Kidney Function, Male Gender, and Aneurysm Diameter Are Predictors of Acute Kidney Injury in Patients with Abdominal Aortic Aneurysms Treated Endovascularly
Abstract
1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boozari, M.; Hosseinzadeh, H. Preventing contrast-induced nephropathy (CIN) with herbal medicines: A review. Phytother. Res. 2021, 35, 1130–1146. [Google Scholar] [CrossRef]
- Stacul, F.; van der Molen, A.J.; Reimer, P.; Webb, J.A.W.; Thomsen, H.S.; Morcos, S.K.; Almén, T.; Aspelin, P.; Bellin, M.-F.; Clement, O.; et al. On behalf of the Contrast Media Safety Committee of the European Society of Urogenital Radiology. Contrast induced nephropathy: Updated ESUR Contrast Media Safety Committee guidelines. Eur. Radiol. 2011, 21, 2527–2541. [Google Scholar] [CrossRef] [PubMed]
- KDIGO Clinical Practice Guideline for Acute Kidney Injury. Summary of Recommendation Statements. Kidney Int. Suppl. 2012, 2, 8–12. [CrossRef]
- Chong, E.; Poh, K.K.; Liang, S.; Tan, H.C. Risk factors and clinical outcomes for contrast-induced nephropathy after percutaneous coronary intervention in patients with normal serum creatinine. Ann. Acad. Med. Singap. 2010, 39, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Hipp, A.; Desai, S.; Lopez, C.; Sinert, R. The incidence of contrast-induced nephropathy in trauma patients. Eur. J. Emerg. Med. 2008, 15, 134–139. [Google Scholar] [CrossRef]
- Owen, R.J.; Hiremath, S.; Myers, A.; Fraser-Hill, M.; Barrett, B.J. Canadian Association of Radiologists consensus guidelines for the prevention of contrast-induced nephropathy: Update 2012. Can. Assoc. Radiol. J. 2014, 65, 96–105. [Google Scholar] [CrossRef]
- Chong, E.; Poh, K.K.; Shen, L.; Chai, P.; Tan, H.C. Diabetic patients with normal baseline renal function are at increased risk of developing contrast-induced nephropathy post-percutaneous coronary intervention. Singap. Med. J. 2009, 50, 250–254. [Google Scholar]
- McCullough, P.A.; Wolyn, R.; Rocher, L.L.; Levin, R.N.; O’Neill, W.W. Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am. J. Med. 1997, 103, 368–375. [Google Scholar] [CrossRef]
- Bang, J.-Y.; Jun, I.-G.; Lee, J.-B.; Ko, Y.-S.; Kim, K.-W.; Jeong, J.-H.; Kim, S.-H.; Song, J.-G. Impact of Sarcopenia on Acute Kidney Injury after Infrarenal Abdominal Aortic Aneurysm Surgery: A Propensity Matching Analysis. Nutrients 2021, 13, 2212. [Google Scholar] [CrossRef]
- Demirjian, S.; Schold, J.D.; Navia, J.; Mastracci, T.M.; Paganini, E.P.; Yared, J.P.; Bashour, C.A. Predictive models for acute kidney injury following cardiac surgery. Am. J. Kidney Dis. 2012, 59, 382–389. [Google Scholar] [CrossRef]
- Tao, S.M.; Wichmann, J.L.; Schoepf, U.J.; Fuller, S.R.; Lu, G.M.; Zhang, L.J. Contrast-induced nephropathy in CT: Incidence, risk factors and strategies for prevention. Eur. Radiol. 2016, 26, 3310–3318. [Google Scholar] [CrossRef] [PubMed]
- Becquemin, J.P.; Pillet, J.C.; Lescalie, F.; Sapoval, M.; Goueffic, Y.; Lermusiaux, P.; Steinmetz, E.; Marzelle, J. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low–to moderate-risk patients. J. Vasc. Surg. 2011, 53, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, J.L.; Baas, A.F.; Buth, J.; Prinssen, M.; Verhoeven, E.L.; Cuypers, P.W.; van Sambeek, M.R.; Balm, R.; Grobbee, D.E.; Blankensteijn, J.D. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N. Engl. J. Med. 2010, 362, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, R.M.; Brown, L.C.; Kwong, G.P.; Powell, J.T.; Thompson, S.G.; EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: Randomised controlled trial. Lancet 2004, 364, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Saratzis, A.; Melas, N.; Mahmood, A.; Sarafidis, P. Incidence of acute kidney injury after endovascular abdominal aortic aneurysm repair and impact on outcome. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 534e40. [Google Scholar] [CrossRef] [PubMed]
- Pisimisis, G.T.; Bechara, C.F.; Barshes, N.R.; Lin, P.H.; Kougias, P. Risk factors and impact of proximal fixation on acute and chronic renal dysfunction after endovascular aortic aneurysm repair using glomerular filtration rate criteria. Ann. Vasc. Surg. 2013, 27, 16–22. [Google Scholar] [CrossRef]
- Saratzis, A.; Nduwayo, S.; Pantelis, S.; Sayers, R.; Brown, M. Renal function is the main predictor of acute kidney injury after endovascular abdominal aortic aneurysm repair. Ann. Vasc. Surg. 2016, 31, 52–59. [Google Scholar] [CrossRef]
- Saratzis, A.; Shakespeare, J.; Jones, O.; Brown, M.J.; Mahmood, A.; Imray, C.H.E. Pre-operative functional cardiovascular reserve is associated with acute kidney injury after intervention. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 717–724. [Google Scholar] [CrossRef]
- Saratzis, A.N.; Goodyear, S.; Sur, H.; Saedon, M.; Imray, C.; Mahmood, A. Acute Kidney Injury After Endovascular Repair of Abdominal Aortic Aneurysm. J. Endovasc. Ther. 2013, 20, 315–330. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.M.; Jung, S.; Cho, W.; Hong, K.C.; Jeon, Y.S.; Cho, S.G.; Lee, J.B. Occurrences and Results of Acute Kidney Injury after Endovascular Aortic Abdominal Repair. Vasc. Spec. Int. 2017, 33, 135–139. [Google Scholar] [CrossRef]
- Brulotte, V.; Leblond, F.A.; Elkouri, S.; Therasse, E.; Pichette, V.; Beaulieu, P. Bicarbonates for the prevention of post-operative renal failure in endovascular aortic aneurysm repair: A randomized pilot trial. Anesth. Res. Pract. 2013, 2013, 467326. [Google Scholar]
- Krasznai, A.G.; Sigterman, T.A.; Bouwman, L.H. Contrast Free Duplex-Assisted EVAR in Patients with Chronic Renal Insufficiency. Ann. Vasc. Dis. 2014, 7, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Faga, T.; Pisani, A.; Sabbatini, M.; Russo, D.; Michael, A. The choice of the iodinated radiographic contrast media to prevent contrast-induced nephropathy. Adv. Nephrol. 2014, 2014, 11. [Google Scholar] [CrossRef]
- Yang, J.S.; Peng, Y.R.; Tsai, S.C.; Tyan, Y.S.; Lu, C.C.; Chiu, H.Y.; Chiu, Y.J.; Kuo, S.C.; Tsai, Y.F.; Lin, P.C.; et al. The molecular mechanism of contrast- induced nephropathy (CIN) and its link to in vitro studies on iodinated contrast media (CM). Biomedicine 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.H.; Kwon, S.K.; Park, J.H.; Chu, W.; Kim, D.H.; Jung, H.J.; Lee, S.S. Renal function-adjusted contrast medium volume is a major risk factor in the occurrence of acute kidney injury after endovascular aneurysm repair. Medicine 2021, 100, e25381. [Google Scholar] [CrossRef] [PubMed]
- Blankensteijn, J.D.; de Jong, S.E.C.A.; Prinssen, M.; van der Ham, A.C.; Buth, J.; van Sterkenburg, S.M.M.; Grobbee, D.E. Two-Year Outcomes after Conventional or Endovascular Repair of Abdominal Aortic Aneurysms. N. Engl. J. Med. 2005, 352, 2398–2405. [Google Scholar] [CrossRef]
- Rastogi, V.; de Bruin, J.L.; Bouwens, E.; Hoeks, S.E.; Ten Raa, S.; van Rijn, M.J.; Fioole, B.; Schermerhorn, M.L.; Verhagen, H.J.M. Incidence, Prognostic Significance, and Risk Factors of Acute Kidney Injury Following Elective Infrarenal and Complex Endovascular Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2022, 64, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Arbănași, E.M.; Mureșan, A.V.; Coșarcă, C.M.; Arbănași, E.M.; Niculescu, R.; Voidăzan, S.T.; Ivănescu, A.D.; Hălmaciu, I.; Filep, R.C.; Mărginean, L.; et al. Computed Tomography Angiography Markers and Intraluminal Thrombus Morphology as Predictors of Abdominal Aortic Aneurysm Rupture. Int. J. Environ. Res. Public Health 2022, 19, 15961. [Google Scholar] [CrossRef] [PubMed]
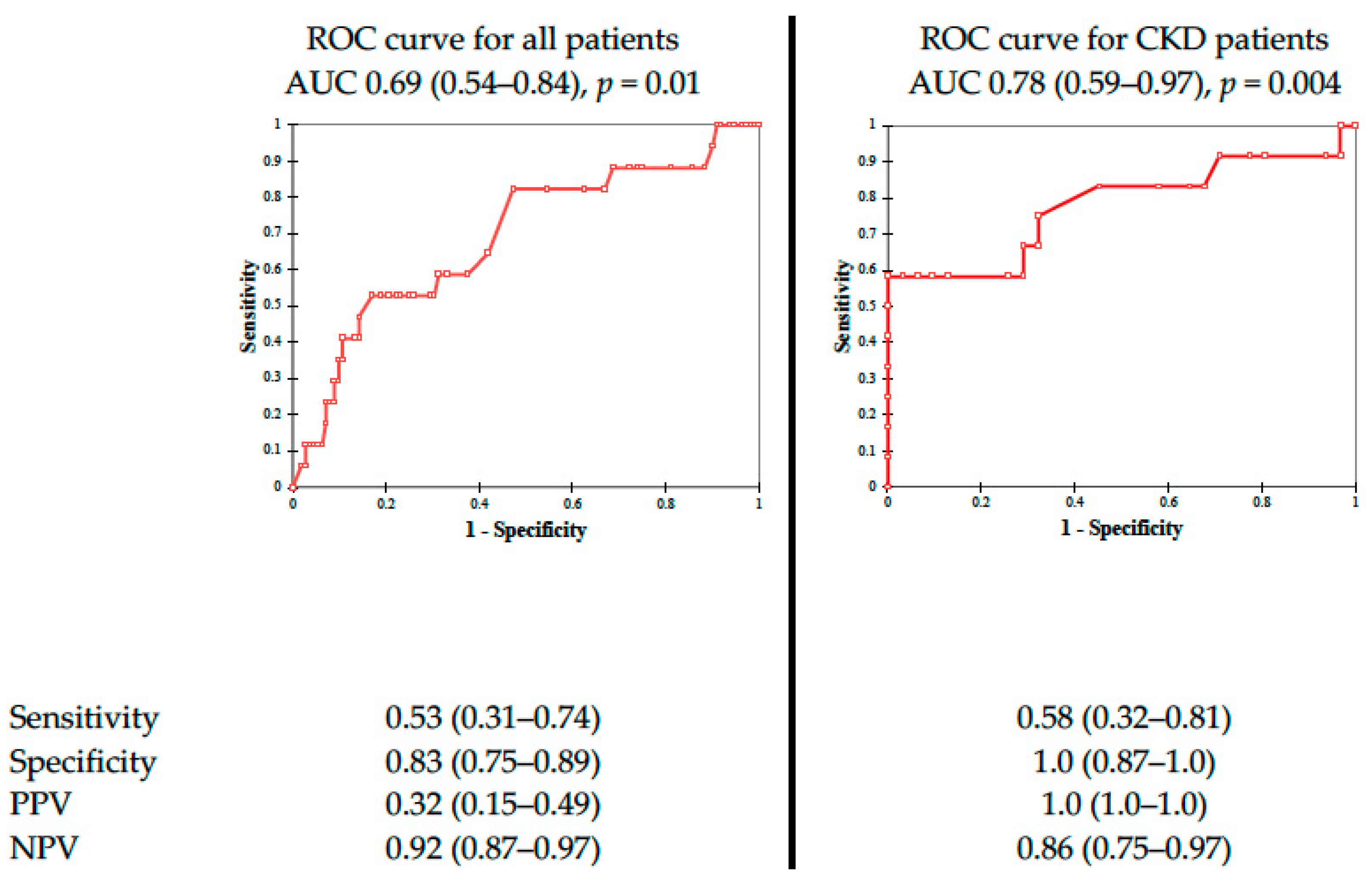
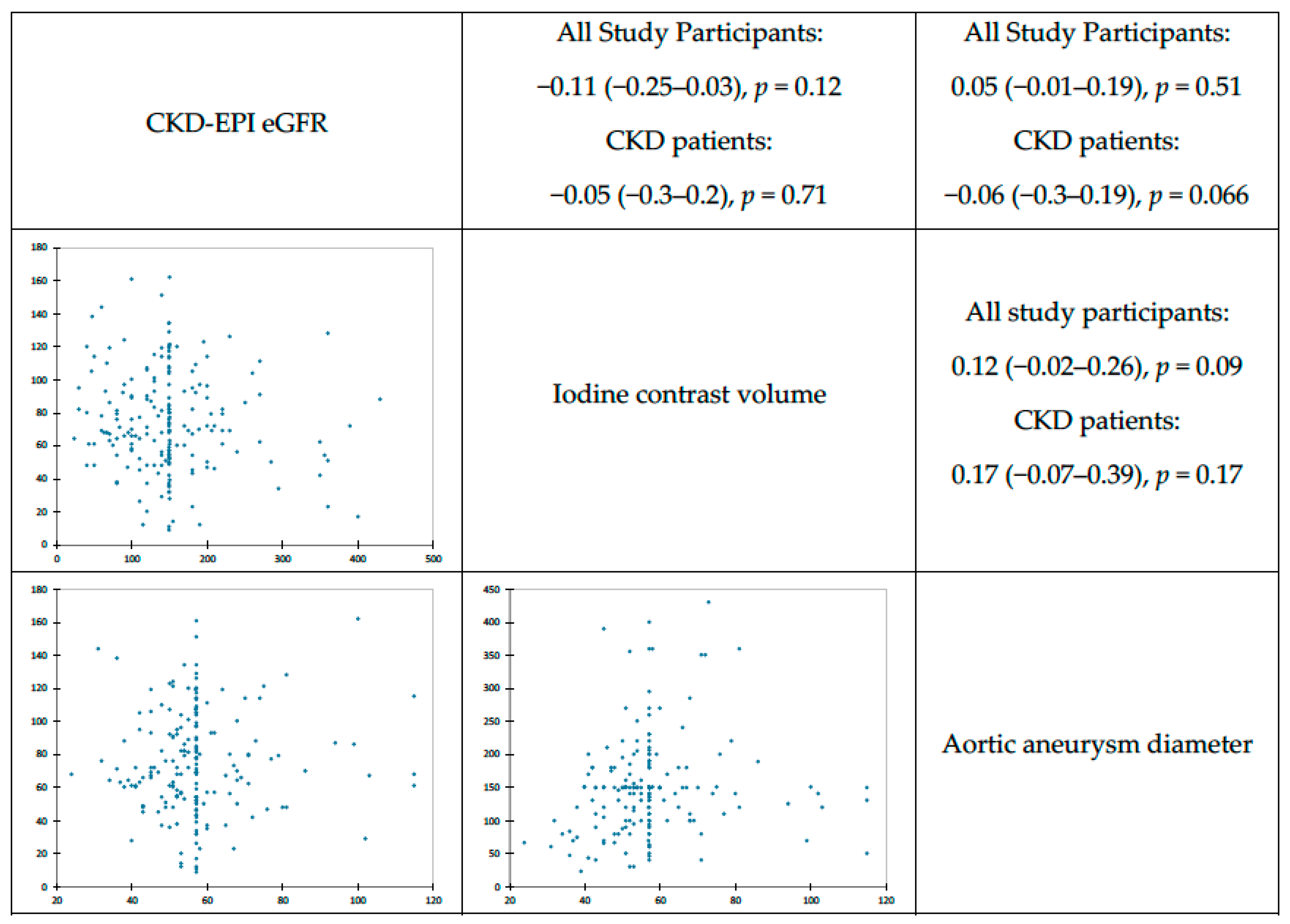
| All Study Participants (N = 192) | Patients with PC–AKI (N = 36) | Patients without PC–AKI (N = 156) | p | |
|---|---|---|---|---|
| Age (years); mean (SD) | 73.3 (7.9) | 74.9 (9.1) | 72.8 (7.5) | 0.12 |
| Male; % (N) | 76 (146) | 86.1 (31) | 73.3 (115) | <0.001 |
| Obesity; % (N) | 7.8 (15) | 11.1 (4) | 7.1 (11) | 0.42 |
| Hypertension; % (N) | 57.8 (111) | 55.6 (20) | 58.3 (91) | 0.76 |
| Diabetes mellitus; % (N) | 15.1 (29) | 13.9 (5) | 15.4 (24) | 0.82 |
| Atrial fibrillation; % (N) | 17.7 (34) | 11.1 (4) | 19.2 (30) | 0.25 |
| Chronic coronary syndrome; % (N) | 32.3 (62) | 33.3 (12) | 32.1 (50) | 0.88 |
| COPD; % (N) | 7.3 (14) | 0 (0) | 9 (14) | 0.06 |
| History of acute coronary syndrome; % (N) | 13.5 (26) | 13.9 (5) | 13.5 (21) | 0.9 |
| History of stroke; % (N) | 9.4 (18) | 16.7 (6) | 7.7 (12) | 0.1 |
| History of neoplasm; % (N) | 7.3 (14) | 5.6 (2) | 11.5 (18) | 0.29 |
| Chronic kidney disease; % (N) | 34.9 (67) | 66.7 (24) | 27.6 (43) | <0.001 |
| Serum creatinine concentration (mg/dL); mean (SD) | 1.18 (0.72) | 1.75 (1.1) | 1.1 (0.5) | <0.001 |
| CKD-EPI eGFR mL/min/1.73 m²; mean (SD) | 74.77 (30.5) | 54.9 (28.7) | 79.4 (29) | <0.001 |
| CKD-EPI eGFR <45 mL/min/1.73 m²; % (N) | 13.5 (26) | 36.1 (13) | 8.3 (13) | <0.001 |
| CKD-EPI eGFR <30 mL/min/1.73 m²; % (N) | 6.3 (12) | 22.2 (8) | 2.6 (4) | |
| CKD-EPI eGFR <15 mL/min/1.73 m²; % (N) | 2.6 (5) | 8.3 (3) | 1.3 (2) | |
| Blood urea nitrogen concentration (mg/dL); mean (SD) | 43.63 (18.9) | 56.4 (23.8) | 40.6 (16.2) | <0.001 |
| Blood sugar level (mg/dL); mean (SD) | 124.5 (49) | 141.3 (60.3) | 120.7 (45.3) | 0.03 |
| Serum sodium concentration (mEq/L); mean (SD) | 140.2 (3.3) | 139.9 (3.4) | 140.3 (3.3) | 0.55 |
| Serum potassium concentration (mEq/L); mean (SD) | 4.5 (0.5) | 4.4 (0.6) | 4.5 (0.5) | 0.22 |
| Serum chloride concentration (mEq/L); mean (SD) | 101.5 (3.8) | 101.3 (4.1) | 101.6 (3.7) | 0.67 |
| Aortic diameter; mean (SD) | 57.2 (17) | 66.9 (19.7) | 55.7 (16) | 0.01 |
| Iodine contrast volume (mL); mean (SD) | 149.6 (81.2) | 179.3 (89.1) | 142.1 (77.3) | 0.03 |
| Aortic Aneurysms ≥67 mm (N = 30) | Aortic Aneurysms <67 mm (N = 99) | p | |
|---|---|---|---|
| Age (years); mean (SD) | 76.6 (6.5) | 73.2 (7) | 0.06 |
| Male; % (N) | 86.7 (26) | 72.7 (72) | <0.001 |
| Obesity; % (N) | 6.7 (2) | 4 (4) | 0.051 |
| Hypertension; % (N) | 56.7 (17) | 62.6 (62) | <0.001 |
| Diabetes mellitus; % (N) | 10 (3) | 18.2 (18) | <0.001 |
| Atrial fibrillation; % (N) | 18.7 (5) | 18.2 (18) | 0.85 |
| Chronic coronary syndrome; % (N) | 30 (9) | 34.3 (34) | 0.66 |
| COPD; % (N) | 6.7 (2) | 10.1 (10) | 0.57 |
| History of acute coronary syndrome; % (N) | 13.3 (4) | 13.1 (13) | 0.98 |
| History of stroke; % (N) | 0 (0) | 8.1 (8) | 0.11 |
| History of neoplasm; % (N) | 13.3 (4) | 14.1 (14) | 0.91 |
| Chronic kidney disease; % (N) | 26.7 (8) | 35.4 (35) | <0.001 |
| CKD-EPI eGFR <45 mL/min/1.73 m²; % (N) | 10 (3) | 11.1 (11) | <0.001 |
| CKD-EPI eGFR <30 mL/min/1.73 m²; % (N) | 6.7 (2) | 5.1 (5) | 79.4 (29) |
| CKD-EPI eGFR <15 mL/min/1.73 m²; % (N) | 0 (0) | 2 (2) | 8.3 (13) |
| Serum creatinine concentration (mg/dL); mean (SD) | 1.11 (0.42) | 1.14 (0.54) | 0.76 |
| CKD-EPI eGFR mL/min/1.73 m²; mean (SD) | 77.3 (30.3) | 73.4 (27.7) | 0.52 |
| Blood urea nitrogen concentration (mg/dL); mean (SD) | 41.9 (17.3) | 42.9 (17.4) | 0.79 |
| Blood glucose level (mg/dL); mean (SD) | 135.5 (46.8) | 113.3 (40.6) | 0.02 |
| Serum sodium concentration (mEq/L); mean (SD) | 139.8 (2.8) | 140.7 (3.3) | 0.19 |
| Serum potassium concentration (mEq/L); mean (SD) | 4.2 (0.3) | 4.5 (0.5) | <0.001 |
| Serum chloride concentration (mEq/L); mean (SD) | 101.6 (3.3) | 101.4 (3.6) | 0.83 |
| Iodine contrast volume (mL); mean (SD) | 168.1 (99.8) | 139.3 (72.1) | 0.11 |
| PC–AKI; % (N) | 30 (9) | 8.1 (8) | <0.001 |
| Variables | OR | 95% CI | p |
|---|---|---|---|
| Serum creatinine concentration | 1.439 | 1.056–1.959 | 0.02 |
| Age | 1.224 | 0.931–1.608 | 0.15 |
| Male | 1.345 | 1.004–1.804 | 0.047 |
| Chronic kidney disease | 1.464 | 1.074–1.995 | 0.02 |
| Obesity | 1.037 | 0.822–1.307 | 0.76 |
| Diabetes mellitus | 0.951 | 0.744–1.214 | 0.69 |
| Hypertension | 0.961 | 0.730–1.265 | 0.78 |
| Chronic coronary syndrome | 1.032 | 0.756–1.407 | 0.84 |
| History of acute coronary syndrome | 0.945 | 0.700–1.277 | 0.71 |
| History of stroke | 1.191 | 0.953–1.489 | 0.13 |
| History of neoplasm | 0.790 | 0.579–1.079 | 0.14 |
| Atrial fibrillation | 0.765 | 0.576–1.017 | 0.07 |
| Aortic aneurysms diameter greater than cut off point (67 mm) | 1.364 | 1.093–1.702 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoń, B.; Nazarewski, S.; Małyszko, J. Kidney Function, Male Gender, and Aneurysm Diameter Are Predictors of Acute Kidney Injury in Patients with Abdominal Aortic Aneurysms Treated Endovascularly. Toxins 2023, 15, 130. https://doi.org/10.3390/toxins15020130
Antoń B, Nazarewski S, Małyszko J. Kidney Function, Male Gender, and Aneurysm Diameter Are Predictors of Acute Kidney Injury in Patients with Abdominal Aortic Aneurysms Treated Endovascularly. Toxins. 2023; 15(2):130. https://doi.org/10.3390/toxins15020130
Chicago/Turabian StyleAntoń, Bartłomiej, Sławomir Nazarewski, and Jolanta Małyszko. 2023. "Kidney Function, Male Gender, and Aneurysm Diameter Are Predictors of Acute Kidney Injury in Patients with Abdominal Aortic Aneurysms Treated Endovascularly" Toxins 15, no. 2: 130. https://doi.org/10.3390/toxins15020130
APA StyleAntoń, B., Nazarewski, S., & Małyszko, J. (2023). Kidney Function, Male Gender, and Aneurysm Diameter Are Predictors of Acute Kidney Injury in Patients with Abdominal Aortic Aneurysms Treated Endovascularly. Toxins, 15(2), 130. https://doi.org/10.3390/toxins15020130






