.9 μg/kg (3-ADON). A similar trend was documented by Lee et al. [116] who found NIV, DON, and DON-3G in Republic of Korea barley samples at incidences of 40.0%, 33.3%, and 13.3%, with contamination ranges of 17.3–230 μg/kg (mean value: 90.2 μg/kg), 11.7–286 μg/kg (mean value: 75.8 μg/kg), 18.0–20.6 μg/kg (mean value: 19.3 μg/kg), respectively. Juan et al. [83] evaluated the contamination of type B trichothecenes in barley samples originating from Italy. 3-ADON and 15-ADON were not detected in any sample. Whereas the frequency of DON, FUS-X, and NIV were 11.0%, 44.4%, and 33.3%, respectively, at levels ranging from <LOQ to 35.3 μg/kg (DON), 27.5 to 47.3 μg/kg (FUS-X), and 21.7 to 106 μg/kg (NIV). In another research, Soleimany et al. [7] from Malaysia found DON in 50% of barley samples with concentrations varying between 27.9 µg/kg to 72.5 µg/kg. In another recent study, Bryła et al. [122] detected DON, DON-3G, and NIV in barley-derived beer collected from Poland. Fractions of positive samples were recorded as 83%, 67%, and 56% for DON, DON-3G, and NIV with mean concentrations of 9.0 µg/L (range: 1.0–73.6 µg/L), 9.2 µg/L (range: 2.0–35.8 µg/L), and 2.4 µg/L (range: 0.50–27.6 µg/L), respectively. They suggested that the higher contamination incidence of DON-3G is attributed to the glucosyltransferase enzyme activity during the grain malting process. Additionally, Tima et al. [109] identified DON and other Fusarium mycotoxins in barley samples originating from Hungary. They found DON in 48% of the samples (range: 240–429 μg/kg). They also noted that DON was the most common mycotoxin in these samples followed by T-2 and zearalenone (ZEN). Mishra et al. [110] investigated 25 barley samples from India. DON was identified in 4 samples at concentrations ranging from 30.0 to 530 μg/kg (mean value 210 μg/kg). From this study, the frequency and contamination levels of DON in barley samples were less extreme than those of wheat and maize [110]. In a more recent study, DON was found in ninety-four percent (94%, n = 76) of Brazilian barley samples with a high contamination level over the range from 310 to 15,500 μg/kg (mean value: 5000 μg/kg) [85]. DON was also found in 53% (18/34) of Croatian barley samples at levels varying between 74.0 µg/kg and 228 µg/kg (mean value: 142 µg/kg) [111]. In Tunisia, a total of 72 commercial barley samples were collected in 2009, where fifty-seven percent of all samples were positive for DON, with contamination levels ranging from 500 to 3500 µg/kg (mean value: 1520 µg/kg).
Based on these occurrence studies, the presence and concentrations of type B trichothecenes in barley from different countries were inconsistent. Further studies are highly required to evaluate their current contamination status in various geographical regions.
5.5. Rice
Table 2 summarizes representative data on the prevalence of type B trichothecenes in rice. Golge et al. [123] from Turkey collected 20 paddy rice samples to explore DON contamination. DON was detected in the samples at an incidence rate of 35% (7/20) with concentrations ranging from 136 to 256 µg/kg (mean value: 195 µg/kg). Among several kinds of cereal tested in that report, paddy rice showed the highest contamination frequency for DON [123]. In recent research focused on one hundred eighty polished rice samples from Pakistan, NIV and DON were present in 28% (<LOQ to 116 μg/kg), and 8% (<LOQ to 115 μg/kg) of the samples, respectively [124]. The mean concentration levels were reported as 13.8 μg/kg for NIV and 6.9 μg/kg for DON. Ok et al. [84] evaluated the contamination of type B trichothecenes (NIV, DON, FUS-X, 15-ADON, and 3-ADON) in rice (n = 65), glutinous rice (n = 11), and brown rice (n = 48) collected from Republic of Korea markets. The highest incidence rates of positive samples were recorded for NIV (from 35 to 64%), followed by 15-ADON (from 25 to 56%) and DON (from 15 to 33%). Maximum contamination levels (up to 45.4 μg/kg) were also registered for NIV. In another recent study from Republic of Korea, Ok et al. [125] investigated NIV and DON in two different rice (i.e., white rice, n = 241 and brown rice, n = 241). Generally, NIV was identified as the predominant mycotoxin in the samples with higher contamination levels than DON, particularly in brown rice samples. In white rice samples, NIV and DON were detected in 21% (12.6 to 2175 μg/kg) and 5% (7.1 to 372 μg/kg) of the samples, respectively. Meanwhile in brown rice, these mycotoxins were detected in 34% and 7%, respectively, with concentrations in the ranges of 17.3–2534 μg/kg (NIV), and 9.1–435 μg/kg (DON). It should be noted that NIV co-occurred with DON in 9.1% and 14.9%, and 41.5% for white rice, brown rice, respectively. In Spain, 23 rice samples were screened for DON, 3-ADON, FUS-X, and NIV [126]. These samples were only positive for DON at a maximum contamination level of 5.5 μg/kg (mean level: 5.0 μg/kg). DON was also detected in 70.7% (30/41) of rice samples originating from Nigeria, but at very low concentrations (below 1.0 μg/kg) [104]. In another study from Nigeria, a total of twenty-one rice samples were screened for DON with only five samples found positive for this mycotoxin and the average value and the range were reported as 18.9 μg/kg and 11.2–112 μg/kg, respectively. Soleimany et al. [119] from Malaysia showed that DON was found in 26% (13/50) of rice samples with concentrations varying between 12.5 µg/kg to 81.2 µg/kg. In another report, Moreira et al. [127] identified DON and acetylated DONs in 5.3% (5/93) and 73.3% (66/93) at levels up to 125 μg/kg and 17.0 μg/kg, respectively, in rice flour samples collected from Brazil.
In summary, the most prevalent type B trichothecenes in rice from the majority of studies could be NIV followed by DON, unlike other previously mentioned cereals. While their contamination levels were moderately low, extreme concentrations were also noticed in some positive samples. Thus, special consideration should be paid to the investigation of their occurrence in this food commodity. Furthermore, studies on monitoring other type B trichothecenes (e.g., ADONs, FUS-X) and DON-3G in rice are rather scarce and further efforts are required.
5.6. Sorghum and Rye
Mycotoxin contamination of sorghum and rye is documented less often than contamination of other grains; however, some occurrence studies investigating certain mycotoxins in these food commodities do exist. Representative data on the prevalence of type B trichothecenes and DON-3G in sorghum and rye are listed in Table 2. More recently, Lee et al. [116] detected DON, DON-3G, and NIV in sorghum samples collected from Republic of Korea at incidence rates of 100%, 41.7%, and 91.7%, respectively. The concentrations of DON, DON-3G, and NIV were in the ranges of 18.9–712 μg/kg (mean level: 119 μg/kg), 10.4–43.4 μg/kg (mean level: 18.8 μg/kg), and 4.6–146 μg/kg (mean level: 45.3 μg/kg), respectively. In another study, the presence of DON, 15-ADON, DON-3G, NIV, and FUS-X was investigated in 110 sorghum samples originating from Nigeria [128]. The percentage of positive samples for DON, 15-ADON, and DON-3G were 3%, 2%, and 23%, respectively, and none of the samples were contaminated with NIV and FUS-X. The maximum contamination levels for DON, 15-ADON, and DON-3G were 119 μg/kg (mean level: 100 μg/kg), 44.0 μg/kg (mean level: 39.0 μg/kg), and 63.0 μg/kg (mean level: 24.0 μg/kg), respectively. In recent research from Nigeria, Olopade et al. [129] analyzed twenty sorghum samples to investigate the occurrence of DON, 3-ADON, and 15-ADON. However, none of the tested samples were found to be positive for these mycotoxins. In another study devoted to assessing the safety, occurrence, and contamination levels of mycotoxins in 1533 sorghum grain from four sub-Saharan African (SSA) countries (Sudan, Ethiopia, Mali, and Burkina Faso), only seven samples (0.46%) contained DON with a contamination range varied between 40.0 μg/kg and 112 μg/kg (mean level: 63 μg/kg) [130]. In Ethiopia, a total of 33 sorghum samples and other grains were collected and screened for DON, NIV, and other major mycotoxins. DON and NIV were identified in 90.9% (range: 50–2340 μg/kg, mean level 70.0 μg/kg), and 9.1% (range: 50–490 μg/kg, mean level: 307 μg/kg), respectively [131]. The results from that study showed high incidence and contamination levels of mycotoxins in sorghum, which might be attributed to elevated grain moisture contents due to inappropriate storage conditions. Out of 12 sorghum samples recently analyzed in Togo, 17% (2/12) were found positive for NIV and DON with concentrations in the ranges of 47.6–51.4 μg/kg (mean level: 49.4 μg/kg), and 19.0–32.8 μg/kg (mean level: 4.8 μg/kg), respectively [132]. Generally, the occurrence of mycotoxins and their contamination levels in sorghum were noticeably lower than those of maize analyzed in the same study. In another study that included four sorghum samples collected from Tunisia, DON was not detected in the samples, while NIV was found in all samples at a contamination range varying between 418 μg/kg and 667 μg/kg [133]. In another study from Tunisia concerned with the analysis of 60 sorghum samples, no type B trichothecene mycotoxins were detected in the samples, whilst the most dominant mycotoxin was enniatin B followed by ochratoxin A and enniatin B1 [134].
Juan et al. [83] evaluated the contamination of type B trichothecenes in 11 rye samples collected from Italy. They detected DON, FUS-X, and NIV in 45.4%, 45.4%, and 27.3% of the samples, respectively, at concentration levels ranging from 16.5 to 79.6 μg/kg (DON), 42.4 to 70.2 μg/kg (FUS-X), and 33.9 to 34.4 μg/kg (NIV). Martos et al. [135] investigated the occurrence of DON and its acetylated derivatives in 15 rye grain samples collected from Canada. All tested samples were positive for DON with concentrations varying between 87.0 μg/kg and 500 μg/kg (mean level 270 μg/kg). However, none of the samples contained detectable levels of the acetylated derivatives of DON. In a study preformed on 61 rye samples from Germany to investigate the presence and contamination levels of trichothecenes, all rye samples were contaminated with DON (100%) at a maximum contamination level of 288 μg/kg (mean value: 28.0 μg/kg) [136]. 3-ADON, 15-ADON, NIV, and FUS-X were identified less frequently in 59%, 80%, 3.3%, and 1.6% of the samples with maximum concentrations at 5.0 μg/kg (mean value: 0.39 μg/kg), 8.6 μg/kg (mean value: 0.73 μg/kg), 1.8 μg/kg (mean value: 0.06 μg/kg), 1.8 μg/kg (mean value: 0.01 μg/kg), respectively. In Poland, Stuper-Szablewska and Perkowski [137] conducted an analysis of type B trichothecenes in 378 grain samples including oats, rye, barley, triticale, and wheat collected during 2006–2008. The results of this study demonstrated that the highest mean concentrations of DON (46.0 μg/kg and 53.0 μg/kg) were detected in rye grain samples in 2006 and 2007, respectively. Other type B trichothecenes (FUS-X, 3-ADON, 15-ADON, and NIV) were detected at noticeably lower mean concentrations in the ranges of 1.0–27.0 μg/kg (2006), <(LOD)–26.0 μg/kg (2007), and <LOD–38.0 μg/kg (2008). Rasmussen et al. [138] from Denmark reported on the contamination of 59.4% and 13.0% rye grain samples with DON and NIV at maximum concentrations for positive samples of 257 μg/kg and 48.0 μg/kg, respectively. Furthermore, high concentration values of DON (up to 10,760 μg/kg) were reported in rye grain from the USA [139].
Based on these occurrence studies, the incidence of type B trichothecenes in sorghum and rye was inconsistent in different countries, while their concentrations seem low (below the regulatory limits of the EU), except for a few studies in which the levels of some positive samples were relatively high. Furthermore, contamination studies on type B trichothecenes in these commodities are rather scarce and further efforts are highly required to compare their recent contamination status in different areas.
6. Sample Preparation Methods for Analysis
Due to the restrictive regulations established regarding the maximum levels of trichothecenes in foodstuffs, the development of sensitive, accurate, precise, and reliable analytical methodologies is increasingly required to enforce the current regulations with a certain confidence. Sample preparation and analytical methods for the determination of trichothecenes in food and feed have been extensively reviewed [7,22,161,162]. The quantitative analysis of type B trichothecenes in food matrices generally comprises several steps: sampling, homogenization, extraction, clean-up that often includes analyte enrichment, and finally separation and detection using various instrumental and non-instrumental techniques (see Figure 3) [161,163]. Eventually, the method’s suitability for the specified analytical objective has to be assessed with appropriately adopted quality assurance procedures [164,165].
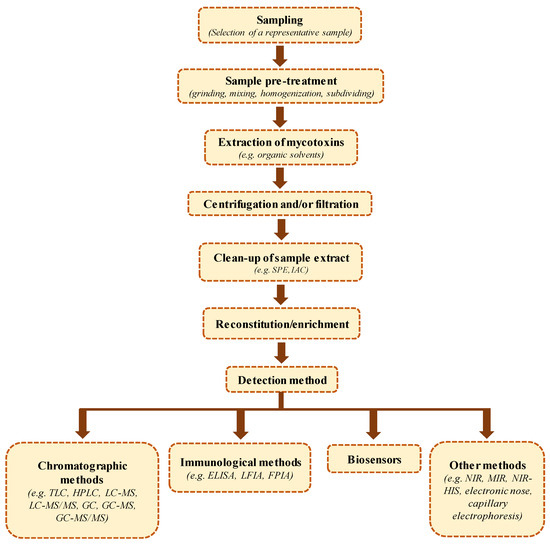
Figure 3.
Diagram of the main steps employed in the analysis of mycotoxins in food commodities. Abbreviation: SPE: solid-phase extraction; IAC: Immunoaffinity column; TLC: thin layer chromatography; HPLC: high-performance liquid chromatography; LC-MS: liquid chromatography–mass spectrometry; LC-MS/MS: liquid chromatography–tandem mass spectrometry; GC: gas chromatography; GC-MS: gas chromatography–mass spectrometry; GC-MS/MS: gas chromatography–tandem mass spectrometry; ELISA: enzyme-linked immunosorbent assay; LFIA: lateral flow immunochromatographic assay; FPIA: fluorescence polarization immunoassay; NIR: near-infrared spectroscopy; MIR: mid-infrared spectroscopy; NIR-HIS: near-infrared spectroscopy–hyperspectral imaging.
6.1. Sampling Tactics
Establishing an appropriate sampling procedure is crucial for analytical methodologies developed for the determination of chemical contaminants in foods due to the complexity of food matrices [166]. Collecting representative samples for mycotoxin measurements is a vital step and has a considerable effect on the final analytical results since the overall testing error includes the sum of errors across all analytical processes starting from sampling [167]. It is important that the sample used for the laboratory analysis be representative of the raw starting material, which is typically challenging for mycotoxins owing to the considerable variability of their distribution in contaminated commodities and inconsistent development of mycotoxigenic fungi on food matrices [161,166]. Therefore, a regular aspect of all sampling procedures is that the entire original sample should be pulverized and blended so that this sample and the test portion used for the analysis can contain the same concentration of the target toxins. This is particularly essential in raw grains because some toxins such as DON are mostly contained in the grain pericarp [166]. By increasing the sample size required for the analysis, the overall analytical bias might be further minimized. The homogenization degree of the sample should be also considered since it impacts the analyte extraction yield [168]. Various sampling procedures have been developed, and the common procedure commonly reported in the scientific literature has been established by the EU (Community Regulation No. 401/2006) for the official control of certain mycotoxins in foodstuffs [169].
6.2. Extraction Methods
The quantitative determination of type B trichothecenes in food matrices is not straightforward. Many factors must be carefully considered such as sample collection, handling, storage, and particularly the sample preparation process. Generally, the development of suitable sample preparation methodologies is often recognized as the most challenging step in the entire analytical chain because it remains the most labor-intensive and bias-prone step [170,171,172,173]. At present, a broad spectrum of extraction and clean-up procedures has been developed for type B trichothecenes to separate these mycotoxins from solid food matrices into a liquid phase and to enable their purification and enrichment before analysis. The choice and optimization of extraction solvent are made wisely by considering the physicochemical properties of mycotoxins and food sample matrices, the type of the selected clean-up procedure, and the employed separation and detection technique [7,161]. During the extraction process, the analytes will move into the extraction solvent and the compounds of interest from the sample extract are separated for detection [174]. Two extraction methods for type B trichothecenes in food are generally employed: solid–liquid extraction (SLE), and liquid–liquid extraction (LLE). The SLE method is one of the earliest sample preparation methodologies to separate analytes by partition between two involved phases: solid matrix and extractant. This method is still one of the most commonly employed approaches for mycotoxin extraction from solid materials such as grains, cereal products, and other solid foodstuffs [15,17,18,19,136,144,145,175,176]. The second extraction method relies on liquid–liquid extraction for liquid samples like juice, milk, wine, and beer to initially separate mycotoxins by transferring them from their original liquid matrix into an extraction solvent so that they can be easily analyzed using suitable techniques [122,163,177]. Type B trichothecenes are polar or relatively polar compounds with good solubility in organic solvents, such as methanol, ethyl acetate, dichloromethane, acetonitrile, chloroform, and acetone. The use of low amounts of diluted acids (formic acid, citric acid, or acetic acid) is often advantageous for the extraction process because they can break potential interactions between the mycotoxins and other matrix constituents, such as sugars or proteins [94,161,166]. Furthermore, the addition of water in small quantities can enhance the substrate wetting and further improve the extraction efficiencies by increasing penetration of the extraction solvent into the hydrophilic material [18,161,175]. Defatting step using hexane or cyclohexane is sometimes included to minimize lipophilic components in the sample extract of high lipid content [17,178,179]. In addition to the type of extraction solvent, other crucial factors such as solvent ratio, temperature, and extraction duration should be thoroughly considered to accomplish more reliable mycotoxin determination. After solvent extraction, the sample extract is usually centrifuged or filtered prior to concentration and/or purification processes.
According to the scientific literature, the most widely used extraction solvents for extracting type B trichothecenes from various food matrices are acetonitrile/water and methanol/water mixtures [7,15,19,93,125,180,181,182,183,184]. Both of these solvent mixtures are volatile and suitable for LC analysis, which is necessary when sample extract is injected directly into the analytical technique [168]. However, in multi-mycotoxin extraction from cereals and their products, more improved efficiency of acetonitrile-based solvents has been demonstrated over methanol-based solvents [94,185,186]. In previous research, the highest recoveries for thirty-four chemically varied mycotoxins (including all major type B trichothecenes) extracted from spiked wheat samples were achieved with a high proportion of acetonitrile [185]. The recoveries of the mycotoxins ranged from 87 to 111% with the acetonitrile/water mixture, except for fumonisins and patulin (17–35%), while the equivalent methanol/water mixture provided more variable and unsatisfactory recoveries, for a large number of mycotoxins, which were generally in the range of 9–204%. Indeed, acetonitrile and methanol have relatively comparable polarities [187]. However, a possible explanation for the enhanced extraction efficiency of acetonitrile might be attributed to its more appropriate selectivity towards the nonionic mycotoxins, which is mostly based upon dipole interactions rather than acid or basic functionalities [187].
Among the acetonitrile/water mixtures, acetonitrile/water (84:16; v/v) was shown to be more efficient than other solvent compositions for extracting trichothecenes of different polarity from cereal commodities because it often provides less co-extracted matrix interferences and also demonstrates acceptable recoveries [19,94,181]. Juan et al. [94] evaluated four different extraction solvent mixtures, i.e., acetonitrile/methanol (40/60, v/v), acetonitrile/methanol (60/40, v/v), acetonitrile/water (84/16, v/v), and acetonitrile/water (16/ 84, v/v) for type B trichothecenes and other Fusarium mycotoxins in spiked cereal grains. The obtained results revealed that the acetonitrile/water (84/16, v/v) mixture was the best extraction solvent in terms of providing high extraction recoveries as well as minimum co-extractive matrix interferences. Moreover, Zhao et al. [19] investigated two different extraction solvents, i.e., acetonitrile/water (50/50, v/v) and acetonitrile/water (84/16, v/v), for the extraction of type B trichothecenes and DON-3G from animal feed and corn. Both extraction solvents achieved satisfactory recoveries for all target mycotoxins; however, the acetonitrile/water (84/16, v/v) mixture yielded a cleaner sample extract than the acetonitrile/water (50/50, v/v) mixture. The same findings were also observed in other similar studies for the extraction of trichothecenes from grains [172,188,189]. On the other hand, type B trichothecenes are water-soluble mycotoxins, and therefore distilled or deionized water has been frequently employed as an extraction solvent for immunochemical and other methods, which also exhibits good results for DON recovery [190,191]. Pascale et al. [192] used pure water for extracting DON and NIV from wheat and achieved good recovery values in the range of 81.0–95.0%. Similarly, Trombete et al. [193] extracted DON, NIV, and DON-3G from wheat grains using pure water with extraction recoveries varying between 84.7% and 112.3%.
In addition to the classical SLE approaches, other instrumental automated solvent extraction approaches, such as accelerated solvent extraction (ASE)/pressurized liquid extraction (PLE), supercritical fluid extraction (SFE), and microwave-assisted extraction (MAE) have been developed in recent years [167,194,195,196,197]. In the conventional SLE approaches, ultrasonic energy and/or mechanical shaking are often employed for facilitating the extraction of analytes, whereas an additional form of energy input is required in these new instrumental approaches. Therefore, they often require lower consumption of harmful solvents, shorter extraction time, and provide higher extraction yield; however, these approaches continue to be cost-intensive due to expensive equipment [198,199,200].
6.3. Clean-Up Methods
Following sample extraction and before analysis of the desired mycotoxins, sample clean-up is a necessary step to minimize the possible co-extracted interferences (undesirable substances such as protein, pigments, sugars, lipids, or fatty acids) from food matrices, and to facilitate reliable and robust measurements of trichothecenes. Worryingly, the presence of these substances in the final sample solutions can impact the sensitivity, selectivity, precision, and accuracy of the analysis [8,18]. Various clean-up approaches have been established, such as the QuEChERS (quick, easy, cheap, effective, rugged, and safe), liquid–liquid partitioning, solid phase extraction (SPE), dispersive solid-phase extraction (DSPE), column chromatography, multifunctional clean-up columns (MFCs), immunoaffinity columns (IACs), and ion-exchange columns. However, SPE, IACs, and MFCs have been extensively used to retain type B trichothecenes from various food matrices [18,94,125,136,175,183,184,189,191,201]. IAC contains activated solid phase support covalently bound with antibodies that can selectively interact with the desired mycotoxins from sample extracts while interfering components can be eliminated by a simple washing step. Organic solvents or antibody denaturation is subsequently used to elute the mycotoxins from the IAC. Therefore, IACs rely on extremely specific antigen–antibody interactions that allow for efficient clean-up and enhanced specificity of analytes in complex food matrices [202]. However, the shortcomings of this application include the possibility of antibody cross-reactivity with other structurally related toxins, high cost, and applicability to a single mycotoxin or fewer chemically related mycotoxins [183,191]. Ok et al. [125] developed a high-performance liquid chromatography with ultraviolet detection (HPLC-UV) method for the analysis of DON and NIV in rice and bran, in which these mycotoxins were extracted with distilled water, and then the sample extract was cleaned-up using immunoaffinity columns, obtaining recoveries for DON and NIV in the ranges of 93.1–106.2% and 86.2–106.6%, respectively. In another study, Pascale et al. [192] determined the same mycotoxins in wheat by UPLC-PDA after water extraction, and they achieved recovery values from 81.0 to 88.0% for NIV and from 85.0 to 95.0% for DON. More recently, Gab-Allah et al. [18] proposed the use of immunoaffinity columns for the clean-up of grain samples that were previously extracted with deionized water. The IAC allowed the simultaneous determination of DON, DON-3G, NIV, and 3-ADON using LC-MS/MS with very acceptable recoveries in the range of 87.0–92.0%. In the same study, FUS-X and 15-ADON were not adequately retained on the IAC and therefore required a more appropriate clean-up tool. In another interesting work conducted by Zuo et al. [191], a novel IAC was synthesized based on hapten 3-O-hemisuccinyl-DON (3-HS-DON) conjugated to bovine serum albumin (BSA) by active ester method to separate and clean-up DON, 3-ADON, and 15-ADON after water extraction from cereals (i.e., maize, wheat, and oatmeal). Using this IAC, the recoveries of the target analytes were in the ranges of 67.5–93.8%, 63.8–113.2%, and 75.5–106.6%, respectively.
When sample analysis includes multiple mycotoxins, even from different families, SPEs and MFCs are more suitable and rather efficient. Currently, SPE and MFC-based approaches have gained more popularity due to their wide range of selectivity, high enrichment factor, simplicity, less solvent consumption, as well as effective elimination of matrix interferences [172]. Various adsorbent materials have been commercially available, including charcoal, silica, modified silica, Florisil (magnesium silicate), silica-based octadecyl silane (C18), polymers, ion-exchange resins, Celite, aluminum oxide, charcoal–alumina, NH2, and the selection of the proper sorbent is mainly governed by several parameters, including the nature of analytes, food matrix, extraction solvent and co-extractive interference components that could be existing in the sample extract [166,203,204]. C18, Celite, Florisil, aluminum oxide, and charcoal–alumina are commonly employed for the extraction of trichothecenes from various food materials [19,39,205]. Zhao et al. [19] established a simple and reliable methodology for the analysis of type B trichothecenes and DON-3G in animal feed by LC-MS/MS. The mycotoxins were extracted with acetonitrile/water (84/16, v/v) and the clean-up was based on improved dispersive solid-phase extraction with C18, Cleanert silica, graphitized carbon black (GCB), and primary secondary amine (PSA), in which the obtained recoveries ranged from 79.0% to 118.4%. Muscarella et al. [190] developed a method based on LC-FLD with online chemical post-column derivatization for the analysis of DON and NIV in cereals. Pure water was used for sample extraction and the performances of four conventional SPE cartridges with different sorbents, including Oasis HLBTM cartridges, silica gel, immunoaffinity columns (DONtest WBTM, Vicam), and multifunctional cartridges were evaluated. Among these SPE cartridges, the polymer-based Oasis HLBTM cartridges provided the best results in terms of purification efficiency and average recoveries (varying between 89% and 101%). Montes et al. [188] reported the application of MycoSep 227 column after extraction of DON, NIV, 3-ADON, 15-ADON, and FUS-X with acetonitrile:water (84:16, v/v) mixture from breakfast cereal samples. They found that this multifunctional column can provide satisfactory recoveries (69–110%) for all tested analytes. Zhang et al. [39] used MycoSep 226 for the cleanup of DON and DON-3G to study their fate during wheat milling and Chinese steamed bread processing, obtaining recoveries in the range of 70.1–109.4%. Additionally, Sasanya et al. [206] achieved recoveries of 96.4% for DON and 70.0% for DON-3G using a C18 cartridge for sample clean-up after extraction with acetonitrile–water (84:16 v/v) mixture to quantify DON and DON-3G in hard spring wheat by LC-UV.
QuEChERS (quick, easy, cheap, effective, rugged, and safe) method has been successfully used for the extraction and clean-up of different mycotoxins in various food matrices [19,207]. Indeed, QuEChERS was originally designed in 2003 for the analysis of pesticides residues in vegetables and fruits, but it rapidly gained broad acceptability in the comprehensive isolation of a broad variety of analytes including mycotoxins in different sample matrices [208,209]. This simple extraction approach includes an initial partitioning with acetonitrile in the presence of salts, such as sodium chloride and magnesium sulfate in either a 2:1 or 4:1 ratio. Following the partitioning step, the sample extract is cleaned-up using dispersive-SPE (d-SPE) [209]. Typically, sodium chloride is utilized to minimize the polar interferences in the extract, and magnesium sulfate is often used to dehydrate the organic phase. For the cleanup step (d-SPE), the most commonly used sorbents, whether applied individually or in combination are C18, PSA, and GCB. C18 is usually used to eliminate high lipid contents, whereas PSA is exploited to remove sugars, fatty acids, lipids, organic acids, as well as some pigments and GCB is particularly effective for eliminating co-extractive pigments [163,166,209]. QuEChERS is a straightforward, quick, and economical methodology that necessitates minimal amounts of solvent in comparison to other approaches. Over the last few years, this methodology has been used for the analysis of type B trichothecenes and other multiple mycotoxins in various food matrices, such as cereal grain, cereal products, plant-based beverages, spices, coffee, and livestock products (meat, milk, and eggs) [210,211,212,213,214,215,216]. For instance, Zhou et al. [216] proposed a facile and sensitive method based on modified QuEChERS that can quantify 10 mycotoxins, including DON, NIV, 3-ADON, 15-ADON, and FUS-X in wheat flour. The samples were mixed with acetonitrile:water (84/16, v/v) mixture followed by dispersive SPE clean-up with 50 mg of C18 and 50 mg of PSA. The method exhibited very acceptable performance characteristics and proved to be a rapid and robust tool for the determination of mycotoxins in wheat flour. QuEChERS method was also adopted for the determination of DON, 15-ADON, NIV, FUS-X, and ZEN in breakfast cereals and flours [214]. The sample was mixed with water and washed with n-hexane. Acetonitrile was then added, and the mixture was subjected to salting-out liquid partitioning using MgSO4, and NaCl, while the dispersive SPE clean-up was carried out with MgSO4 and C18. This method was favorable for its simplicity, selectivity, sensitivity, fast analysis, good recovery, and high robustness.
In recent times, the role of sample clean-up has changed due to the advancement in LC-MS/MS technology, and substantial purification is not usually required due to the high selectivity and sensitivity of modern analytical techniques. This has enabled the development of methodologies with reduced, or no sample purification and injection of unpurified sample extracts (i.e., dilute and shoot approaches). Using this strategy, rapid and straightforward multi-mycotoxin methods have been successfully established, also in the area of mycotoxin analysis [8,94,217].
7. Separation and Detection Techniques
Due to the widespread occurrence and serious toxicological effects of type B trichothecenes, analytical methods devoted to their detection in foods should be robust, rapid, accurate, and selective. This necessity has driven the scientific community to establish a variety of analytical methods for these mycotoxins, which have eased their monitoring and surveillance in many food materials. The analytical technique should be chosen based on the purpose of analysis; meanwhile, sensitive analytical methods are often necessary for low tolerance levels of mycotoxins in food commodities. Currently, quantitative and qualitative analyses of type B trichothecenes are usually performed using different chromatographic techniques, immunochemical methods, rapid methods, or other emerging detection technology. Table 3 provides a summary of the abovementioned methods.

Table 3.
Advantages and disadvantages of analytical methods for type B trichothecenes.
7.1. Chromatographic Methods
Chromatographic techniques are the most widely employed for quantitative analysis of type B trichothecenes in cereals and other food matrices. These techniques are based on the separation of a mixture of chemical substances into their individual components by distribution between two phases: mobile phase and stationary phase [161]. Currently used chromatographic techniques are thin layer chromatography (TLC), high-performance liquid chromatography (HPLC), or ultra-performance liquid chromatography (UPLC) with smaller column packing material (particle size 1–2 µm), coupled with diode array detection (DAD), ultraviolet detection (UV), or fluorescence detection (FLD), liquid chromatography–tandem mass spectrometry (LC-MS/MS) and gas chromatography (GC) coupled with flame ionization detection (FID), electron capture detection (ECD), or mass spectrometry (MS) detection.
TLC is considered the oldest chromatographic technique for qualitative or semiquantitative analysis of mycotoxins by visual assessment or instrumental densitometry [163]. In previous reports, TLC was used for the detection of DON in cereal-based bakeries [218]; wheat, rye, barley, and maize [219]; maize [100]; and other human food commodities [220]. This method can provide easy, rapid, low-cost and simultaneous analysis of multiple mycotoxins; however, low selectivity and poor sensitivity and precision are the major drawbacks of this application [6]. Recently, high-efficiency thin-layer chromatography (HETLC) using nano silica gel TLC plates by fluorescence visualization under ultraviolet (UV) light has been successfully employed to survey DON in wheat flour with satisfactory recovery values and repeatability [221].
The recent trend in the determination of type B trichothecenes in food relies on the application of reliable, rapid, and simple technologies for multi-mycotoxin analysis with lower detection limits, and enhanced selectivity. As one of the most widely used separation techniques, HPLC has found widespread uses in a variety of sectors, such as scientific research, clinical analysis, environmental analysis, food analysis, and diagnostics. Coupled with UV detection, DAD, or FLD, this technique can be widely employed for the confirmatory analysis of various types of mycotoxins, including type B trichothecenes in foods. Such methods offer good sensitivity, selectivity, and precision, and are hence occasionally used as official reference methods for the validation and verification of immunochemical tests [43]. Since type B trichothecenes can absorb UV light of a certain wavelength, various studies proposed HPLC with DAD or UV for the quantification of these mycotoxins, particularly DON, NIV, and DON-3G. In recent times, HPLC-DAD and HPLC-UV have been applied for analyzing DON, DON-3G, and NIV in wheat grains [193]; DON, DON-3G, and NIV in rice and baby formula [222]; DON and NIV in rice and bran [125], DON in wheat flour, instant noodle, and biscuits [223]; DON, NIV, and DON-3G in wheat and maize [102]; and DON, NIV, together with their glucosides in cereals and related products [116]. On the other hand, HPLC-FLD in combination with appropriate extraction and purification procedures can exhibit comparable sensitivity to that attained by LC-MS/MS, as well as very good selectivity [163,224]. However, due to the lack of natural fluorescence in trichothecenes, this method necessitates a derivatization step to improve the analyte detection signal. Furthermore, detection by this technique is only applicable to a single toxin or a group of chemically related toxins [190,224]. It is worth mentioning that the HPLC-FLD method has been recommended by the European Committee of Standardization (CEN) and AOAC International for the analysis of mycotoxins in grain cereals [225]. Recently, the HPLC-FLD method has been used to determine type B trichothecenes in different agriculture commodities, including DON and NIV in wheat [226]; DON and NIV in various cereals [190]; DON and its major conjugates (DON-3G, 3-ADON, 15-ADON) in maize, wheat, and barley [224]; and DON in cereals intended for human consumption [227].
To fulfill the increasing demands of testing laboratories and to facilitate compliance with the current regulations, rapid and high throughput multi-mycotoxin analytical methods are highly required. In recent times, LC-MS/MS analysis has played an increasingly significant role in the analysis of chemically diverse mycotoxins in complex food matrices [2,10,166,228]. The most commonly used analyzer in the mycotoxin field is triple quadrupole (QqQ), which enable tandem mass spectrometry in the so-called multiple reaction monitoring (MRM) modes [166]. High chromatographic separation capacity, outstanding detection sensitivity, fast acquisition features, compatibility with a broad range of sample preparation procedures, and wide linear dynamic range are the main features of LC-MS/MS [166,172]. As a consequence, LC-MS/MS method has become the main choice for regulatory testing agencies in various countries, and the majority of research studies evolved in recent years have used this method for quantifying type B trichothecenes in various foods: DON and DON-3G in maize, groundnuts, and their products [151]; DON, NIV, DON-3G, 3-ADON, 15-ADON, and FUS-X in corn and wheat [18]; DON, NIV, 3-ADON, and 15-ADON in cereals, infant formula, spices, nuts, oil, and cocoa [229]; DON and DON-3G in wheat and maize [101]; DON and acetylated derivatives in DON wheat and maize [144]; DON, DON-3G, and NIV in animal feed and maize [148]; DON, NIV, 3-ADON, 15-ADON, and FUS-X in wheat grains [142]; DON, DON-3G, 3-ADON, NIV, and FUS-X in wheat, oat, barley, and rye triticale [24]. On the other side, the introduction of high-resolution mass spectrometry (HRMS) allows for the analysis of numerous contaminants with a single extraction, including pesticides, mycotoxins, veterinary drugs, and ergot alkaloids [167,230]. Due to its outstanding sensitivity, high resolving power, and accurate mass measurement, LC coupled with HRMS stands as a suitable method for the detection of non-targeted mycotoxins or new members of known mycotoxin groups, which is particularly useful for the discovery of metabolites or other masked forms whenever an analytical standard is not available [230,231,232]. In an interesting work, UPLC coupled with quadrupole Orbitrap HRMS was applied for the first time for the identification and detection of fusarenon X-glucoside in wheat grain artificially infected with Fusarium species [233]. This method was also proposed to investigate the occurrence of 54 mycotoxins in ready-to-eat tree nut products [234], and to analyze various Fusarium mycotoxins, including DON, NIV, DON-3G, 3-ADON, and 15-ADON in cereals (wheat, maize, and barley) [235].
The gas chromatographic (GC) method is a useful tool for evaluating volatile substances that can be vaporized without decomposition. In the past, the use of GC-FID or GC-ECD for the identification and determination of type B trichothecenes was a common practice; however, GC has been recently considered a rarely utilized technique because of its narrow scope of analysis. Eke et al. [236], and Schothorst et al. [237] proposed GC-FID methods for the simultaneous determination of type A and B trichothecenes in food and feed (wheat, semolina, and corn grits feed). Furlong et al. [238] applied a GC-FID method for the confirmation and quantification of DON, NIV, and other mycotoxins belonging to type A trichothecenes in wheat. In other studies, GC-ECD was used for the determination of DON and DON-3G in wheat [239]; DON, NIV, 3-ADON, 15-ADON and FUS-X in wheat [195]; and DON and NIV in polished rice, corn, and wheat [240]. To enhance the volatility of type B trichothecenes and enable more sensitive detection, GC techniques often require derivatizing the trichothecenes’ hydroxyl groups via trimethylsilylation or fluoroacylation processes. When GC is coupled to an MS detector (GC-MS) or tandem MS detector (GC-MS/MS), this method can serve as a very valuable analytical tool for the determination of various toxins in food with maintaining high levels of selectivity and sensitivity. In this regard, GC-MS/MS has been successfully employed in the analysis of type B trichothecenes, including DON, NIV, 3-ADON, and FUS-X in wheat semolina [241], cereal-based products (wheat, rice and maize) [126], and more recently in breadsticks [242].
7.2. Immunochemical Techniques
Although the abovementioned physicochemical detection approaches for type B trichothecenes benefit from high sensitivity and selectivity, lengthy sample preparation, high-priced instruments, as well as the necessity for highly qualified technicians are their major shortages [10,166]. Therefore, different immunochemical techniques have been developed as rapid and cost-effective alternatives to overcome the above limitations.
7.2.1. Enzyme-Linked Immunosorbent Assay
Enzyme-linked immunosorbent assay (ELISA) approach for detecting trichothecenes is typically based on a highly specific molecular interaction between the required target and the biorecognition element (anti-trichothecene antibody or an enzyme-labeled trichothecene antibody) [166,243]. Thereafter, the resulting complex can react with a chromogenic substrate to produce a quantifiable result. Recently, ELISA methods have gained more popularity in identifying and measuring various mycotoxins in food due to their easy manipulation, fast detection, and relatively low cost; however, their inefficiency in detection at low concentrations has limited their application in certain situations [88,163]. Moreover, ELIZA kits are designed for one-time use only, which would increase the cost of detecting multiple mycotoxins in contaminated food samples [243]. Pleadin et al. [100] proposed an ELISA method for the determination of DON and ZEN in contaminated maize using a particular and sensitive anti-DON/ZEA monoclonal antibody. Regarding the DON’s sensitivity, a detection limit of 10 µg/kg was achieved, and the average recovery was as high as 92%. Moreover, a good correlation (r = 0.96) between DON concentrations determined by ELISA and HPLC-UV was obtained. Santos et al. [244] reported the development of an indirect competitive ELISA (ic-ELISA) using an anti-DON.3 monoclonal antibody (mAb) to investigate the occurrence of DON in wheat samples. The detection limit of the developed method was 177.1 µg/kg, and the average recovery of DON was 108.4%. Other recent studies have also reported on the use of ELIZA methods for detecting type B trichothecenes in different foods, including NIV and 15-acetylnivalenol in wheat and other grains [245]; DON in cereals and derived products (wheat, barley, and malt) [246]; DON in maize, barley, rice, wheat, oat, flour, and milk [247]; NIV and DON in wheat kernels [248]; and DON and DON-3G in cereal-based beer [25]. Nevertheless, more efforts are required to improve their sensitivity. It is noteworthy to mention that the detection accuracy with the ELISA method may be influenced by potential matrix interferences and cross-reactivity with structurally related mycotoxins or other specific matrix components co-present in sample extracts [25,41,247]. For instance, the developed antibodies for DON or DON-3G showed high cross-reactivity with acetyl derivatives of DON since they may share similar immunodominance [246,247]. Consequently, confirmatory methods based on HPLC are often needed to meet regulatory standards.
7.2.2. Lateral Flow Immunochromatographic Assay
Lateral flow immunochromatographic assay (LFIA) is a quick, unique and straightforward (single-step) test format that requires no special equipment, additional chemicals, or laborious preparation processes [249,250]. LFIA depends on a competitive or “sandwiched” immunoassay using a labeled antibody as a signal reagent. It consists of three parts: a conjugate pad containing antibodies, a porous membrane (chromatographic material) and an absorbent pad [251]. After applying the liquid components of the test onto the conjugate pad, it moves across the membrane by capillary force to the absorbent pad, which absorbs the excess reagents and prevents the backflow of the liquid. Antibody-labeling tag conjugates, such as quantum dots, gold nanoparticles, and luminescent nanoparticles are currently used as the labeled reagents that could interact with the pathogens in the moving liquid sample. Visual detection results can be obtained in a short time through direct color markers or enzymatic color reactions, which makes this application an excellent onsite detection technology [252].
Although LFIA has been mainly employed for qualitative detection, the emergence of modern technologies allows for the generation of quantitative measurements. Due to the aforementioned features, the quantitative LFIA is particularly attractive in the food safety area for deoxynivalenol measurements, particularly for onsite testing and first-level screening. Liu et al. [249] established an LFIA test using DON-V lateral flow strips (consisting of a reader, barcode scanner, and strip cassette) for the detection of DON in durum wheat, semolina, and pasta. Limit of detection (LOD) was recorded as 0.30 mg/kg, and the results obtained from this simple approach were comparable to those of LC-MS measurements. Overall, the developed LFIA test proved to be a rapid, easy, and inexpensive onsite screening method for the quantitative detection of DON in wheat. In a more recent study, Yu et al. [250] reported the development of an enhanced LFIA test using silver staining as a signal amplification strategy to simultaneously detect DON and fumonisin B1 in maize. The test was characterized by exploiting gold nanoparticle (AuNPs)-labeled antibodies to serve as a detection probe, as well as a catalyst. The method was validated by the analysis of naturally contaminated maize samples, obtaining a detection limit of 40 ng/mL for DON, and the results were in good agreement with HPLC-MS/MS measurement results. The major attributes of this system were analytical rapidity, simplicity, low detection cost, and suitability for onsite detection. Recently, the LFIA assay has been widely developed for rapid and simultaneous detection of multiple analytes. In a pioneering work, Song et al. [253] proposed a novel and rapid LFIA test using three class-specific monoclonal antibodies for the qualitative and/or semiquantitative determination of DON, ZEN, aflatoxin B1 (AFB1), and their main metabolites (DONs, ZENs, AFs) in maize and wheat. No cross-reactivity to other mycotoxin groups was observed, and the recoveries of all analytes from spiked maize and wheat varied between 80% and 120%. Furthermore, the detection limit for DON was 3 µg/kg. Other previous studies reported the application of LFIA tests for the detection of DON in different food materials, including barley [254]; rice and corn [255]; wheat, maize, and bran [256].
7.2.3. Fluorescence Polarization Immunoassay
Fluorescence polarization immunoassay (FPIA), unlike the previously mentioned assays, is a homogeneous method performed in the solution phase without the need for attaching immunoreagents to solid surfaces [251]. Therefore, this method provides faster detection with no additional separation and washing steps, making it convenient for monitoring large-scale samples. In this technique, the analyte and fluorescent-labeled analyte (tracer) compete for specific antibody-binding sites in the solution. The detection is based on the fluorescence polarization of the fluorophore tracers, which is inversely proportional to their molecular rotation. In the presence of free toxins in the sample solution, the toxins bind with the available antibodies, while free tracers exist in the solution. Thus, yielding a high rate of rotation and consequently a lower fluorescence polarization value and vice versa [251,257]. Various fluorophore tracers and mAbs have been investigated for the development of FPIA tests for trichothecene detection. Maragos et al. [258] developed an FPIA technique for the determination of DON-contaminated wheat using a DON-specific monoclonal antibody and a fluorescently tagged DON (DON-fluorescein, DON-FL). The assays were very rapid and user-friendly, requiring only mixing the aqueous extract of wheat samples with the antibody and DON-FL. The authors noted that the sensitivity of the assay decreased with increasing the incubation time of the sample and the tracer. The IC50 for the assay reached 30 ng/mL with a tracer incubation of 15 s, while IC50 higher than 1000 ng/mL was recorded at an incubation time of 10 min. The results from this assay were compared favorably to an HPLC reference method; however, high cross-reactivity of the DON monoclonal antibody to 15-ADON was observed in the assay. Currently, multi-analyte FPIAs have gained growing attention because of their high throughput detection, inexpensive costs per assay, and minimal sample consumption. Li et al. [257] have recently established a novel homologous multi-wavelength fluorescence polarization immunoassay (MWFPIA) for the rapid, simple, and high-throughput detection of DON, T2 toxin, and FB1 in maize. Three different dye-labeled tracers and specific monoclonal antibodies were employed to perform the MWFPIA. Under optimal conditions, recoveries of DON from spiked maize were in the range of 78.7–103.1%, and the LOD was 242 μg/kg. Upon comparison of the measurement results of naturally contaminated maize samples obtained using the developed MWFPIA with those from the HPLC-MS/MS technique, good agreement between the two techniques was obtained with a correlation coefficient of 0.97 for DON. According to the obtained results, this MWFPIA technique proved to be a versatile strategy for food safety analysis.
7.3. Biosensors
Biosensors are measuring devices consisting of receptors (specific bio-recognition elements) connected to a physicochemical element (transducer), that transforms the signal generated by the bio-recognition element into a detectable response (thermal, optical, electrochemical, or piezoelectric signal) [243,259]. Antibodies, antigens, nucleic acids, proteins, cells, and enzymes have been frequently exploited as bio-recognition elements. Utilizing adsorption, trapping, or covalent bonding, these bioreceptors are successfully immobilized in the biosensor. When the toxin interacts with bioreceptors, a physicochemical signal is produced from the biochemical reaction, which can be amplified and processed by a detector before displaying it on an electronic display system [251]. This is an area of growing interest in recent years for the detection of deoxynivalenol and other trichothecenes in foodstuffs. High transmission, good selectivity, reproducibility, sensitivity, speed of analysis, and low-cost operation are the main characteristics of biosensors [260,261]. For detecting trichothecenes in food matrices, electrochemical aptasensors are often utilized, which typically comprised toxin-specific aptamers (peptide, antibody, oligonucleotide, or peptide nucleic acid) and highly sensitive transducer elements. Ong et al. [261] established a high-performance biosensing system (DON-aptasensor) using DON-aptamer (a tailor-made aptamer sequence) as the biorecognition element, and iron nanoflorets on 3D graphene–nickel substrate (INFGN) of large surface area as the transducer element. This developed system allowed for simple, selective, sensitive, and cost-effective detection of DON in food and feed with a minimum LOD of 2.11 pg/mL. Wen et al. [262] developed a facile and novel electrochemical aptasensor using a multifunctional N-doped Cu-metallic organic framework (N–Cu–MOF) nanomaterial and DON-specific aptamer for the detection of DON in wheat samples. The great electrical conductivity and the large specific surface area of N–Cu–MOF significantly enhanced stability, selectivity, and sensitivity of the electrochemical biosensing probe. The established electrochemical aptasensor device exhibited a wide linear concentration range (0.2–20 ng/mL), very low LOD (0.008 ng/mL), and good reproducibility for DON, being therefore efficient and reliable in rapid and sensitive analysis of this mycotoxin in various food materials. Moreover, the authors demonstrated that this proposed approach can be readily extended to other toxins by changing the target recognition aptamers. In recent times, aptamers have been folded into three-dimensional (3D) structures such as 3D DNA Walker, which often demonstrate smart dynamic interaction, multiple binding signal events, and outstanding predictability. For potential signal amplification, enzyme amplification strategies are often introduced into aptasensor since they are highly efficient and easy to use. Wang et al. [260] reported the development of an electrochemical aptasensing platform based on Exonuclease III (Exo III)-assisted triple-amplified for measuring DON in maize using PtPd nanoparticles polyethyleneimine-functionalized reduced graphene oxide (PtPd NPs/PEIrGO) as the transducer element, which enhanced the surface area and conductivity of the electrode. Satisfactory results in terms of stability and reproducibility were obtained, and the developed technique was applied to the detection of DON in real maize samples with an LOD of 6.9 × 10−9 mg mL−1. On the same track, a DNAzyme-assisted triple-amplified electrochemical aptasensor has recently been reported for ultra-sensitive detection of trichothecenes, through the signal amplification of nanomaterials and the interaction of Ag+ and DNA. The incorporation of metal ions as cofactors of high selectivity can enhance aptamer availability by cleaving nucleic acid substrate of DNA molecules [243]. Compared with protein enzymes, DNAzyme offers the benefits of economic synthesis, high stability, and easy modification [263].
Another distinctive feature of biosensors over the one-time-use ELISA kits and other fast screening strip techniques is their ability to be recycled. Although various biosensing systems have gained extensive application as efficient tools in mycotoxin analysis, the majority of these methods still require extensive sample preparation procedures and are incapable of performing simultaneous quantification of multiple analytes.
7.4. Infrared Spectroscopy
Infrared spectroscopy (IR spectroscopy) is an optical technique that measures the absorption of infrared radiation (800–25,000 nm) by chemical bonds in a given molecule [243]. Different chemical functional groups tend to absorb IR radiation at different frequencies. Therefore, the light transmitted through the molecule can be used for chemical structure identification, chemical fingerprinting, and quantitative measurements of chemical species. Combined with appropriate mathematical tools such as principal component analysis (PCA), the IR spectroscopy technique can provide a promising tool for screening and quantification of mycotoxins in food industries due to its attractive features, such as analytical rapidity, easy operation, and non-destructive testing with minimal or no sample manipulation [264,265]. For detecting type B trichothecenes in foods, different IR spectroscopic techniques have been successfully utilized, such as near-infrared spectroscopy (NIR), mid-infrared spectroscopy (MIR), and hyperspectral imaging [266,267,268]. In a recent study, the application of Fourier transform NIR (FTNIR) and MIR spectroscopy was investigated for the rapid analysis of DON in wheat bran samples aiming at grouping them into two classes based on the maximum regulatory limit of 750 µg/kg established for DON in cereal products by the EU [264]. Based on this classification model, overall discrimination rates were in the ranges of 87–91% and 86–87% for the FTNIR and FTMIR spectroscopy, respectively. Therefore, the results suggested that FTNIR spectroscopy coupled with an appropriate classification model is a promising and efficient approach for the quick classification of several bran wheat samples according to DON contamination. In another study, Tyska et al. [268] investigated the applicability and effectiveness of NIR (dispersive NIR and FTNIR) to analyze DON in wheat flour samples (n = 267) using various chemometrics techniques such as discriminatory methods, and to classify the samples into two classes according to the maximum regulatory limit of 750 µg/kg established for DON in cereal products in Brazil. The results showed overall discrimination rates in the range of 85.0–87.5% with a 10–15% error, thereby indicating that NIR can be an outstanding alternative strategy for the classification of wheat flour samples based on the levels of DON in the tested samples.
It is well-established that conventional IR spectroscopic techniques enable only a mean spectrum (average measurement) of a sample without offering information about the spatial distribution of chemical constituents across the sample since they are considered point-based scanning methods [266,267]. To address this drawback, NIR hyperspectral imaging (NIR-HIS), which integrates conventional digital imaging and NIR spectroscopy into a unique system has recently been introduced, presenting improvements on conventional NIR devices. This advanced and high-performance analytical technology provides images in a 3D form called “hypercube”, which simultaneously exhibits spatial (localization) and spectral (identification) information for each pixel in the image [266,267]. In recent years, NIR-HIS has gained wide application in the assessment of various contaminants in different food materials, as well as evaluating their quality and safety based on classification and defects detection strategies. Shen et al. [266] studied the feasibility of NIR-HSI coupled with chemometrics, and partial least squares discriminant analysis (PLS-DA) for analyzing DON in 120 wheat kernels (severely damaged, moderately damaged, and asymptomatic wheat kernels). The results revealed that this technique can provide rapid, high-throughput, and nondestructive analysis of DON. Moreover, it can identify the distribution of DON content in wheat kernels and classify these samples into different grades based on the degree of Fusarium infection. Another similar study based on NIR-HSI and chemometrics was developed to investigate DON presence and Fusarium damage in wheat kernels. The study was also devoted to the classification of the grains into two groups based on the EU maximum limit (1250 µg/kg) [267]. Based on the obtained results, the technique showed a substantial contribution to the management of Fusarium and DON in single wheat kernels, and also overcame their contamination heterogeneity since the classification accuracies according to symptomatology and DON levels were 100% and 98.9%, respectively.
7.5. Other Emerging Detection Technologies
7.5.1. Electronic Nose
A further example of a rapid detection method for mycotoxins is based on the electronic sense of smell. Numerous volatile compounds are found in food odors and aromas, which can be employed as sensory markers of food quality [269]. An electronic nose (EN) is a variant of gas chromatography that attempts to mimic the human olfactory sensory system, which can exhibit rapid, non-destructive, and cost-effective detection of mycotoxins in various food matrices [163]. This approach is based on the interaction of a volatile mycotoxin with chemical sensors with different specificities, leading to the generation of a signal, which is then utilized effectively as a fingerprint of the volatile mycotoxin rising from the tested samples, which serves its identification by means of pattern recognition system [270]. Current EN applications for mycotoxins have focused on the identification of toxigenic fungal species, instead of detecting the mycotoxin itself. The ability to distinguish between toxic and nontoxic fungi is practically beneficial using this approach. A few reports have been published on the application of EN in the detection of DON in grains; wheat bran [270] and durum wheat [269,271]. Theses EN methods have been mainly based on metal oxide semiconductors (MOS) sensors and proved to be suitable for rapid, inexpensive, and user-friendly screening of large numbers of wheat samples to investigate DON contamination and distinguish the microbiological quality of these samples.
Using this technology for mycotoxin detection in food samples is still in the early development phase. Techniques need to be further improved or optimized to enhance their selectivity, reduce interferences, and enable mycotoxin detection in food samples at low concentration levels. Furthermore, many mycotoxins are nonvolatile organic substances that raise major difficulties for EN-based detection.
7.5.2. Capillary Electrophoresis
Capillary electrophoresis (CE) is a chromatographic technique in which the electrochemical potential of mycotoxins is used as the basis of their separation, and UV absorbance or laser-induced fluorescence as detection systems [2,163]. The separation is achieved by the migration of charged particles in the run buffer, where cations migrate to the cathode and anions migrate to the anode under the influence of an electroosmotic flow. Different features such as reduced consumption of hazardous solvents and buffers, the use of less expensive capillaries, and reduced analysis time make this technique a feasible alternative to those necessitating HPLC [272]. Unfavorably, this approach may not be very sensitive since only small sample volumes can be tested; however, capillary zone electrophoresis in combination with laser-based fluorescence detection and appropriate purification procedures, such as IAC, can exhibit comparable accuracy, sensitivity, and precision to that acquired by HPLC for the analysis of trichothecenes in grains [273].
8. Advances in Management and Control Strategies
In fact, the infestation of agricultural commodities with type B trichothecenes poses a significant threat to human and animal health, as well as financial losses to the agro-food systems worldwide. These mycotoxins can be produced by mycotoxigenic Fusarium species that contaminate agricultural crops at the pre-harvest stages of production; hence, it might be difficult to avoid their formation due to the considerable influence of abiotic conditions [18]. Generally, mycotoxins can contaminate crops along the entire food management chain from pre-harvest to post-harvest phases and the existence of fungi does not necessarily imply mycotoxin infection, since mycotoxins are produced under conditions that are specific and distinct from fungal development [276]. In light of their deleterious impacts, the effective control of these compounds at least to the levels established by regulations has become increasingly essential. To date, various control measures and decontamination techniques have been established to minimize the growth of fungi in field crops and to detoxify mycotoxin-contaminated foods. These typically include management policies based on good hygiene practices (GHPs), good agricultural practices (GAPs), good management practices (GMPs), good storage practices (GSPs), bio-safe post-harvest decontamination methods, and appropriate quality assurance programs [274].
8.1. Pre-Harvest Practices
Pre-harvest precautions, for reducing Fusarium spp. growth and mycotoxin contamination in all phases of crop production, require the integration of different GAP measures, such as wise use of fungicides/insecticides, appropriate crop rotation, selection of plant cultivars that are resistant to insect damage or Fusarium spp. infection, proper irrigation and fertilization practices for nutrient enrichment, soil tillage, planting pathogen-free seeds, planting and weed eradication, planting date and harvest times, development of forecasting models, insect management, and production of genetically engineered plants for mycotoxin inhibition.
The use of agricultural chemicals (i.e., fungicides) such as triazoles, strobilurins, and carbendazim is beneficial for the management and control of Fusarium head blight (FHB) disease. Because different Fusarium species may cause FHB, the sensitivity to fungicide is often an important element in identifying the efficacy of a fungicide group [277]. Recent research has shown that plant pathogenic fungi may adapt to fungicides due to mutation, which results in their resistance against fungicides and decreased efficiency in some instances [278]. For a long time, farmers have relied on fungicides in conventional agriculture to protect their crops; however, other health hazards linked with the prevalence of fungicides in foods should be addressed via risk management protocols [279].
Crop rotation (growing alternative crops successively) can have a significant effect on enhancing soil health and reducing pathogen levels by breaking the production chain of infectious material due to the unavailability of the host plants [243]. In fact, the majority of pathogens that are present during cropping season gradually die between the interval of two or three years [243]. Therefore, crop rotation with plants that are not hosts to Fusarium species such as clover, alfalfa, potatoes, and other types of vegetables should be used to reduce the inoculum in the field. When wheat was planted after crops other than maize, the concentration of DON decreased by 31% when compared to maize as the pre-crop [280]. In another study, it was observed that the degree of FHB infection and DON contamination in grains varied greatly depending on whether the previous crop was maize, wheat, or soya bean [281]. The minimum concentration of DON in grains was detected after soya bean and the maximum concentration of this mycotoxin was observed after maize due to the carry-over of pathogens onto grains. In Poland, wheat planted after corn or wheat showed a greater frequency of FHB infection compared to sugar beet in pre-crop [282]. Management of planting date is another critical pre-harvesting practice for controlling mycotoxin contamination because it often determines the date of flowering and weather conditions at the flowering stage; if the date of planting coincidences with spore dispersal, the possibility of fungal attack will be more serious [278]. On the other hand, planting maize, wheat, and other grains should be also planned to prevent increased rainfall and excessive moisture during the flowering stages since these conditions often have a favorable effect on FHB infection and type B trichothecenes production [283].
Excessive heat and soil dryness during the seed’s growth and maturity can induce plant stress, which adversely affects the resistance of grain to fungal infection and mycotoxins contamination [284]. The application of fertilizers, especially those that are rich in nitrogen was found to promote the development of Fusarium species while also promoting plant growth, which in turn leads to an increase in trichothecene contamination [285]. For instance, maize planted in organic farming has a 50% lower Fusarium infection rate than that grown in conventional farming [286]. In former research, Lemmens and coworkers in Austria found a substantial rise in FHB infection and DON contamination in grains by increasing a mineral N fertilizer from 0 to 80 kg/ha [287].
Recent studies have shown that land preparation strategies such as crop rotation and soil tillage are among the most essential pre-harvest practices that can influence/control DON production in wheat and maize [283]. Soil tillage with exposure to autumn sunshine was revealed to be a promising strategy that can assist in destroying Fusarium inoculum and decreasing the production of trichothecene mycotoxins due to the destruction of infected crop residues that can transfer the infection to the subsequent crop [288]. The selection of plant cultivars is very critical in disease management and should be performed based on their resistance to mold infestations. Recent investigations have shown that planting pathogen-free seeds or selecting genetically modified plant cultivars that are resistant to Fusarium spp. infection can lessen the invasion of mycotoxigenic fungi in crops [289]. In addition, some recent and pioneering techniques such as gene silencing through RNA interference (RNAi) have been employed as an efficient strategy based on genetic engineering to mitigate DON production by F. graminearum, and this strategy can be used for the mitigation of other mycotoxins as well [290]. However, the introduction of foods derived from genetically modified crops should be accompanied by adequate policies to guarantee consumer safety and these foods are still not well-accepted by many countries around the world [291].
8.2. Harvest and Post-Harvest Practices
The selection of an appropriate time for grain harvesting is important to ensure the production of healthy grains with a good degree of maturity to maintain the integrity of the seeds since the presence of a large number of immature and overmature seeds can enhance mycotoxin contamination in the final products [292]. In rainy seasons, harvesting should be accomplished in the shortest time possible to reduce fungal development. During harvesting, crop stress should also be reduced by avoiding earlier harvesting, mechanical damage, collection of the damaged kernel, and contact of kernels with soil [293].
Post-harvest management strategies are important in preventing mycotoxin contamination in food raw materials, particularly control of storage and distribution conditions, sorting (i.e., elimination of damaged or infected crops), and application of various decontamination strategies [278]. During transportation, the transport vehicle and containers should be dry, clean, and free from fungi, insects, or any other contaminants. After grain harvesting, moisture content and temperature play a significant role in determining the occurrence of mycotoxigenic fungi, the level of colonization, and the subsequent accumulation of relevant mycotoxins in the grains [29,30,31]. Therefore, appropriate grain drying and proper storage under controlled conditions of temperature, moisture, relative humidity of the grains (<15%), as well as pest or insect management are among the most important measures that control/mitigate fungal growth and mycotoxin formation in foods [88]. Drying groundnuts up to 6.6% moisture content ensured the nuts were free of fungi species and mycotoxins for six months while reducing the moisture content of corn to 15.5% lowered the hazard of fungal growth and mycotoxin formation [294]. The drying process should be performed using solar dryers rather than sun drying because slow drying enhances mycotoxin contamination [295]. It has been demonstrated that high storage temperatures and water activity can promote the growth of F. boothii, F. graminearum sensu stricto, and F. meridionale, and further generation of DON and NIV [296]. The effect of water activity and temperature on the growth of various Fusarium species, including F. culmorum, F. avenaceum, F. tricinctum, and F. poae was also studied with temperatures of 5–10 °C, and water activity of 0.90–0.91 aw yielding the lowest mold growth [297].
Various decontamination techniques have been explored to minimize or remove type B trichothecenes in raw food materials and are mainly categorized into physical, chemical, and biological methods [182].
Physical Methods: the most commonly used physical methods to detoxify trichothecenes include washing, cleaning, density segregation, sorting, milling, dehulling, sieving, thermal inactivation, ultrasound treatment, radiation treatment, bonding with the bacterial cell walls, and adsorption [182,243]. However, the concentration and dispersion of mycotoxins throughout the seed can affect the effectiveness of these approaches. Fusarium infection often initiates from the external layer of the grain; hence, the mycotoxins are mostly found at the surface of the grain. Therefore, milling procedures can be efficient for the removal of mycotoxins from grains [175]. Vidal et al. [40] observed a reduction of DON content in the final flour or semolina after milling wheat grains, while an increase in DON content was detected in the external parts of the grains (shorts and bran).
Thermal treatment in normal food processing operations can be performed either by dry processes (roasting, baking, and frying) or wet processes (cooking and steaming). The degree of mycotoxin degradation by heat treatments is highly influenced by multiple factors such as temperature, moisture content, and heating duration. Trichothecenes are normally stable at 120 °C; however, they will partly decompose when the temperature rises over 200 °C. Vidal et al. [298] detected a considerable decrease in DON content due to the thermal process of baking. DON reduction was more pronounced at greater initial DON concentrations and higher temperatures. They also found an increase in DON-3G content (>300%) under mild baking conditions, while it was rapidly reduced under harsh conditions. In another study, Zhang et al. [42] reported on the reduction of DON and DON-3G levels in noodles after cooking due to the leaching of theses mycotoxins into the cooking water. Generally, this process is regarded as an important strategy for the mitigation of type B trichothecenes in food materials; however, the residual degradation products can have harmful effects that are different from those induced by the original toxin [299]. On the other hand, UV irradiation has been commonly utilized as an efficient approach to remove mycotoxins because of its reduced secondary contamination and lesser influence on degradation production. On the same track, gamma radiation (γ) has also been employed as a viable tool for the preservation and maintenance of food quality [228]. This process serves as an easy and attractive decontamination strategy since it successfully destroys the microorganisms that cause food deterioration and hence reduces mycotoxins. The main benefit of γ radiation is that it can compromise the quality of food in respect of its nutritional values and sensory properties; however, the efficiency of this strategy is governed by various factors, such as type and amount of fungal lineages, radiation dose, food composition, and humidity of surroundings [228].
Different adsorbents and binders with a large specific surface area are used as decontaminating agents that can bind toxins, from contaminated feed, in the gastrointestinal tract of animals. Thus, these toxins can be eliminated through feces and their bioavailability and systemic absorption would be diminished [300,301]. Macroporous resins activated carbon and diatomite are the most commonly used physical adsorbents for the removal of mycotoxins. The interaction between the adsorbent and mycotoxin takes place through hydrophobic bonding, hydrogen bonds, coordination bonds, or electrostatic attraction/repulsion. However, this approach is incapable of degrading toxins and may decrease the nutrient content of the feed, thereby impacting its nutritional value and taste [243]. Up to now, no adsorbent has been approved by the EC for DON removal, probably due to the ineffectiveness of several materials, including clay-based minerals. For instance, Murugesan et al. [53] examined twenty-seven commonly used feed additives for binding mycotoxins and found that the absorption rates of DON were less than 25% in in vitro for all items. Recently, it was found that specific activated carbon can limit DON absorption in the GIT of pigs [302]. Although this approach is promising, it is only suitable for short-term usage since activated carbon is a nonspecific adsorbent that also binds and removes essential nutrients.
Chemical Methods: chemical methods for detoxification of type B trichothecenes include the application of various chemical agents, such as oxidizing agents (e.g., chlorine, moist and dry ozone, or sodium hypochlorite), reducing agents (e.g., sodium bisulfite and sodium metabisulfite), alkaline agents (e.g., ammonia, sodium, or calcium hydroxide), and other agents [88,182,243,303]. Ozone has a high oxidizing potential, which makes it a highly reactive gas. It has been successfully utilized to prevent mold growth and control different mycotoxins in food products since it is considered safe and environmentally friendly [175,304]. Recently, the ozonation process (62 mg/L ozone for 240 min) proved to be an efficient strategy in the detoxification of DON and ZEN in naturally contaminated wheat bran without influencing the total phenolic compound content and antioxidant activity of the wheat bran [304].
Cromey et al. [305] utilized tebuconazole chemical, a triazole fungicide, and succeeded to mitigate mycotoxin levels and FHB in cereal grains. Although most of the physical and chemical methods are economically affordable, they also have certain limitations. For instance, heat treatment processes that are performed at elevated temperatures can adversely affect the quality of raw food materials [306]. Rinsing and washing food materials leaves trichothecenes and their byproducts in solution, which may cause secondary contamination [307]. On the other hand, the use of chemicals such as acids, bases, aldehydes, and oxidizing agents for the decontamination of toxins can alter their structures, and result in the formation of degradation products with toxic chemical residues in foodstuffs and feed, thereby raising deleterious side effects and further public health issues [182,278]. Changing the grain’s nutritional value and quality is also another limitation associated with the application of chemical methods, which limits their application at a large scale [88]. For instance, ammonia or any powerful oxidizing chemicals can efficiently mitigate toxin contamination, and also decreases the nutritional quality of the foodstuffs at the same time [308]. As a result, chemical methods are somewhat restricted by manufacturers, while eco-friendly biological methods using microorganisms and enzymes have been commonly proposed as favorable and reliable alternative strategies for controlling mycotoxin contamination in foods.
Biological Methods: biological decontamination techniques involve the use of microorganisms (bacteria, fungi, yeast, actinomycetes, etc.) and enzymes to mitigate or remove type B trichothecenes from food materials using two main mechanisms; adsorption or enzymatic degradation [306]. Currently, biological degradation has become a hotspot issue on a global scale due to its high efficiency, specificity, minimal negative effects, and environmental friendliness. For biological decontamination of type B trichothecenes, microbial modification can be performed using various processes, such as acetylation, deacetylation, oxidation, glucosylation, de-epoxidation, and epimerization [309]. It is generally recognized that the modification products of trichothecene are less toxic for humans and animals compared to its parent form.
In recent times, it has been found that certain fungi are capable of degrading type B trichothecenes into less harmful compounds. An inspired work was conducted by He and coworkers for the isolation of a strain of Aspergillus Tubingensis (NJA-1) from soil samples by inorganic salt culture media supplemented with DON [310]. The NJA-1 showed high efficiency to hydrolyze DON with an average DON biotransformation rate of 94.4% after two weeks of cultivation, and thus managed to improve the state of DON poisoning in mice. Tian et al. [311] evaluated a biological control-based strategy for DON produced by F. graminearum 5035 in dual culture tests using eight selected Trichoderma strains. They found that T. harzianum Q710613, T. atroviride Q710251, and T. asperellum Q710682 are efficient to reduce DON content at high rates (more than 90% compared with the control). Interestingly, the authors also detected DON-3G when incubating Trichoderma with F. graminearum, which indicated that Trichoderma strains bound DON with glucose moiety to enable its transformation into a less toxic metabolite (i.e., masked mycotoxin) through a self-defense mechanism. Garda-buffon et al. [312] found that Aspergillus oryzae and Rhizopus oryzae were effective not only to degrade DON but also to enhance the activity of the peroxidase enzyme at the time interval when the greatest degradation of DON occurred.
Various bacteria have been explored for the detoxification of DON and other trichothecenes in food materials. The bacterial strain BBSH 797, an anaerobic bacterium isolated from rumen fluid, was successfully evaluated by EFSA for its safety and effectiveness for DON decontamination in pig feed additive, and accordingly, it was approved by the EC [313]. This bacterium (strain no. 11798) was assigned as a member of a genus novus of the family Coriobacteriaceae. In a recent study, lactic acid bacteria showed good efficiency in reducing the concentrations of DON and T2 in malting wheat by 58% and 34%, respectively [314]. Wang et al. [315] isolated Bacillus licheniformis strain YB9 from a moldy soil sample and found that it can degrade more than 82.7% of 1 mg/L DON within 48 h at 37 °C. Zhang et al. [316] reported that Pelagibacterium halotolerans ANSP101 from ocean sources can transform DON into a less toxic product, 3-keto-DON. Another novel bacterium (Eggerthella sp. DII-9) that was isolated from a chicken intestinal tract exhibited great efficiency in the detoxification of trichothecene mycotoxins (DON, T2, HT2, and T2 tetraol) [317]. Yeasts and other fermenting organisms are capable of binding certain trichothecenes from agricultural commodities onto their cell wall surface constituents (e.g., glucomannans, β-D-glucans, mannan oligosaccharides), thus efficiently decontaminating food and feed. Garda et al. [318] tested the alcoholic fermentation of malt with Saccharomyces cerevisiae and succeeded in reaching a total decontamination rate of 53% for DON and T-2 toxin. Various studies have shown that biological detoxification methods are efficient and cost-effective with minor effects on the nutritional value of food; however, the applicability of biological control agents has been commonly investigated in in vitro experiments or at the laboratory scale [175]. Therefore, their uses on mycotoxins should be extended to large-scale detoxification applications. On the other hand, various bioactive compounds, such as phytochemicals and their essential oils (cinnamon oil, propyl paraben, butylated hydroxy anisole, and clove essential oils) have been recently explored as natural, safe, and eco-friendly preservatives with antimicrobial properties for inhibition of trichothecenes production and enhancing the shelf life of foodstuffs [274]. Other innovative technologies based on photocatalytic degradation and electrochemical oxidation have been also established for easy, effective, eco-friendly, and inexpensive degradation or elimination of various mycotoxins, including type B trichothecenes in food materials [88].
9. Conclusions and Future Outlook
Due to the widespread geographical occurrence of type B trichothecenes and DON-3G in cereal grains, as well as the increasing concerns regarding their health impacts, an in-depth understanding of various aspects related to their toxicology, distribution, quantitative analysis, and management strategies is of paramount importance. Acute and chronic exposure to type B trichothecenes can elicit a variety of adverse health effects, such as food refusal, vomiting, weight loss, neural disturbances, gastrointestinal discomfort, hematological disorders, inhibition of protein synthesis, and immunosuppression. Trichothecene-generating Fusarium fungi colonize crops at the pre-harvest stages of production, which makes it difficult to avoid their contamination. Type B trichothecenes and DON-3G have been found in various cereal grains, especially in wheat, maize, barley, and oats. Their concentrations may vary due to complex interactions of biotic and abiotic conditions, geographical locations, storage management, etc. More research in this area is still needed to better understand the impacts of mycotoxin co-contamination and their potential synergism.
Currently, SPE, IACs, and MFCs are the preferred clean-up procedures to retain and purify type B trichothecenes from various food matrices. A variety of detection techniques have been established for type B trichothecenes. Among them, LC-MS/MS is the most popular technique due to its high separation capacity, outstanding detection sensitivity, fast acquisition features, and compatibility with a broad range of sample preparation procedures. However, it has been observed that researchers are migrating toward biosensor-based approaches due to their ease of use, rapidity, low cost, high sensitivity, and selectivity. Even still, some conjugated forms of mycotoxins are still unnoticed and are hardly detected by routine determination, thereby inducing a serious hazard to food safety. Therefore, further studies on conjugated trichothecenes for easy detection and control will necessitate more comprehensive and in-depth research. Considering the continuous evolution of analytical techniques, it is anticipated that conventional chromatographic techniques will be replaced by HRMS, such as quadrupole Orbitrap and time of flight, which are particularly useful for the identification and non-targeted detection of metabolites or other forms of conjugated mycotoxins.
From the perspective of toxin control technology, various pre-harvest and post-harvest strategies have been employed to minimize the growth of Fusarium toxigenic fungi in field crops and to detoxify type B trichothecene in contaminated foods. GAP measures such as appropriate crop rotation, soil tillage, variety selection, planting date, and production of genetically engineered plants are the most reliable approaches to hindering the growth of the toxigenic fungi in the farm. For post-harvest management strategies, appropriate grain drying, sorting, and storage under controlled environmental conditions are important measures to control the development of mycotoxins in grains. Although various decontamination techniques relying on physical and chemical methods have been proposed, most of them are not technically feasible. The growing awareness of the ecological effects of synthetic chemicals has focused the interest of researchers on using more advanced and eco-friendly biotechnology approaches based on gene silencing through RNA interference (RNAi) and protein engineering, which may offer a promising perspective for the biological control of type B trichothecenes in various food materials. In addition, the use of phytochemicals and their essential oils have been proven natural, safe, and eco-friendly for type B trichothecene detoxification. Ultimately, the availability of absolute measures for the removal of these mycotoxins from the food chain is still missing; however, potential hazards associated with their presence in food can be reduced by a combination of efficient pre-harvest, harvest, and post-harvest strategies together with appropriate quality assurance programs.
Author Contributions
M.A.G.-A. designed; wrote and edited the manuscript; K.C. evaluated and edited the manuscript; B.K. critically edited and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Republic of Korea Research Institute of Standards and Science under the project “Establishing measurement standards for organic analysis” (Grant no. 22011072). The funding source had no further detailed involvement in this study.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 15-ADON | 15-Acetyldeoxynivalenol |
| 3-ADON | 3-Acetyldeoxynivalenol |
| AFs | Aflatoxins |
| ARfD | Acute reference dose |
| ASE | Accelerated solvent extraction |
| CAs | Chromosomal aberrations |
| CE | Capillary electrophoresis |
| CEN | European Committee of Standardization |
| DON-3G | Deoxynivalenol-3-glucoside |
| DNA | Deoxyribonucleic acid |
| DON | Deoxynivalenol |
| DSPE | Dispersive solid-phase extraction |
| dsRNA | Double-stranded RNA |
| EC | European Commission |
| ECD | Electron capture detection |
| EFSA | European Food Safety Authority |
| EN | Electronic nose |
| ELIZA | Enzyme-linked immunosorbent assay |
| EU | European Union |
| FAO | Food and Agriculture Organization |
| FHB | Fusarium head blight |
| FID | Flame ionization detection |
| FPIA | Fluorescence polarization immunoassay |
| FTNIR | Fourier transform NIR |
| FUS-X | Fusarenon-X |
| GAPs | Good agricultural practices |
| GC | Gas chromatography |
| GCB | Graphitized carbon black |
| GC-MS | Gas chromatography–mass spectrometry |
| GC-MS/MS | Gas chromatography–tandem mass spectrometry |
| GHPs | Good hygiene practices |
| GMPs | Good management practices |
| GSPs | Good storage practices |
| Hck | Hematopoietic cell kinase |
| HLB | Hydrophilic–lipophilic balanced |
| HPLC-PDA | High-performance liquid chromatography–photodiode array detection |
| HPLC-UV | High-performance liquid chromatography–ultraviolet detection |
| HRMS | High-resolution mass spectrometry |
| HS-GC/MS | Headspace–gas chromatography/mass spectrometry |
| HT2 | HT-2 toxin |
| IACs | Immunoaffinity columns |
| IARC | International Agency for Research on Cancer |
| IECs | Intestinal epithelial cells |
| JECFA | Joint FAO/WHO Expert Committee on Food Additives |
| LC | Liquid chromatography |
| LC-FLD | Liquid chromatography–fluorescence detection |
| LC-MS | Liquid chromatography–mass spectrometry |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| LFIA | Lateral flow immunochromatographic assay |
| LLE | Liquid–liquid extraction |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| mAb | Monoclonal antibody |
| MAE | Microwave-assisted extraction |
| MAPKs | Mitogen-activated protein kinases |
| MOS | Metal oxide semiconductors |
| MFC | Multifunctional clean-up columns |
| MIR | Mid-infrared spectroscopy |
| MOF | Metallic organic framework |
| MRLs | Maximum regulatory limits |
| mRNA | Messenger ribonucleic acid |
| MS | Mass spectrometry |
| MWFPIA | Multi-wavelength fluorescence polarization immunoassay |
| NA | Not available |
| ND | Not detected |
| NIR | Near-infrared spectroscopy |
| NIR-HIS | Near-infrared spectroscopy–hyperspectral imaging |
| NIV | Nivalenol |
| PCA | Principal component analysis |
| PLE | Pressurized liquid extraction |
| PLS-DA | Partial least squares discriminant analysis |
| PMTDI | Provisional maximum tolerable daily intake |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| PSA | Primary secondary amine |
| PTC | Peptidyl transferase center |
| QuEChERS | Quick, easy, cheap, effective, rugged, and safe |
| RNA | Ribonucleic acid |
| RSD | Relative standard deviation |
| SCF | Scientific Committee on Food |
| SFE | Supercritical fluid extraction |
| SLE | Solid–liquid extraction |
| SPE | Solid-phase extraction |
| T2 | T-2 toxin |
| TDI | Tolerable daily intake |
| TJP | Tight junction proteins |
| TLC | Thin layer chromatography |
| UPLC | Ultra-performance liquid chromatography |
| UPLC-MS/MS | Ultra-performance liquid chromatography–tandem mass spectrometry |
| WHO | World Health Organization |
| ZEN | Zearalenone |
References
- Leite, M.; Freitas, A.; Silva, A.S.; Barbosa, J.; Ramos, F. Maize (Zea mays L.) and mycotoxins: A review on optimization and validation of analytical methods by liquid chromatography coupled to mass spectrometry. Trends Food Sci. Technol. 2020, 99, 542–565. [Google Scholar] [CrossRef]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.X.; Marchal, J.L.M.; van der Poel, A.F.B. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim. Feed Sci. Technol. 2018, 237, 129–153. [Google Scholar] [CrossRef]
- de Nijs, M.; van den Top, H.; de Stoppelaar, J.; Lopez, P.; Mol, H. Fate of enniatins and deoxynivalenol during pasta cooking. Food Chem. 2016, 213, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Gab-Allah, M.A.; Choi, K.; Kim, B. Development of isotope dilution–liquid chromatography/tandem mass spectrometry as a candidate reference method for the accurate determination of patulin in apple products. Anal. Bioanal. Chem. 2022, 414, 1867–1879. [Google Scholar] [CrossRef]
- Lijalem, Y.G.; Gab-Allah, M.A.; Choi, K.; Kim, B. Development of isotope dilution-liquid chromatography/tandem mass spectrometry for the accurate determination of zearalenone and its metabolites in corn. Food Chem. 2022, 384, 132483. [Google Scholar] [CrossRef]
- Ran, R.; Wang, C.; Han, Z.; Wu, A.; Zhang, D.; Shi, J. Determination of deoxynivalenol (DON) and its derivatives: Current status of analytical methods. Food Control 2013, 34, 138–148. [Google Scholar] [CrossRef]
- Romera, D.; Mateo, E.M.; Mateo-Castro, R.; Gomez, J.V.; Gimeno-Adelantado, J.V.; Jimenez, M. Determination of multiple mycotoxins in feedstuffs by combined use of UPLC–MS/MS and UPLC–QTOF–MS. Food Chem. 2018, 267, 140–148. [Google Scholar] [CrossRef]
- Arraché, E.R.S.; Fontes, M.R.V.; Buffon, J.G.; Badiale-Furlong, E. Trichothecenes in wheat: Methodology, occurrence and human exposure risk. J. Cereal Sci. 2018, 82, 129–137. [Google Scholar] [CrossRef]
- Haque, M.A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Yoshinari, T.; Ohashi, H.; Abe, R.; Kaigome, R.; Ohkawa, H.; Sugita-Konishi, Y. Development of a rapid method for the quantitative determination of deoxynivalenol using Quenchbody. Anal. Chim. Acta 2015, 888, 126–130. [Google Scholar] [CrossRef]
- Ibáñez-Vea, M.; Martínez, R.; González-Peñas, E.; Lizarraga, E.; López de Cerain, A. Co-occurrence of aflatoxins, ochratoxin A and zearalenone in breakfast cereals from spanish market. Food Control 2011, 22, 1949–1955. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Syvähuoko, J.; Malachová, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sieviläinen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Sarikaya, E.; Jestoi, M.; Godula, M.; Peltonen, K. Determination of deoxynivalenol and deoxynivalenol-3-glucoside in wheat and barley using liquid chromatography coupled to mass spectrometry: On-line clean-up versus conventional sample preparation techniques. J. Chromatogr. A 2014, 1374, 31–39. [Google Scholar] [CrossRef]
- Gab-Allah, M.A.; Choi, K.; Kim, B. Development of isotope dilution-liquid chromatography/tandem mass spectrometry for the accurate determination of type-A trichothecenes in grains. Food Chem. 2021, 344, 128698. [Google Scholar] [CrossRef]
- Zou, Z.; He, Z.; Li, H.; Han, P.; Tang, J.; Xi, C.; Li, Y.; Zhang, L.; Li, X. Development and application of a method for the analysis of two trichothecenes: Deoxynivalenol and T-2 toxin in meat in China by HPLC–MS/MS. Meat Sci. 2012, 90, 613–617. [Google Scholar] [CrossRef]
- Gab-Allah, M.A.; Choi, K.; Kim, B. Accurate determination of type B trichothecenes and conjugated deoxynivalenol in grains by isotope dilution–liquid chromatography tandem mass spectrometry. Food Control 2021, 121, 107557. [Google Scholar] [CrossRef]
- Zhao, Z.; Rao, Q.; Song, S.; Liu, N.; Han, Z.; Hou, J.; Wu, A. Simultaneous determination of major type B trichothecenes and deoxynivalenol-3-glucoside in animal feed and raw materials using improved DSPE combined with LC-MS/MS. J. Chromatogr. B 2014, 963, 75–82. [Google Scholar] [CrossRef]
- Berthiller, F.; Dall’Asta, C.; Schuhmacher, R.; Lemmens, M.; Adam, G.; Krska, R. Masked mycotoxins: Determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography−tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 3421–3425. [Google Scholar] [CrossRef]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Sulyok, M.; Krska, R.; Schuhmacher, R. Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. Int. J. Food Microbiol. 2007, 119, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Lancova, K.; Hajslova, J.; Poustka, J.; Krplova, A.; Zachariasova, M.; Dostálek, P.; Sachambula, L. Transfer of Fusarium mycotoxins and ‘masked’deoxynivalenol (deoxynivalenol-3-glucoside) from field barley through malt to beer. Food Addit. Contam. 2008, 25, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.; Nielsen, K.F.; Ghorbani, F.; Spliid, N.H.; Nielsen, G.; Jørgensen, L. Occurrence of different trichothecenes and deoxynivalenol-3-β-D-glucoside in naturally and artificially contaminated Danish cereal grains and whole maize plants. Mycotoxin Res. 2012, 28, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zachariasova, M.; Hajslova, J.; Kostelanska, M.; Poustka, J.; Krplova, A.; Cuhra, P.; Hochel, I. Deoxynivalenol and its conjugates in beer: A critical assessment of data obtained by enzyme-linked immunosorbent assay and liquid chromatography coupled to tandem mass spectrometry. Anal. Chim. Acta 2008, 625, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Gab-Allah, M.A.; Mekete, K.G.; Choi, K.; Kim, B. Occurrence of major type-B trichothecenes and deoxynivalenol-3-glucoside in cereal-based products from Republic of Korea. J. Food Compos. Anal. 2021, 99, 103851. [Google Scholar] [CrossRef]
- Awad, W.; Ghareeb, K.; Böhm, J.; Zentek, J. The toxicological impacts of the Fusarium mycotoxin, deoxynivalenol, in poultry flocks with special reference to immunotoxicity. Toxins 2013, 5, 912–925. [Google Scholar] [CrossRef]
- Cinar, A.; Onbaşı, E. Mycotoxins: The hidden danger in foods. Mycotoxins Food Saf. 2019, 1–21. [Google Scholar] [CrossRef]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Magan, N.; Olsen, M. Mycotoxins in Food: Detection and Control; Woodhead Publishing: Cambridge, UK, 2004. [Google Scholar]
- Shwab, E.K.; Keller, N.P. Regulation of secondary metabolite production in filamentous ascomycetes. Mycol. Res. 2008, 112, 225–230. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology 2017, 45, 240–254. [Google Scholar] [CrossRef]
- D’Mello, J. Contaminants and toxins in animal feeds. FAO Feed and Food Safety page. In Animal Production and Health Division; FAO: Rome, Italy, 2001; Available online: https://agris.fao.org/agris-search/search.do?recordID=XF2013001395 (accessed on 2 December 2022).
- Degirmencioglu, N.; Esecali, H.; Cokal, Y.; Bilgic, M. From safety feed to safety food: The application of HACCP in mycotoxin control. Arch Zootech 2005, 8, 19–32. [Google Scholar]
- Devegowda, G.; Murthy, T. Mycotoxins: Their effects in poultry and some practical solutions. In The Mycotoxin Blue Book; Nottingham University Press: Nottingham, UK, 2005; pp. 25–56. [Google Scholar]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; El Khoury, A. Mycotoxins: Factors influencing production and control strategies. AIMS Agric. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Ksieniewicz-Woźniak, E.; Szymczyk, K.; Jędrzejczak, R. Modified Fusarium mycotoxins in cereals and their products—Metabolism, occurrence, and toxicity: An updated review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B. Fate of deoxynivalenol and deoxynivalenol-3-glucoside during wheat milling and Chinese steamed bread processing. Food Control 2014, 44, 86–91. [Google Scholar] [CrossRef]
- Vidal, A.; Sanchis, V.; Ramos, A.J.; Marín, S. The fate of deoxynivalenol through wheat processing to food products. Curr. Opin. Food Sci. 2016, 11, 34–39. [Google Scholar] [CrossRef]
- Vidal, A.; Ambrosio, A.; Sanchis, V.; Ramos, A.J.; Marín, S. Enzyme bread improvers affect the stability of deoxynivalenol and deoxynivalenol-3-glucoside during breadmaking. Food Chem. 2016, 208, 288–296. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B. Fates of deoxynivalenol and deoxynivalenol-3-glucoside during bread and noodle processing. Food Control 2015, 50, 754–757. [Google Scholar] [CrossRef]
- Polak-Śliwińska, M.; Paszczyk, B. Trichothecenes in Food and Feed, Relevance to Human and Animal Health and Methods of Detection: A Systematic Review. Molecules 2021, 26, 454. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Pinton, P.; Accensi, F.; Beauchamp, E.; Cossalter, A.-M.; Callu, P.; Grosjean, F.; Oswald, I.P. Ingestion of deoxynivalenol (DON) contaminated feed alters the pig vaccinal immune responses. Toxicol. Lett. 2008, 177, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Khaneghah, A.M.; Martins, L.M.; von Hertwig, A.M.; Bertoldo, R.; Sant’Ana, A.S. Deoxynivalenol and its masked forms: Characteristics, incidence, control and fate during wheat and wheat based products processing-A review. Trends Food Sci. Technol. 2018, 71, 13–24. [Google Scholar] [CrossRef]
- Arunachalam, C.; Doohan, F.M. Trichothecene toxicity in eukaryotes: Cellular and molecular mechanisms in plants and animals. Toxicol. Lett. 2013, 217, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Pestka, J.J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. 2008, 25, 1128–1140. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. 2016, 2, 63–68. [Google Scholar] [CrossRef]
- Savard, C.; Pinilla, V.; Provost, C.; Gagnon, C.A.; Chorfi, Y. In vivo effect of deoxynivalenol (DON) naturally contaminated feed on porcine reproductive and respiratory syndrome virus (PRRSV) infection. Vet. Microbiol. 2014, 174, 419–426. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Varasteh, S.; Alizadeh, A.; Garssen, J.; Fink-Gremmels, J. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch. Toxicol. 2017, 91, 1007–1029. [Google Scholar] [CrossRef]
- Murugesan, G.; Ledoux, D.; Naehrer, K.; Berthiller, F.; Applegate, T.; Grenier, B.; Phillips, T.; Schatzmayr, G. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult. Sci. 2015, 94, 1298–1315. [Google Scholar] [CrossRef]
- Van De Walle, J.; Sergent, T.; Piront, N.; Toussaint, O.; Schneider, Y.-J.; Larondelle, Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl. Pharmacol. 2010, 245, 291–298. [Google Scholar] [CrossRef]
- Hsia, C.C.; Wu, Z.Y.; Li, Y.S.; Zhang, F.; Sun, Z.T. Nivalenol, a main Fusarium toxin in dietary foods from high-risk areas of cancer of esophagus and gastric cardia in China, induced benign and malignant tumors in mice. Oncol. Rep. 2004, 12, 449–456. [Google Scholar] [CrossRef]
- Wu, W.; Flannery, B.M.; Sugita-Konishi, Y.; Watanabe, M.; Zhang, H.; Pestka, J.J. Comparison of murine anorectic responses to the 8-ketotrichothecenes 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, fusarenon X and nivalenol. Food Chem. Toxicol. 2012, 50, 2056–2061. [Google Scholar] [CrossRef]
- Wu, W.; Bates, M.A.; Bursian, S.J.; Link, J.E.; Flannery, B.M.; Sugita-Konishi, Y.; Watanabe, M.; Zhang, H.; Pestka, J.J. Comparison of emetic potencies of the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon X, and nivalenol. Toxicol. Sci. 2013, 131, 279–291. [Google Scholar] [CrossRef]
- Ueno, Y.; Nakajima, M.; Sakai, K.; Ishii, K.; Sato, N.; Shimada, N. Comparative toxicology of trichothec mycotoxins: Inhibition of protein synthesis in animal cells. J. Biochem. 1973, 74, 285–296. [Google Scholar]
- Garaleviciene, D.; Pettersson, H.; Elwinger, K. Effects on health and blood plasma parameters of laying hens by pure nivalenol in the diet. J. Anim. Physiol. Anim. Nutr. 2002, 86, 389–398. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J. 2014, 12, 3916. [Google Scholar]
- Pettersson, H.; Hedman, R.; Engström, B.; Elwinger, K.; Fossum, O. Nivalenol in Swedish cereals1—Occurrence, production and toxicity towards chickens. Food Addit. Contam. 1995, 12, 373–376. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on risks for animal and public health related to the presence of nivalenol in food and feed. EFSA J. 2013, 11, 3262. [Google Scholar] [CrossRef]
- Claeys, L.; Romano, C.; De Ruyck, K.; Wilson, H.; Fervers, B.; Korenjak, M.; Zavadil, J.; Gunter, M.J.; De Saeger, S.; De Boevre, M. Mycotoxin exposure and human cancer risk: A systematic review of epidemiological studies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1449–1464. [Google Scholar] [CrossRef]
- Maul, R.; Müller, C.; Rieß, S.; Koch, M.; Methner, F.-J.; Irene, N. Germination induces the glucosylation of the Fusarium mycotoxin deoxynivalenol in various grains. Food Chem. 2012, 131, 274–279. [Google Scholar] [CrossRef]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Ajandouz, E.H.; Berdah, S.; Moutardier, V.; Bege, T.; Birnbaum, D.J.; Perrier, J.; Di Pasquale, E.; Maresca, M. Hydrolytic fate of 3/15-acetyldeoxynivalenol in humans: Specific deacetylation by the small intestine and liver revealed using in vitro and ex vivo approaches. Toxins 2016, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Braicu, C.; Nougayrede, J.-P.; Laffitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Juan-García, A.; Juan, C.; Tolosa, J.; Ruiz, M.-J. Effects of deoxynivalenol, 3-acetyl-deoxynivalenol and 15-acetyl-deoxynivalenol on parameters associated with oxidative stress in HepG2 cells. Mycotoxin Res. 2019, 35, 197–205. [Google Scholar] [CrossRef]
- Juan-García, A.; Taroncher, M.; Font, G.; Ruiz, M.-J. Micronucleus induction and cell cycle alterations produced by deoxynivalenol and its acetylated derivatives in individual and combined exposure on HepG2 cells. Food Chem. Toxicol. 2018, 118, 719–725. [Google Scholar] [CrossRef]
- Sugita-Konishi, Y.; Pestka, J.J. Differential upregulation of TNF-α, IL-6, and IL-8 production by deoxynivalenol (vomitoxin) and other 8-ketotrichothecenes in a human macrophage model. J. Toxicol. Environ. Health Part A 2001, 64, 619–636. [Google Scholar] [CrossRef]
- Tomar, R.; Blakley, B.; Schiefer, H.; DeCoteau, W. In vitro effects of 3-acetyl-deoxynivalenol on the immune response of human peripheral blood lymphocytes. Int. J. Immunopharmacol. 1986, 8, 125–130. [Google Scholar] [CrossRef]
- Forsell, J.; Jensen, R.; Tai, J.-H.; Witt, M.; Lin, W.; Pestka, J. Comparison of acute toxicities of deoxynivalenol (vomitoxin) and 15-acetyldeoxynivalenol in the B6C3F1 mouse. Food Chem. Toxicol. 1987, 25, 155–162. [Google Scholar] [CrossRef]
- Kadota, T.; Furusawa, H.; Hirano, S.; Tajima, O.; Kamata, Y.; Sugita-Konishi, Y. Comparative study of deoxynivalenol, 3-acetyldeoxynivalenol, and 15-acetyldeoxynivalenol on intestinal transport and IL-8 secretion in the human cell line Caco-2. Toxicol. Vitr. 2013, 27, 1888–1895. [Google Scholar] [CrossRef]
- He, Y.; Yin, X.; Dong, J.; Yang, Q.; Wu, Y.; Gong, Z. Transcriptome Analysis of Caco-2 Cells upon the Exposure of Mycotoxin Deoxynivalenol and Its Acetylated Derivatives. Toxins 2021, 13, 167. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Puel, O.; Oswald, I.P. Toxicological interactions between the mycotoxins deoxynivalenol, nivalenol and their acetylated derivatives in intestinal epithelial cells. Arch. Toxicol. 2015, 89, 1337–1346. [Google Scholar] [CrossRef]
- Yin, S.; Liu, X.; Fan, L.; Hu, H. Mechanisms of cell death induction by food-borne mycotoxins. Crit. Rev. Food Sci. Nutr. 2018, 58, 1406–1417. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, H.-R.; Bursian, S.J.; Pan, X.; Link, J.E.; Berthiller, F.; Adam, G.; Krantis, A.; Durst, T.; Pestka, J.J. Comparison of anorectic and emetic potencies of deoxynivalenol (vomitoxin) to the plant metabolite deoxynivalenol-3-glucoside and synthetic deoxynivalenol derivatives EN139528 and EN139544. Toxicol. Sci. 2014, 142, 167–181. [Google Scholar] [CrossRef]
- Wu, W.; He, K.; Zhou, H.-R.; Berthiller, F.; Adam, G.; Sugita-Konishi, Y.; Watanabe, M.; Krantis, A.; Durst, T.; Zhang, H. Effects of oral exposure to naturally-occurring and synthetic deoxynivalenol congeners on proinflammatory cytokine and chemokine mRNA expression in the mouse. Toxicol. Appl. Pharmacol. 2014, 278, 107–115. [Google Scholar] [CrossRef]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.-P.F.; Liaubet, L.; Schatzmayr, G.; Berthiller, F.; Moll, W.-D. Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-D-glucoside. Arch. Toxicol. 2016, 90, 2037–2046. [Google Scholar] [CrossRef]
- Dall’Erta, A.; Cirlini, M.; Dall’Asta, M.; Del Rio, D.; Galaverna, G.; Dall’Asta, C. Masked mycotoxins are efficiently hydrolyzed by human colonic microbiota releasing their aglycones. Chem. Res. Toxicol. 2013, 26, 305–312. [Google Scholar] [CrossRef]
- Gareis, M.; Bauer, J.; Thiem, J.; Plank, G.; Grabley, S.; Gedek, B. Cleavage of zearalenone-glycoside, a “masked” mycotoxin, during digestion in swine. J. Vet. Med. Ser. B 1990, 37, 236–240. [Google Scholar] [CrossRef]
- Joint, F.; World Health Organization; WHO Expert Committee on Food Additives. Evaluation of Certain Contaminants in Food: Seventy-Second [72nd] Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Juan, C.; Ritieni, A.; Mañes, J. Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chem. 2013, 141, 1747–1755. [Google Scholar] [CrossRef]
- Ok, H.E.; Choi, S.-W.; Chung, S.H.; Kang, Y.-W.; Kim, D.-S.; Chun, H.S. Natural occurrence of type-B trichothecene mycotoxins in Republic of Korea cereal-based products. Food Addit. Contam. Part B 2011, 4, 132–140. [Google Scholar] [CrossRef]
- Piacentini, K.C.; Rocha, L.; Savi, G.; Carnielli-Queiroz, L.; Almeida, F.; Minella, E.; Corrêa, B. Occurrence of deoxynivalenol and zearalenone in brewing barley grains from Brazil. Mycotoxin Res. 2018, 34, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Kyprianou, M. Commission Regulation (EC) No 1126/2007. of 28 September 2007. Amending regulation (EC) no 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. J. Eur. Union 2007, 255, 14–17. [Google Scholar]
- Mishra, S.; Srivastava, S.; Dewangan, J.; Divakar, A.; Kumar Rath, S. Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: A survey. Crit. Rev. Food Sci. Nutr. 2022, 60, 1346–1374. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Sharma, B.; Dhawan, K.; Mishra, S.; Kumar, M.; Tripathi, A.D.; Rasane, P. Deoxynivalenol: An Overview on Occurrence, Chemistry, Biosynthesis, Health Effects and Its Detection, Management, and Control Strategies in Food and Feed. Microbiol. Res. 2022, 13, 292–314. [Google Scholar] [CrossRef]
- Scientific Committee on Food (SCF). Opinion of the Scientific Committee on Food on Fusarium Toxins. Part 6: Group Evaluation of T-2 Toxin, HT-2 Toxin, Nivalenol and Deoxynivalenol; Scientific Opinions European Commission: Brussels, Belgium, 2002; pp. 1–12. [Google Scholar]
- Authority, E.F.S. Deoxynivalenol in food and feed: Occurrence and exposure. EFSA J. 2013, 11, 3379. [Google Scholar]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar]
- Chun, H. Risk assessment of mycotoxin contamination in Republic of Korea foods. In Proceedings of FFTCeKU 2011 Conference, International Seminar on Risk Assessment and Risk Management of Mycotoxins for Food Safety in Asia; Kasetsart University: Bangkok, Thailand, 2011. [Google Scholar]
- Ji, F.; Xu, J.; Liu, X.; Yin, X.; Shi, J. Natural occurrence of deoxynivalenol and zearalenone in wheat from Jiangsu province, China. Food Chem. 2014, 157, 393–397. [Google Scholar] [CrossRef]
- Juan, C.; Ritieni, A.; Mañes, J. Determination of trichothecenes and zearalenones in grain cereal, flour and bread by liquid chromatography tandem mass spectrometry. Food Chem. 2012, 134, 2389–2397. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Wang, L.; Chang, F.; Yang, L. Occurrence of deoxynivalenol in wheat, Hebei Province, China. Food Chem. 2016, 197, 1271–1274. [Google Scholar] [CrossRef]
- Xu, W.; Han, X.; Li, F. Co-occurrence of multi-mycotoxins in wheat grains harvested in Anhui province, China. Food Control 2019, 96, 180–185. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Natural occurrence of nivalenol, deoxynivalenol, and deoxynivalenol-3-glucoside in Polish winter wheat. Toxins 2018, 10, 81. [Google Scholar] [CrossRef]
- Palacios, S.A.; Erazo, J.G.; Ciasca, B.; Lattanzio, V.M.; Reynoso, M.M.; Farnochi, M.C.; Torres, A.M. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat from Argentina. Food Chem. 2017, 230, 728–734. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Usman, S.; Razis, A.F.A.; Basheir Ali, N.; Saif, T.; Asi, M.R. Assessment of deoxynivalenol in wheat, corn and its products and estimation of dietary intake. Int. J. Environ. Res. Public Health 2020, 17, 5602. [Google Scholar] [CrossRef]
- Pleadin, J.; Sokolović, M.; Perši, N.; Zadravec, M.; Jaki, V.; Vulić, A. Contamination of maize with deoxynivalenol and zearalenone in Croatia. Food Control 2012, 28, 94–98. [Google Scholar] [CrossRef]
- Berthiller, F.; Dall’Asta, C.; Corradini, R.; Marchelli, R.; Sulyok, M.; Krska, R.; Adam, G.; Schuhmacher, R. Occurrence of deoxynivalenol and its 3-β-D-glucoside in wheat and maize. Food Addit. Contam. 2009, 26, 507–511. [Google Scholar] [CrossRef]
- Gab-Allah, M.A.; Tahoun, I.F.; Yamani, R.N.; Rend, E.A.; Shehata, A.B. Natural occurrence of deoxynivalenol, nivalenol and deoxynivalenol-3-glucoside in cereal-derived products from Egypt. Food Control 2022, 137, 108974. [Google Scholar] [CrossRef]
- Adejumo, T.O.; Hettwer, U.; Karlovsky, P. Occurrence of Fusarium species and trichothecenes in Nigerian maize. Int. J. Food Microbiol. 2007, 116, 350–357. [Google Scholar] [CrossRef]
- Egbuta, M.; Wanza, M.; Dutton, M. Evaluation of five major mycotoxins co-contaminating two cereal grains from Nigeria. Int. J. Biochem. Res. Rev. 2015, 6, 160–169. [Google Scholar] [CrossRef]
- Jajić, I.; Jurić, V.; Glamočić, D.; Abramović, B. Occurrence of deoxynivalenol in maize and wheat in Serbia. Int. J. Mol. Sci. 2008, 9, 2114–2126. [Google Scholar] [CrossRef]
- Camardo Leggieri, M.; Bertuzzi, T.; Pietri, A.; Battilani, P. Mycotoxin occurrence in maize produced in Northern Italy over the years 2009–2011: Focus on the role of crop related factors. Phytopathol. Mediterr. 2015, 212–221. [Google Scholar] [CrossRef]
- Kamala, A.; Ortiz, J.; Kimanya, M.; Haesaert, G.; Donoso, S.; Tiisekwa, B.; De Meulenaer, B. Multiple mycotoxin co-occurrence in maize grown in three agro-ecological zones of Tanzania. Food Control 2015, 54, 208–215. [Google Scholar] [CrossRef]
- Czembor, E.; Stępień, Ł.; Waśkiewicz, A. Effect of environmental factors on Fusarium species and associated mycotoxins in maize grain grown in Poland. PLoS ONE 2015, 10, e0133644. [Google Scholar] [CrossRef] [PubMed]
- Tima, H.; Brückner, A.; Mohácsi-Farkas, C.; Kiskó, G. Fusarium mycotoxins in cereals harvested from Hungarian fields. Food Addit. Contam. Part B 2016, 9, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Ansari, K.M.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Occurrence of deoxynivalenol in cereals and exposure risk assessment in Indian population. Food Control 2013, 30, 549–555. [Google Scholar] [CrossRef]
- Pleadin, J.; Vahčić, N.; Perši, N.; Ševelj, D.; Markov, K.; Frece, J. Fusarium mycotoxins’ occurrence in cereals harvested from Croatian fields. Food Control 2013, 32, 49–54. [Google Scholar]
- Tarazona, A.; Gómez, J.V.; Mateo, F.; Jiménez, M.; Mateo, E.M. Potential Health Risk Associated with Mycotoxins in Oat Grains Consumed in Spain. Toxins 2021, 13, 421. [Google Scholar] [CrossRef]
- Schöneberg, T.; Jenny, E.; Wettstein, F.E.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Musa, T.; Seifert, K.; Gräfenhan, T.; Keller, B. Occurrence of Fusarium species and mycotoxins in Swiss oats—Impact of cropping factors. Eur. J. Agron. 2018, 92, 123–132. [Google Scholar] [CrossRef]
- Ji, X.; Yang, H.; Wang, J.; Li, R.; Zhao, H.; Xu, J.; Xiao, Y.; Tang, B.; Qian, M. Occurrence of deoxynivalenol (DON) in cereal-based food products marketed through e-commerce stores and an assessment of dietary exposure of Chinese consumers to DON. Food Control 2018, 92, 391–398. [Google Scholar] [CrossRef]
- Islam, M.N.; Tabassum, M.; Banik, M.; Daayf, F.; Fernando, W.D.; Harris, L.J.; Sura, S.; Wang, X. Naturally Occurring Fusarium Species and Mycotoxins in Oat Grains from Manitoba, Canada. Toxins 2021, 13, 670. [Google Scholar] [CrossRef]
- Lee, S.Y.; Woo, S.Y.; Tian, F.; Song, J.; Michlmayr, H.; Kim, J.-B.; Chun, H.S. Occurrence of deoxynivalenol, nivalenol, and their glucosides in Republic of Korea market foods and estimation of their population exposure through food consumption. Toxins 2020, 12, 89. [Google Scholar] [CrossRef]
- Fredlund, E.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Lindblad, M. Deoxynivalenol and other selected Fusarium toxins in Swedish oats—Occurrence and correlation to specific Fusarium species. Int. J. Food Microbiol. 2013, 167, 276–283. [Google Scholar] [CrossRef]
- Edwards, S.G. Impact of agronomic and climatic factors on the mycotoxin content of harvested oats in the United Kingdom. Food Addit. Contam. Part A 2017, 34, 2230–2241. [Google Scholar] [CrossRef]
- Soleimany, F.; Jinap, S.; Abas, F. Determination of mycotoxins in cereals by liquid chromatography tandem mass spectrometry. Food Chem. 2012, 130, 1055–1060. [Google Scholar] [CrossRef]
- Sliková, S.; Gavurníková, S.; Hasana, R.; Mináriková, M.; Gregová, E. Deoxynivalenol in grains of oats and wheat produced in Slovakia. Poljopr. I Sumar. 2016, 62, 343. [Google Scholar] [CrossRef]
- Ok, H.; Chang, H.-J.; Choi, S.-W.; Cho, T.; Oh, K.; Chun, H. Occurrence and intake of deoxynivalenol in cereal-based products marketed in Republic of Korea during 2007–2008. Food Addit. Contam. 2009, 2, 154–161. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Co-occurrence of nivalenol, deoxynivalenol and deoxynivalenol-3-glucoside in beer samples. Food Control 2018, 92, 319–324. [Google Scholar] [CrossRef]
- Golge, O.; Kabak, B. Occurrence of deoxynivalenol and zearalenone in cereals and cereal products from Turkey. Food Control 2020, 110, 106982. [Google Scholar] [CrossRef]
- Majeed, S.; De Boevre, M.; De Saeger, S.; Rauf, W.; Tawab, A.; Rahman, M.; Iqbal, M. Multiple mycotoxins in rice: Occurrence and health risk assessment in children and adults of Punjab, Pakistan. Toxins 2018, 10, 77. [Google Scholar] [CrossRef]
- Ok, H.E.; Lee, S.Y.; Chun, H.S. Occurrence and simultaneous determination of nivalenol and deoxynivalenol in rice and bran by HPLC-UV detection and immunoaffinity cleanup. Food Control 2018, 87, 53–59. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Berrada, H.; Mañes, J. A survey of trichothecenes, zearalenone and patulin in milled grain-based products using GC–MS/MS. Food Chem. 2014, 146, 212–219. [Google Scholar] [CrossRef]
- Moreira, G.M.; Nicolli, C.P.; Gomes, L.B.; Ogoshi, C.; Scheuermann, K.K.; Silva-Lobo, V.L.; Schurt, D.A.; Ritieni, A.; Moretti, A.; Pfenning, L.H. Nationwide survey reveals high diversity of Fusarium species and related mycotoxins in Brazilian rice: 2014 and 2015 harvests. Food Control 2020, 113, 107171. [Google Scholar] [CrossRef]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Occurrence of Fusarium mycotoxins in cereal crops and processed products (Ogi) from Nigeria. Toxins 2016, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Olopade, B.K.; Oranusi, S.U.; Nwinyi, O.C.; Gbashi, S.; Njobeh, P.B. Occurrences of Deoxynivalenol, Zearalenone and some of their masked forms in selected cereals from Southwest Nigeria. NFS J. 2021, 23, 24–29. [Google Scholar] [CrossRef]
- Ssepuuya, G.; Van Poucke, C.; Ediage, E.N.; Mulholland, C.; Tritscher, A.; Verger, P.; Kenny, M.; Bessy, C.; De Saeger, S. Mycotoxin contamination of sorghum and its contribution to human dietary exposure in four sub-Saharan countries. Food Addit. Contam. Part A 2018, 35, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, A.; Fehrmann, H.; Lepschy, J.; Beck, R.; Abate, D. Natural occurrence of mycotoxins in staple cereals from Ethiopia. Mycopathologia 2006, 162, 57–63. [Google Scholar] [CrossRef]
- Hanvi, D.M.; Lawson-Evi, P.; De Boevre, M.; Goto, C.; De Saeger, S.; Eklu-Gadegbeku, K. Natural occurrence of mycotoxins in maize and sorghum in Togo. Mycotoxin Res. 2019, 35, 321–327. [Google Scholar] [CrossRef]
- Serrano, A.; Font, G.; Ruiz, M.; Ferrer, E. Co-occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chem. 2012, 135, 423–429. [Google Scholar] [CrossRef]
- Oueslati, S.; Blesa, J.; Moltó, J.C.; Ghorbel, A.; Mañes, J. Presence of mycotoxins in sorghum and intake estimation in Tunisia. Food Addit. Contam. Part A 2014, 31, 307–318. [Google Scholar] [CrossRef]
- Martos, P.; Thompson, W.; Diaz, G. Multiresidue mycotoxin analysis in wheat, barley, oats, rye and maize grain by high-performance liquid chromatography-tandem mass spectrometry. World Mycotoxin J. 2010, 3, 205–223. [Google Scholar] [CrossRef]
- Gottschalk, C.; Barthel, J.; Engelhardt, G.; Bauer, J.; Meyer, K. Simultaneous determination of type A, B and D trichothecenes and their occurrence in cereals and cereal products. Food Addit. Contam. 2009, 26, 1273–1289. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Perkowski, J. Level of contamination with mycobiota and contents of mycotoxins from the group of trichothecenes in grain of wheat, oats, barley, rye and triticale harvested in Poland in 2006–2008. Ann. Agric. Environ. Med. 2017, 24, 49–55. [Google Scholar] [CrossRef]
- Rasmussen, P.H.; Ghorbani, F.; Berg, T. Deoxynivalenol and other Fusarium toxins in wheat and rye flours on the Danish market. Food Addit. Contam. 2003, 20, 396–404. [Google Scholar] [CrossRef]
- Jin, Z.; Gillespie, J.; Barr, J.; Wiersma, J.J.; Sorrells, M.E.; Zwinger, S.; Gross, T.; Cumming, J.; Bergstrom, G.C.; Brueggeman, R. Malting of Fusarium head blight-infected rye (Secale cereale): Growth of Fusarium graminearum, trichothecene production, and the impact on malt quality. Toxins 2018, 10, 369. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, T.; Xu, W.; Han, X.; Zhang, J.; Zhang, H.; Wang, C.; Fanning, S.; Li, F. Natural co-occurrence of multi-mycotoxins in unprocessed wheat grains from China. Food Control 2021, 130, 108321. [Google Scholar] [CrossRef]
- Vidal, A.; Marín, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Determination of aflatoxins, deoxynivalenol, ochratoxin A and zearalenone in wheat and oat based bran supplements sold in the Spanish market. Food Chem. Toxicol. 2013, 53, 133–138. [Google Scholar] [CrossRef]
- Alkadri, D.; Rubert, J.; Prodi, A.; Pisi, A.; Mañes, J.; Soler, C. Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chem. 2014, 157, 111–118. [Google Scholar] [CrossRef]
- Blesa, J.; Moltó, J.-C.; El Akhdari, S.; Mañes, J.; Zinedine, A. Simultaneous determination of Fusarium mycotoxins in wheat grain from Morocco by liquid chromatography coupled to triple quadrupole mass spectrometry. Food Control 2014, 46, 1–5. [Google Scholar] [CrossRef]
- Yan, P.; Liu, Z.; Liu, S.; Yao, L.; Liu, Y.; Wu, Y.; Gong, Z. Natural occurrence of deoxynivalenol and its acetylated derivatives in Chinese maize and wheat collected in 2017. Toxins 2020, 12, 200. [Google Scholar] [CrossRef]
- Tralamazza, S.M.; Bemvenuti, R.H.; Zorzete, P.; de Souza Garcia, F.; Corrêa, B. Fungal diversity and natural occurrence of deoxynivalenol and zearalenone in freshly harvested wheat grains from Brazil. Food Chem. 2016, 196, 445–450. [Google Scholar] [CrossRef]
- Tanaka, H.; Sugita-Konishi, Y.; Takino, M.; Tanaka, T.; Toriba, A.; Hayakawa, K. A survey of the occurrence of Fusarium mycotoxins in biscuits in Japan by using LC/MS. J. Health Sci. 2010, 56, 188–194. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Musa, T.; Bänziger, I.; Kägi, A.; Bucheli, T.D.; Wettstein, F.E.; Pasquali, M.; Forrer, H.-R. Fusarium mycotoxins in Swiss wheat: A survey of growers’ samples between 2007 and 2014 shows strong year and minor geographic effects. Toxins 2017, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.F.; Girgin, G.; Baydar, T.; Krska, R.; Sulyok, M. Occurrence of multiple mycotoxins and other fungal metabolites in animal feed and maize samples from Egypt using LC-MS/MS. J. Sci. Food Agric. 2017, 97, 4419–4428. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.; Tan, L.; Chin, L.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Castillo, M.-Á.; Montes, R.; Navarro, A.; Segarra, R.; Cuesta, G.; Hernández, E. Occurrence of deoxynivalenol and nivalenol in Spanish corn-based food products. J. Food Compos. Anal. 2008, 21, 423–427. [Google Scholar] [CrossRef]
- Abia, W.A.; Warth, B.; Sulyok, M.; Krska, R.; Tchana, A.N.; Njobeh, P.B.; Dutton, M.F.; Moundipa, P.F. Determination of multi-mycotoxin occurrence in cereals, nuts and their products in Cameroon by liquid chromatography tandem mass spectrometry (LC-MS/MS). Food Control 2013, 31, 438–453. [Google Scholar] [CrossRef]
- Xing, F.; Liu, X.; Wang, L.; Selvaraj, J.N.; Jin, N.; Wang, Y.; Zhao, Y.; Liu, Y. Distribution and variation of fungi and major mycotoxins in pre-and post-nature drying maize in North China Plain. Food Control 2017, 80, 244–251. [Google Scholar] [CrossRef]
- Tutelyan, V.; Zakharova, L.; Sedova, I.; Perederyaev, O.; Aristarkhova, T.; Eller, K. Fusariotoxins in Russian Federation 2005–2010 grain harvests. Food Addit. Contam. Part B 2013, 6, 139–145. [Google Scholar] [CrossRef]
- Hofgaard, I.; Aamot, H.; Torp, T.; Jestoi, M.; Lattanzio, V.; Klemsdal, S.; Waalwijk, C.; Van der Lee, T.; Brodal, G. Associations between Fusarium species and mycotoxins in oats and spring wheat from farmers’ fields in Norway over a six-year period. World Mycotoxin J. 2016, 9, 365–378. [Google Scholar] [CrossRef]
- Bensassi, F.; Rjiba, I.; Zarrouk, A.; Rhouma, A.; Hajlaoui, M.; Bacha, H. Deoxynivalenol contamination in Tunisian barley in the 2009 harvest. Food Addit. Contam. Part B 2011, 4, 205–211. [Google Scholar] [CrossRef]
- Al-Julaifi, M.Z.; Al-Falih, A.M. Detection of trichothecenes in animal feeds and foodstuffs during the years 1997 to 2000 in Saudi Arabia. J. Food Prot. 2001, 64, 1603–1606. [Google Scholar] [CrossRef]
- Makun, H.A.; Dutton, M.F.; Njobeh, P.B.; Mwanza, M.; Kabiru, A.Y. Natural multi-occurrence of mycotoxins in rice from Niger State, Nigeria. Mycotoxin Res. 2011, 27, 97–104. [Google Scholar] [CrossRef]
- Roscoe, V.; Lombaert, G.; Huzel, V.; Neumann, G.; Melietio, J.; Kitchen, D.; Kotello, S.; Krakalovich, T.; Trelka, R.; Scott, P. Mycotoxins in breakfast cereals from the Canadian retail market: A 3-year survey. Food Addit. Contam. 2008, 25, 347–355. [Google Scholar] [CrossRef]
- Lee, M.J.; Wee, C.-D.; Ham, H.; Choi, J.-H.; Baek, J.S.; Lim, S.B.; Lee, T.; Kim, J.-S.; Jang, J.Y. Survey on Fusarium mycotoxin contamination in oat, sorghum, adlay, and proso millet during the harvest season in Republic of Korea. J. Food Hyg. Saf. 2020, 35, 13–22. [Google Scholar] [CrossRef]
- Błajet-Kosicka, A.; Twarużek, M.; Kosicki, R.; Sibiorowska, E.; Grajewski, J. Co-occurrence and evaluation of mycotoxins in organic and conventional rye grain and products. Food Control 2014, 38, 61–66. [Google Scholar] [CrossRef]
- Rahmani, A.; Jinap, S.; Soleimany, F. Qualitative and quantitative analysis of mycotoxins. Compr. Rev. Food Sci. Food Saf. 2009, 8, 202–251. [Google Scholar] [CrossRef]
- Meneely, J.P.; Ricci, F.; van Egmond, H.P.; Elliott, C.T. Current methods of analysis for the determination of trichothecene mycotoxins in food. TrAC Trends Anal. Chem. 2011, 30, 192–203. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.L.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Shehata, A.; Mohamed, G.; Gab-Allah, M. Development of crude oil reference material certified for the concentrations of sulfur, iron, nickel, vanadium and magnesium. Mapan 2017, 32, 101–112. [Google Scholar] [CrossRef]
- Pereira, V.; Fernandes, J.; Cunha, S. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Shanakhat, H.; Sorrentino, A.; Raiola, A.; Romano, A.; Masi, P.; Cavella, S. Current methods for mycotoxins analysis and innovative strategies for their reduction in cereals: An overview. J. Sci. Food Agric. 2018, 98, 4003–4013. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.K.; Basic, C.; Bethem, R.A. Trace Quantitative Analysis by Mass Spectrometry; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Commission Regulation (EC). Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, 70, 12–34. [Google Scholar]
- Gab-Allah, M.; Goda, E.S.; Shehata, A.; Gamal, H. Critical review on the analytical methods for the determination of sulfur and trace elements in crude oil. Crit. Rev. Anal. Chem. 2020, 50, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.; Mohamed, G.; Gab-Allah, M. Simple spectrophotometric method for determination of iron in crude oil. Pet. Chem. 2017, 57, 1007–1011. [Google Scholar] [CrossRef]
- Tahoun, I.F.; Gab-Allah, M.A.; Yamani, R.N.; Shehata, A.B. Development and validation of a reliable LC-MS/MS method for simultaneous determination of deoxynivalenol and T-2 toxin in maize and oats. Microchem. J. 2021, 169, 106599. [Google Scholar] [CrossRef]
- Tahoun, I.F.; Rend, E.A.; Gab-Allah, M.A. Preparation and value assignment of parabens and phenoxyethanol in cosmetic cream certified reference material. J. Chem. Metrol. 2021, 15, 1–10. [Google Scholar] [CrossRef]
- Tahoun, I.F.; Gab-Allah, M.A. Development of ledipasvir and sofosbuvir pure certified reference materials for improving quality of pharmaceutical analysis. J. Chem. Metrol. 2022, 16, 68–77. [Google Scholar] [CrossRef]
- Chen, P.; Xiang, B.; Shi, H.; Yu, P.; Song, Y.; Li, S. Recent advances on type A trichothecenes in food and feed: Analysis, prevalence, toxicity, and decontamination techniques. Food Control 2020, 118, 107371. [Google Scholar] [CrossRef]
- Dong, M.; Si, W.; Jiang, K.; Nie, D.; Wu, Y.; Zhao, Z.; De Saeger, S.; Han, Z. Multi-walled carbon nanotubes as solid-phase extraction sorbents for simultaneous determination of type A trichothecenes in maize, wheat and rice by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2015, 1423, 177–182. [Google Scholar] [CrossRef]
- Kirimker, S.E.; Turksoy, S.; Kabak, B. Assessment of dietary exposure to deoxynivalenol and fumonisin in the population of infants and toddlers in Turkey. Food Chem. Toxicol. 2020, 140, 111304. [Google Scholar] [CrossRef]
- Giménez, I.; Herrera, M.; Escobar, J.; Ferruz, E.; Lorán, S.; Herrera, A.; Ariño, A. Distribution of deoxynivalenol and zearalenone in milled germ during wheat milling and analysis of toxin levels in wheat germ and wheat germ oil. Food Control 2013, 34, 268–273. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Y.; Deng, Q.; Sun, L.; Wang, R.; Wang, X.; Liao, J.; Gooneratne, R. Simultaneous quantification of aflatoxin B1, T-2 toxin, ochratoxin A and deoxynivalenol in dried seafood products by LC-MS/MS. Toxins 2020, 12, 488. [Google Scholar] [CrossRef]
- González-Osnaya, L.; Cortés, C.; Soriano, J.; Moltó, J.; Mañes, J. Occurrence of deoxynivalenol and T-2 toxin in bread and pasta commercialised in Spain. Food Chem. 2011, 124, 156–161. [Google Scholar] [CrossRef]
- Jin, P.; Han, Z.; Cai, Z.; Wu, Y.; Ren, Y. Simultaneous determination of 10 mycotoxins in grain by ultra-high-performance liquid chromatography–tandem mass spectrometry using 13C15-deoxynivalenol as internal standard. Food Addit. Contam. Part A 2010, 27, 1701–1713. [Google Scholar] [CrossRef]
- Nagl, V.; Schatzmayr, G. Deoxynivalenol and its masked forms in food and feed. Curr. Opin. Food Sci. 2015, 5, 43–49. [Google Scholar] [CrossRef]
- Barros, G.; García, D.; Oviedo, M.; Ramirez, M.; Torres, A.; Lattanzio, V.; Pascale, M.; Chulze, S. Survey of T-2 and HT-2 toxins in soybean and soy meal from Argentina using immunoaffinity clean-up and high performance liquid chromatography. World Mycotoxin J. 2011, 4, 189–197. [Google Scholar] [CrossRef]
- Valle-Algarra, F.M.; Mateo, E.M.; Mateo, R.; Gimeno-Adelantado, J.V.; Jiménez, M. Determination of type A and type B trichothecenes in paprika and chili pepper using LC-triple quadrupole–MS and GC–ECD. Talanta 2011, 84, 1112–1117. [Google Scholar] [CrossRef]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef]
- Beltrán, E.; Ibáñez, M.; Sancho, J.V.; Hernández, F. Determination of mycotoxins in different food commodities by ultra-high-pressure liquid chromatography coupled to triple quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1801–1809. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Montes, R.; Segarra, R.; Castillo, M.-Á. Trichothecenes in breakfast cereals from the Spanish retail market. J. Food Compos. Anal. 2012, 27, 38–44. [Google Scholar] [CrossRef]
- Ibáñez-Vea, M.; Lizarraga, E.; González-Peñas, E. Simultaneous determination of type-A and type-B trichothecenes in barley samples by GC–MS. Food Control 2011, 22, 1428–1434. [Google Scholar] [CrossRef]
- Muscarella, M.; Iammarino, M.; Nardiello, D.; Palermo, C.; Centonze, D. Determination of deoxynivalenol and nivalenol by liquid chromatography and fluorimetric detection with on-line chemical post-column derivatization. Talanta 2012, 97, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.G.; Zhu, J.X.; Shi, L.; Zhan, C.R.; Guo, P.; Wang, Y.; Zhang, Y.; Liu, J. Development of a novel immunoaffinity column for the determination of deoxynivalenol and its acetylated derivatives in cereals. Food Anal. Methods 2018, 11, 2252–2260. [Google Scholar] [CrossRef]
- Pascale, M.; Panzarini, G.; Powers, S.; Visconti, A. Determination of deoxynivalenol and nivalenol in wheat by ultra-performance liquid chromatography/photodiode-array detector and immunoaffinity column cleanup. Food Anal. Methods 2014, 7, 555–562. [Google Scholar] [CrossRef]
- Trombete, F.; Barros, A.; Vieira, M.; Saldanha, T.; Venâncio, A.; Fraga, M. Simultaneous determination of deoxynivalenol, deoxynivalenol-3-glucoside and nivalenol in wheat grains by HPLC-PDA with immunoaffinity column cleanup. Food Anal. Methods 2016, 9, 2579–2586. [Google Scholar] [CrossRef]
- Royer, D.; Humpf, H.-U.; Guy, P. Quantitative analysis of Fusarium mycotoxins in maize using accelerated solvent extraction before liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. Food Addit. Contam. 2004, 21, 678–692. [Google Scholar] [CrossRef]
- Josephs, R.; Krska, R.; Grasserbauer, M.; Broekaert, J. Determination of trichothecene mycotoxins in wheat by use of supercritical fluid extraction and high-performance liquid chromatography with diode array detection or gas chromatography with electron capture detection. J. Chromatogr. A 1998, 795, 297–304. [Google Scholar] [CrossRef]
- Panasiuk, Ł.; Jedziniak, P.; Pietruszka, K.; Posyniak, A. Simultaneous determination of deoxynivalenol, its modified forms, nivalenol and fusarenone-X in feedstuffs by the liquid chromatography–tandem mass spectrometry method. Toxins 2020, 12, 362. [Google Scholar] [CrossRef]
- Razzazi-Fazeli, E.; Reiter, E. Sample preparation and clean up in mycotoxin analysis: Principles, applications and recent developments. Determ. Mycotoxins Mycotoxigenic Fungi Food Feed 2011, 37–70. [Google Scholar] [CrossRef]
- Tomczyk, Ł.; Szablewski, T.; Stuper-Szablewska, K.; Nowaczewski, S.; Cegielska-Radziejewska, R. The influence of the conditions of acquisition and storage of table eggs on changes in their quality and the presence of mycobiota and Fusarium mycotoxins. Poult. Sci. 2019, 98, 2964–2971. [Google Scholar] [CrossRef]
- Twarużek, M.; Błajet-Kosicka, A.; Kosicki, R.; Grajewski, J. Mycotoxin Analytical Methods. In Environmental Mycology in Public Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 363–386. [Google Scholar]
- Han, Z.; Ren, Y.; Zhu, J.; Cai, Z.; Chen, Y.; Luan, L.; Wu, Y. Multianalysis of 35 mycotoxins in traditional Chinese medicines by ultra-high-performance liquid chromatography–tandem mass spectrometry coupled with accelerated solvent extraction. J. Agric. Food Chem. 2012, 60, 8233–8247. [Google Scholar] [CrossRef]
- Mahmoud, A.F.; Escrivá, L.; Rodríguez-Carrasco, Y.; Moltó, J.C.; Berrada, H. Determination of trichothecenes in chicken liver using gas chromatography coupled with triple-quadrupole mass spectrometry. LWT 2018, 93, 237–242. [Google Scholar] [CrossRef]
- Gab-Allah, M.A.; Tahoun, I.F.; Yamani, R.N.; Rend, E.A.; Shehata, A.B. Eco-friendly and sensitive analytical method for determination of T-2 toxin and HT-2 toxin in cereal products using UPLC-MS/MS. J. Food Compos. Anal. 2022, 107, 104395. [Google Scholar] [CrossRef]
- Dong, H.; Xian, Y.; Xiao, K.; Wu, Y.; Zhu, L.; He, J. Development and comparison of single-step solid phase extraction and QuEChERS clean-up for the analysis of 7 mycotoxins in fruits and vegetables during storage by UHPLC-MS/MS. Food Chem. 2019, 274, 471–479. [Google Scholar] [CrossRef]
- Klötzel, M.; Lauber, U.; Humpf, H.U. A new solid phase extraction clean-up method for the determination of 12 type A and B trichothecenes in cereals and cereal-based food by LC-MS/MS. Mol. Nutr. Food Res. 2006, 50, 261–269. [Google Scholar] [CrossRef]
- McMaster, N.; Acharya, B.; Harich, K.; Grothe, J.; Mehl, H.L.; Schmale, D.G. Quantification of the mycotoxin deoxynivalenol (DON) in sorghum using GC-MS and a stable isotope dilution assay (SIDA). Food Anal. Methods 2019, 12, 2334–2343. [Google Scholar] [CrossRef]
- Sasanya, J.; Hall, C.; Wolf-Hall, C. Analysis of deoxynivalenol, masked deoxynivalenol, and Fusarium graminearum pigment in wheat samples, using liquid chromatography–UV–mass spectrometry. J. Food Prot. 2008, 71, 1205–1213. [Google Scholar] [CrossRef]
- Pereira, V.; Fernandes, J.; Cunha, S. Comparative assessment of three cleanup procedures after QuEChERS extraction for determination of trichothecenes (type A and type B) in processed cereal-based baby foods by GC–MS. Food Chem. 2015, 182, 143–149. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS-Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- Tamura, M.; Mochizuki, N.; Nagatomi, Y.; Harayama, K.; Toriba, A.; Hayakawa, K. A method for simultaneous determination of 20 Fusarium toxins in cereals by high-resolution liquid chromatography-orbitrap mass spectrometry with a pentafluorophenyl column. Toxins 2015, 7, 1664–1682. [Google Scholar] [CrossRef] [PubMed]
- Miró-Abella, E.; Herrero, P.; Canela, N.; Arola, L.; Borrull, F.; Ras, R.; Fontanals, N. Determination of mycotoxins in plant-based beverages using QuEChERS and liquid chromatography–tandem mass spectrometry. Food Chem. 2017, 229, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carrasco, Y.; Fattore, M.; Albrizio, S.; Berrada, H.; Manes, J. Occurrence of Fusarium mycotoxins and their dietary intake through beer consumption by the European population. Food Chem. 2015, 178, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Dall’Erta, A.; Mantovani, P.; Massi, A.; Galaverna, G. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat. World Mycotoxin J. 2013, 6, 83–91. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Development and validation of a method based on a QuEChERS procedure and heart-cutting GC-MS for determination of five mycotoxins in cereal products. J. Sep. Sci. 2010, 33, 600–609. [Google Scholar] [CrossRef]
- Frenich, A.G.; Romero-González, R.; Gómez-Pérez, M.L.; Vidal, J.L.M. Multi-mycotoxin analysis in eggs using a QuEChERS-based extraction procedure and ultra-high-pressure liquid chromatography coupled to triple quadrupole mass spectrometry. J. Chromatogr. A 2011, 1218, 4349–4356. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, F.; Chen, L.; Jiang, D. Quantitative analysis of 10 mycotoxins in wheat flour by ultrahigh performance liquid chromatography-tandem mass spectrometry with a modified QuEChERS strategy. J. Food Sci. 2016, 81, T2886–T2890. [Google Scholar] [CrossRef]
- Lim, C.W.; Tai, S.H.; Lee, L.M.; Chan, S.H. Analytical method for the accurate determination of tricothecenes in grains using LC-MS/MS: A comparison between MRM transition and MS3 quantitation. Anal. Bioanal. Chem. 2012, 403, 2801–2806. [Google Scholar] [CrossRef]
- Neira, M.; Pacin, A.; Martinez, E.; Moltó, G.; Resnik, S. The effects of bakery processing on natural deoxynivalenol contamination. Int. J. Food Microbiol. 1997, 37, 21–25. [Google Scholar] [CrossRef]
- Tutelyan, V.A. Deoxynivalenol in cereals in Russia. Toxicol. Lett. 2004, 153, 173–179. [Google Scholar] [CrossRef]
- Njobeh, P.B.; Dutton, M.F.; Koch, S.H.; Chuturgoon, A.A.; Stoev, S.D.; Mosonik, J.S. Simultaneous occurrence of mycotoxins in human food commodities from Cameroon. Mycotoxin Res. 2010, 26, 47–57. [Google Scholar] [CrossRef]
- Rocha, D.F.d.L.; Oliveira, M.d.S.; Furlong, E.B.; Junges, A.; Paroul, N.; Valduga, E.; Backes, G.T.; Zeni, J.; Cansian, R.L. Evaluation of the TLC quantification method and occurrence of deoxynivalenol in wheat flour of southern Brazil. Food Addit. Contam. Part A 2017, 34, 2220–2229. [Google Scholar] [CrossRef]
- Lee, S.Y.; Woo, S.Y.; Malachová, A.; Michlmayr, H.; Kim, S.-H.; Kang, G.J.; Chun, H.S. Simple validated method for simultaneous determination of deoxynivalenol, nivalenol, and their 3-β-D-glucosides in baby formula and Republic of Korea rice wine via HPLC-UV with immunoaffinity cleanup. Food Addit. Contam. Part A 2019, 36, 964–975. [Google Scholar] [CrossRef]
- Almeida, A.P.d.; Lamardo, L.C.A.; Shundo, L.; Silva, S.A.d.; Navas, S.A.; Alaburda, J.; Ruvieri, V.; Sabino, M. Occurrence of deoxynivalenol in wheat flour, instant noodle and biscuits commercialised in Brazil. Food Addit. Contam. Part B 2016, 9, 251–255. [Google Scholar] [CrossRef]
- Gonçalves, C.; Mischke, C.; Stroka, J. Determination of deoxynivalenol and its major conjugates in cereals using an organic solvent-free extraction and IAC clean-up coupled in-line with HPLC-PCD-FLD. Food Addit. Contam. Part A 2020, 37, 1765–1776. [Google Scholar] [CrossRef]
- Pascale, M.N. Detection methods for mycotoxins in cereal grains and cereal products. Zbornik Matice Srpske za Prirodne Nauke 2009, 15–25. [Google Scholar] [CrossRef]
- Zheng, Y.; Hossen, S.M.; Sago, Y.; Yoshida, M.; Nakagawa, H.; Nagashima, H.; Okadome, H.; Nakajima, T.; Kushiro, M. Effect of milling on the content of deoxynivalenol, nivalenol, and zearalenone in Japanese wheat. Food Control 2014, 40, 193–197. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.N.; Papageorgiou, M.D. Optimized and validated high-performance liquid chromatography method for the determination of deoxynivalenol and aflatoxins in cereals. J. Sep. Sci. 2016, 39, 1425–1432. [Google Scholar] [CrossRef]
- Montanha, F.P.; Anater, A.; Burchard, J.F.; Luciano, F.B.; Meca, G.; Manyes, L.; Pimpão, C.T. Mycotoxins in dry-cured meats: A review. Food Chem. Toxicol. 2018, 111, 494–502. [Google Scholar] [CrossRef]
- Desmarchelier, A.; Tessiot, S.; Bessaire, T.; Racault, L.; Fiorese, E.; Urbani, A.; Chan, W.-C.; Cheng, P.; Mottier, P. Combining the quick, easy, cheap, effective, rugged and safe approach and clean-up by immunoaffinity column for the analysis of 15 mycotoxins by isotope dilution liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2014, 1337, 75–84. [Google Scholar] [CrossRef]
- Narváez, A.; Rodríguez-Carrasco, Y.; Castaldo, L.; Izzo, L.; Ritieni, A. Ultra-high-performance liquid chromatography coupled with quadrupole Orbitrap high-resolution mass spectrometry for multi-residue analysis of mycotoxins and pesticides in botanical nutraceuticals. Toxins 2020, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Gab-Allah, M.A.; Lijalem, Y.G.; Yu, H.; Lee, S.; Baek, S.-Y.; Han, J.; Choi, K.; Kim, B. Development of a certified reference material for the accurate determination of type B trichothecenes in corn. Food Chem. 2022, 404, 134542. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Shi, L.; Zhang, F.; Fan, C.; Chang, J.; Chu, X. Multiplexing data independent untargeted workflows for mycotoxins screening on a quadrupole-Orbitrap high resolution mass spectrometry platform. Food Chem. 2019, 278, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Ohmichi, K.; Sakamoto, S.; Sago, Y.; Kushiro, M.; Nagashima, H.; Yoshida, M.; Nakajima, T. Detection of a new Fusarium masked mycotoxin in wheat grain by high-resolution LC–Orbitrap™ MS. Food Addit. Contam. Part A 2011, 28, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Narváez, A.; Rodríguez-Carrasco, Y.; Castaldo, L.; Izzo, L.; Graziani, G.; Ritieni, A. Occurrence and exposure assessment of mycotoxins in ready-to-eat tree nut products through ultra-high performance liquid chromatography coupled with high resolution q-orbitrap mass spectrometry. Metabolites 2020, 10, 344. [Google Scholar] [CrossRef]
- Zachariasova, M.; Lacina, O.; Malachova, A.; Kostelanska, M.; Poustka, J.; Godula, M.; Hajslova, J. Novel approaches in analysis of Fusarium mycotoxins in cereals employing ultra performance liquid chromatography coupled with high resolution mass spectrometry. Anal. Chim. Acta 2010, 662, 51–61. [Google Scholar] [CrossRef]
- Eke, Z.; Kende, A.; Torkos, K. Simultaneous detection of A and B trichothecenes by gas chromatography with flame ionization or mass selective detection. Microchem. J. 2004, 78, 211–216. [Google Scholar] [CrossRef]
- Schothorst, R.C.; Jekel, A.A. Determination of trichothecenes in wheat by capillary gas chromatography with flame ionisation detection. Food Chem. 2001, 73, 111–117. [Google Scholar] [CrossRef]
- Furlong, E.B.; Valente Soares, L.M. Gas chromatographic method for quantitation and confirmation of trichothecenes in wheat. J. AOAC Int. 1995, 78, 386–390. [Google Scholar] [CrossRef]
- Simsek, S.; Burgess, K.; Whitney, K.L.; Gu, Y.; Qian, S.Y. Analysis of deoxynivalenol and deoxynivalenol-3-glucoside in wheat. Food Control 2012, 26, 287–292. [Google Scholar] [CrossRef]
- Tanaka, T.; Hasegawa, A.; Matsuki, Y.; Ishii, K.; Ueno, Y. Improved methodology for the simultaneous detection of the trichothecene mycotoxins deoxynivalenol and nivalenol in cereals. Food Addit. Contam. 1985, 2, 125–137. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Berrada, H.; Font, G.; Mañes, J. Multi-mycotoxin analysis in wheat semolina using an acetonitrile-based extraction procedure and gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1270, 28–40. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Font, G.; Moltó, J.C.; Berrada, H. Quantitative determination of trichothecenes in breadsticks by gas chromatography-triple quadrupole tandem mass spectrometry. Food Addit. Contam. Part A 2014, 31, 1422–1430. [Google Scholar] [CrossRef]
- Mahato, D.K.; Pandhi, S.; Kamle, M.; Gupta, A.; Sharma, B.; Panda, B.K.; Srivastava, S.; Kumar, M.; Selvakumar, R.; Pandey, A.K. Trichothecenes in food and feed: Occurrence, impact on human health and their detection and management strategies. Toxicon 2022, 208, 62–77. [Google Scholar] [CrossRef]
- dos Santos, J.S.; Souza, T.M.; Ono, E.Y.S.; Hashimoto, E.H.; Bassoi, M.C.; de Miranda, M.Z.; Itano, E.N.; Kawamura, O.; Hirooka, E.Y. Natural occurrence of deoxynivalenol in wheat from Paraná State, Brazil and estimated daily intake by wheat products. Food Chem. 2013, 138, 90–95. [Google Scholar] [CrossRef]
- Noda, K.; Hirakawa, Y.; Nishino, T.; Sekizuka, R.; Kishimoto, M.; Furukawa, T.; Sawane, S.; Matsunaga, A.; Kobayashi, N.; Sugita, K. Preparation of Monoclonal Antibodies Specifically Reacting with the Trichothecene Mycotoxins Nivalenol and 15-Acetylnivalenol via the Introduction of a Linker Molecule into Its C-15 Position. Toxins 2022, 14, 747. [Google Scholar] [CrossRef]
- Dzuman, Z.; Vaclavikova, M.; Polisenska, I.; Veprikova, Z.; Fenclova, M.; Zachariasova, M.; Hajslova, J. Enzyme-linked immunosorbent assay in analysis of deoxynivalenol: Investigation of the impact of sample matrix on results accuracy. Anal. Bioanal. Chem. 2014, 406, 505–514. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Lu, Y.; Ma, D.-Y.; Qi, M.G.; Wang, S. A competitive direct enzyme-linked immunosorbent assay for the rapid detection of deoxynivalenol: Development and application in agricultural products and feedstuff. Food Agric. Immunol. 2017, 28, 516–527. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Kohno, H.; Ikeda, K.; Shinoda, T.; Yokohama, H.; Morita, K.; Kusada, O.; Kobayashi, Y. A practical method for measuring deoxynivalenol, nivalenol, and T-2+ HT-2 toxin in foods by an enzyme-linked immunosorbent assay using monoclonal antibodies. Biosci. Biotechnol. Biochem. 2004, 68, 2076–2085. [Google Scholar] [CrossRef]
- Liu, J.; Zanardi, S.; Powers, S.; Suman, M. Development and practical application in the cereal food industry of a rapid and quantitative lateral flow immunoassay for deoxynivalenol. Food Control 2012, 26, 88–91. [Google Scholar] [CrossRef]
- Yu, Q.; Li, H.; Li, C.; Zhang, S.; Shen, J.; Wang, Z. Gold nanoparticles-based lateral flow immunoassay with silver staining for simultaneous detection of fumonisin B1 and deoxynivalenol. Food Control 2015, 54, 347–352. [Google Scholar] [CrossRef]
- Goryacheva, I.Y.; Saeger, S.d.; Eremin, S.A.; Peteghem, C.V. Immunochemical methods for rapid mycotoxin detection: Evolution from single to multiple analyte screening: A review. Food Addit. Contam. 2007, 24, 1169–1183. [Google Scholar] [CrossRef] [PubMed]
- Nan, M.; Xue, H.; Bi, Y. Contamination, Detection and Control of Mycotoxins in Fruits and Vegetables. Toxins 2022, 14, 309. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, N.; Zhao, Z.; Njumbe Ediage, E.; Wu, S.; Sun, C.; De Saeger, S.; Wu, A. Multiplex lateral flow immunoassay for mycotoxin determination. Anal. Chem. 2014, 86, 4995–5001. [Google Scholar] [CrossRef] [PubMed]
- Foubert, A.; Beloglazova, N.V.; Gordienko, A.; Tessier, M.D.; Drijvers, E.; Hens, Z.; De Saeger, S. Development of a rainbow lateral flow immunoassay for the simultaneous detection of four mycotoxins. J. Agric. Food Chem. 2017, 65, 7121–7130. [Google Scholar] [CrossRef]
- Kim, K.Y.; Shim, W.B.; Kim, J.S.; Chung, D.H. Development of a simultaneous lateral flow strip test for the rapid and simple detection of deoxynivalenol and zearalenone. J. Food Sci. 2014, 79, M2048–M2055. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, Q.; Wang, J.; Liang, Z.; Li, J.; Wu, J.; Shen, X.; Lei, H.; Li, X. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens. Bioelectron. 2020, 158, 112178. [Google Scholar] [CrossRef]
- Li, C.; Wen, K.; Mi, T.; Zhang, X.; Zhang, H.; Zhang, S.; Shen, J.; Wang, Z. A universal multi-wavelength fluorescence polarization immunoassay for multiplexed detection of mycotoxins in maize. Biosens. Bioelectron. 2016, 79, 258–265. [Google Scholar] [CrossRef]
- Maragos, C.; Jolley, M.; Nasir, M. Fluorescence polarization as a tool for the determination of deoxynivalenol in wheat. Food Addit. Contam. 2002, 19, 400–407. [Google Scholar] [CrossRef]
- Goda, E.S.; Gab-Allah, M.; Singu, B.S.; Yoon, K.R. Halloysite nanotubes based electrochemical sensors: A review. Microchem. J. 2019, 147, 1083–1096. [Google Scholar] [CrossRef]
- Wang, K.; He, B.; Xie, L.; Li, L.; Yang, J.; Liu, R.; Wei, M.; Jin, H.; Ren, W. Exonuclease III-assisted triple-amplified electrochemical aptasensor based on PtPd NPs/PEI-rGO for deoxynivalenol detection. Sens. Actuators B Chem. 2021, 349, 130767. [Google Scholar] [CrossRef]
- Ong, C.C.; Sangu, S.S.; Illias, N.M.; Gopinath, S.C.B.; Saheed, M.S.M. Iron nanoflorets on 3D-graphene-nickel: A ‘Dandelion’nanostructure for selective deoxynivalenol detection. Biosens. Bioelectron. 2020, 154, 112088. [Google Scholar] [CrossRef]
- Wen, X.; Huang, Q.; Nie, D.; Zhao, X.; Cao, H.; Wu, W.; Han, Z. A multifunctional n-doped cu–mofs (N–cu–mof) nanomaterial-driven electrochemical aptasensor for sensitive detection of deoxynivalenol. Molecules 2021, 26, 2243. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, Y.; Li, H.; Kong, D.-M.; Shen, H.-X. Sensitive dual DNAzymes-based sensors designed by grafting self-blocked G-quadruplex DNAzymes to the substrates of metal ion-triggered DNA/RNA-cleaving DNAzymes. Biosens. Bioelectron. 2012, 38, 331–336. [Google Scholar] [CrossRef]
- De Girolamo, A.; Cervellieri, S.; Cortese, M.; Porricelli, A.C.R.; Pascale, M.; Longobardi, F.; von Holst, C.; Ciaccheri, L.; Lippolis, V. Fourier transform near-infrared and mid-infrared spectroscopy as efficient tools for rapid screening of deoxynivalenol contamination in wheat bran. J. Sci. Food Agric. 2019, 99, 1946–1953. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; ElMasry, G.; Sun, D.-W.; Allen, P. Non-destructive prediction and visualization of chemical composition in lamb meat using NIR hyperspectral imaging and multivariate regression. Innov. Food Sci. Emerg. Technol. 2012, 16, 218–226. [Google Scholar] [CrossRef]
- Shen, G.; Cao, Y.; Yin, X.; Dong, F.; Xu, J.; Shi, J.; Lee, Y.-W. Rapid and nondestructive quantification of deoxynivalenol in individual wheat kernels using near-infrared hyperspectral imaging and chemometrics. Food Control 2022, 131, 108420. [Google Scholar] [CrossRef]
- Femenias, A.; Bainotti, M.B.; Gatius, F.; Ramos, A.J.; Marín, S. Standardization of near infrared hyperspectral imaging for wheat single kernel sorting according to deoxynivalenol level. Food Res. Int. 2021, 139, 109925. [Google Scholar] [CrossRef]
- Tyska, D.; Mallmann, A.; Gressler, L.T.; Mallmann, C.A. Near-infrared spectroscopy as a tool for rapid screening of deoxynivalenol in wheat flour and its applicability in the industry. Food Addit. Contam. Part A 2021, 38, 1958–1968. [Google Scholar] [CrossRef]
- Lippolis, V.; Pascale, M.; Cervellieri, S.; Damascelli, A.; Visconti, A. Screening of deoxynivalenol contamination in durum wheat by MOS-based electronic nose and identification of the relevant pattern of volatile compounds. Food Control 2014, 37, 263–271. [Google Scholar] [CrossRef]
- Lippolis, V.; Cervellieri, S.; Damascelli, A.; Pascale, M.; Di Gioia, A.; Longobardi, F.; De Girolamo, A. Rapid prediction of deoxynivalenol contamination in wheat bran by MOS-based electronic nose and characterization of the relevant pattern of volatile compounds. J. Sci. Food Agric. 2018, 98, 4955–4962. [Google Scholar] [CrossRef] [PubMed]
- Campagnoli, A.; Cheli, F.; Polidori, C.; Zaninelli, M.; Zecca, O.; Savoini, G.; Pinotti, L.; Dell’Orto, V. Use of the electronic nose as a screening tool for the recognition of durum wheat naturally contaminated by deoxynivalenol: A preliminary approach. Sensors 2011, 11, 4899–4916. [Google Scholar] [CrossRef] [PubMed]
- Bueno, D.; Istamboulie, G.; Muñoz, R.; Marty, J.L. Determination of mycotoxins in food: A review of bioanalytical to analytical methods. Appl. Spectrosc. Rev. 2015, 50, 728–774. [Google Scholar] [CrossRef]
- Pascale, M.; Lippolis, V.; Maragos, C.M.; Visconti, A. Recent Developments in Trichothecene Analysis; ACS Publications: Washington, DC, USA, 2008. [Google Scholar]
- Kumar, P.; Mahato, D.K.; Gupta, A.; Pandey, S.; Paul, V.; Saurabh, V.; Pandey, A.K.; Selvakumar, R.; Barua, S.; Kapri, M. Nivalenol Mycotoxin Concerns in Foods: An Overview on Occurrence, Impact on Human and Animal Health and Its Detection and Management Strategies. Toxins 2022, 14, 527. [Google Scholar] [CrossRef] [PubMed]
- Beloglazova, N.V.; Sobolev, A.M.; Tessier, M.D.; Hens, Z.; Goryacheva, I.Y.; De Saeger, S. Fluorescently labelled multiplex lateral flow immunoassay based on cadmium-free quantum dots. Methods 2017, 116, 141–148. [Google Scholar] [CrossRef]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A review of recent innovative strategies for controlling mycotoxins in foods. Food Control 2022, 144, 109350. [Google Scholar] [CrossRef]
- Leslie, J.F.; Moretti, A.; Mesterházy, Á.; Ameye, M.; Audenaert, K.; Singh, P.K.; Richard-Forget, F.; Chulze, S.N.; Ponte, E.M.D.; Chala, A. Key global actions for mycotoxin management in wheat and other small grains. Toxins 2021, 13, 725. [Google Scholar] [CrossRef]
- Peivasteh-Roudsari, L.; Pirhadi, M.; Shahbazi, R.; Eghbaljoo-Gharehgheshlaghi, H.; Sepahi, M.; Mirza Alizadeh, A.; Tajdar-oranj, B.; Jazaeri, S. Mycotoxins: Impact on health and strategies for prevention and detoxification in the food chain. Food Rev. Int. 2021, 1–32. [Google Scholar] [CrossRef]
- Paul, P.; Lipps, P.; Hershman, D.; McMullen, M.; Draper, M.; Madden, L. Efficacy of triazole-based fungicides for Fusarium head blight and deoxynivalenol control in wheat: A multivariate meta-analysis. Phytopathology 2008, 98, 999–1011. [Google Scholar] [CrossRef]
- Beyer, M.; Klix, M.B.; Klink, H.; Verreet, J.-A. Quantifying the effects of previous crop, tillage, cultivar and triazole fungicides on the deoxynivalenol content of wheat grain—A review. J. Plant Dis. Prot. 2006, 113, 241–246. [Google Scholar] [CrossRef]
- Dill-Macky, R.; Jones, R. The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant Dis. 2000, 84, 71–76. [Google Scholar] [CrossRef]
- Wenda-Piesik, A.; Lemańczyk, G.; Twarużek, M.; Błajet-Kosicka, A.; Kazek, M.; Grajewski, J. Fusarium head blight incidence and detection of Fusarium toxins in wheat in relation to agronomic factors. Eur. J. Plant Pathol. 2017, 149, 515–531. [Google Scholar] [CrossRef]
- Nada, S.; Nikola, T.; Bozidar, U.; Ilija, D.; Andreja, R. Prevention and practical strategies to control mycotoxins in the wheat and maize chain. Food Control 2022, 136, 108855. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Codex Alimentarius Commission. Code of practice for the prevention and reduction of mycotoxin contamination in cereals, including annexes on ochratoxin A, zearalenone, fumonisins and tricothecenes (CAC/RCP 51-2003). In Prevention and Reduction of Food and Feed Contamination; FAO/WHO: Rome Italy, 2003; pp. 1–13. [Google Scholar]
- Hofer, K.; Barmeier, G.; Schmidhalter, U.; Habler, K.; Rychlik, M.; Hückelhoven, R.; Hess, M. Effect of nitrogen fertilization on Fusarium head blight in spring barley. Crop Prot. 2016, 88, 18–27. [Google Scholar] [CrossRef]
- Ariño, A.; Juan, T.; Estopañan, G.; González-Cabo, J.F. Natural occurrence of Fusarium species, fumonisin production by toxigenic strains, and concentrations of fumonisins B1 and B2 in conventional and organic maize grown in Spain. J. Food Prot. 2007, 70, 151–156. [Google Scholar] [CrossRef]
- Lemmens, M.; Haim, K.; Lew, H.; Ruckenbauer, P. The effect of nitrogen fertilization on Fusarium head blight development and deoxynivalenol contamination in wheat. J. Phytopathol. 2004, 152, 1–8. [Google Scholar] [CrossRef]
- Aldred, D.; Magan, N. Prevention strategies for trichothecenes. Toxicol. Lett. 2004, 153, 165–171. [Google Scholar] [CrossRef]
- Bai, G.H.; Plattner, R.; Desjardins, A.; Kolb, F.; McIntosh, R. Resistance to Fusarium head blight and deoxynivalenol accumulation in wheat. Plant Breed. 2001, 120, 1–6. [Google Scholar] [CrossRef]
- Gaffar, F.Y.; Imani, J.; Karlovsky, P.; Koch, A.; Kogel, K.-H. Different components of the RNA interference machinery are required for conidiation, ascosporogenesis, virulence, deoxynivalenol production, and fungal inhibition by exogenous double-stranded RNA in the head blight pathogen Fusarium graminearum. Front. Microbiol. 2019, 10, 1662. [Google Scholar] [CrossRef]
- Bawa, A.; Anilakumar, K. Genetically modified foods: Safety, risks and public concerns—A review. J. Food Sci. Technol. 2013, 50, 1035–1046. [Google Scholar] [CrossRef]
- Torres, A.M.; Barros, G.G.; Palacios, S.A.; Chulze, S.N.; Battilani, P. Review on pre-and post-harvest management of peanuts to minimize aflatoxin contamination. Food Res. Int. 2014, 62, 11–19. [Google Scholar] [CrossRef]
- Munkvold, G. Crop management practices to minimize the risk of mycotoxins contamination in temperate-zone maize. Mycotoxin Reduct. Grain Chain. 2014, 1, 59–77. [Google Scholar]
- Awuah, R.; Ellis, W. Effects of some groundnut packaging methods and protection with Ocimum and Syzygium powders on kernel infection by fungi. Mycopathologia 2002, 154, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.; Taniwaki, M.H.; Cole, M. Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of Food Safety Objectives. Food Control 2013, 32, 205–215. [Google Scholar] [CrossRef]
- Belizán, M.M.; Gomez, A.d.l.A.; Baptista, Z.P.T.; Jimenez, C.M.; Matías, M.d.H.S.; Catalán, C.A.; Sampietro, D.A. Influence of water activity and temperature on growth and production of trichothecenes by Fusarium graminearum sensu stricto and related species in maize grains. Int. J. Food Microbiol. 2019, 305, 108242. [Google Scholar] [CrossRef]
- Magan, N.; Lacey, J. Water relations of some Fusarium species from infected wheat ears and grain. Trans. Br. Mycol. Soc. 1984, 83, 281–285. [Google Scholar] [CrossRef]
- Vidal, A.; Sanchis, V.; Ramos, A.J.; Marín, S. Thermal stability and kinetics of degradation of deoxynivalenol, deoxynivalenol conjugates and ochratoxin A during baking of wheat bakery products. Food Chem. 2015, 178, 276–286. [Google Scholar] [CrossRef]
- Stadler, D.; Berthiller, F.; Suman, M.; Schuhmacher, R.; Krska, R. Novel analytical methods to study the fate of mycotoxins during thermal food processing. Anal. Bioanal. Chem. 2020, 412, 9–16. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef]
- Elella, M.H.A.; Goda, E.S.; Gab-Allah, M.A.; Hong, S.E.; Pandit, B.; Lee, S.; Gamal, H.; ur Rehman, A.; Yoon, K.R. Xanthan gum-derived materials for applications in environment and eco-friendly materials: A review. J. Environ. Chem. Eng. 2021, 9, 104702. [Google Scholar] [CrossRef]
- Devreese, M.; Antonissen, G.; De Backer, P.; Croubels, S. Efficacy of active carbon towards the absorption of deoxynivalenol in pigs. Toxins 2014, 6, 2998–3004. [Google Scholar] [CrossRef]
- Gab-Allah, M.A.; Shehata, A.B. Determination of iron, nickel, and vanadium in crude oil by inductively coupled plasma optical emission spectrometry following microwave-assisted wet digestion. Chem. Pap. 2021, 75, 4239–4248. [Google Scholar] [CrossRef]
- Santos Alexandre, A.P.; Vela-Paredes, R.S.; Santos, A.S.; Costa, N.S.; Canniatti-Brazaca, S.G.; Calori-Domingues, M.A.; Augusto, P.E.D. Ozone treatment to reduce deoxynivalenol (DON) and zearalenone (ZEN) contamination in wheat bran and its impact on nutritional quality. Food Addit. Contam. Part A 2018, 35, 1189–1199. [Google Scholar] [CrossRef]
- Cromey, M.; Lauren, D.; Parkes, R.; Sinclair, K.; Shorter, S.; Wallace, A. Control of Fusarium head blight of wheat with fungicides. Australas. Plant Pathol. 2001, 30, 301–308. [Google Scholar] [CrossRef]
- Yao, Y.; Long, M. The biological detoxification of deoxynivalenol: A review. Food Chem. Toxicol. 2020, 145, 111649. [Google Scholar] [CrossRef]
- Elella, M.H.A.; Goda, E.; Gab-Allah, M.; Hong, S.E.; Lijalem, Y.G.; Yoon, K.R. Biodegradable Polymeric Nanocomposites for Wastewater Treatment. In Advances in Nanocomposite Materials for Environmental and Energy Harvesting Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 245–298. [Google Scholar]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A 2011, 28, 1590–1609. [Google Scholar] [CrossRef]
- Wachowska, U.; Packa, D.; Wiwart, M. Microbial inhibition of Fusarium pathogens and biological modification of trichothecenes in cereal grains. Toxins 2017, 9, 408. [Google Scholar] [CrossRef]
- He, C.; Fan, Y.; Liu, G.; Zhang, H. Isolation and identification of a strain of Aspergillus tubingensis with deoxynivalenol biotransformation capability. Int. J. Mol. Sci. 2008, 9, 2366–2375. [Google Scholar] [CrossRef]
- Tian, Y.; Tan, Y.; Liu, N.; Yan, Z.; Liao, Y.; Chen, J.; De Saeger, S.; Yang, H.; Zhang, Q.; Wu, A. Detoxification of deoxynivalenol via glycosylation represents novel insights on antagonistic activities of Trichoderma when confronted with Fusarium graminearum. Toxins 2016, 8, 335. [Google Scholar] [CrossRef]
- Garda-Buffon, J.; Kupski, L.; Badiale-Furlong, E. Deoxynivalenol (DON) degradation and peroxidase enzyme activity in submerged fermentation. Food Sci. Technol. 2011, 31, 198–203. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the safety and efficacy of micro-organism DSM 11798 when used as a technological feed additive for pigs. EFSA J. 2013, 11, 3203. [Google Scholar]
- Juodeikiene, G.; Bartkiene, E.; Cernauskas, D.; Cizeikiene, D.; Zadeike, D.; Lele, V.; Bartkevics, V. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT 2018, 89, 307–314. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Q.; Guo, Q.; Zhang, J.; Sun, Y.; Wei, H.; Shen, L. Isolation and characterization of a deoxynivalenol-degrading bacterium Bacillus licheniformis YB9 with the capability of modulating intestinal microbial flora of mice. Toxins 2020, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qin, X.; Guo, Y.; Zhang, Q.; Ma, Q.; Ji, C.; Zhao, L. Enzymatic degradation of deoxynivalenol by a novel bacterium, Pelagibacterium halotolerans ANSP101. Food Chem. Toxicol. 2020, 140, 111276. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Mu, P.; Wen, J.; Sun, Y.; Chen, Q.; Deng, Y. Detoxification of trichothecene mycotoxins by a novel bacterium, Eggerthella sp. DII-9. Food Chem. Toxicol. 2018, 112, 310–319. [Google Scholar] [CrossRef]
- Garda, J.; Macedo, R.M.; Faria, R.; Bernd, L.; Dors, G.C.; Badiale-Furlong, E. Alcoholic fermentation effects on malt spiked with trichothecenes. Food Control 2005, 16, 423–428. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).