Seasonal and Geographical Impact on the Mycotoxigenicity of Aspergillus and Fusarium Species Isolated from Smallholder Dairy Cattle Feeds and Feedstuffs in Free State and Limpopo Provinces of South Africa
Abstract
1. Introduction
2. Results
2.1. Method Validation
2.2. Toxigenicity of Aspergillus and Fusarium Species
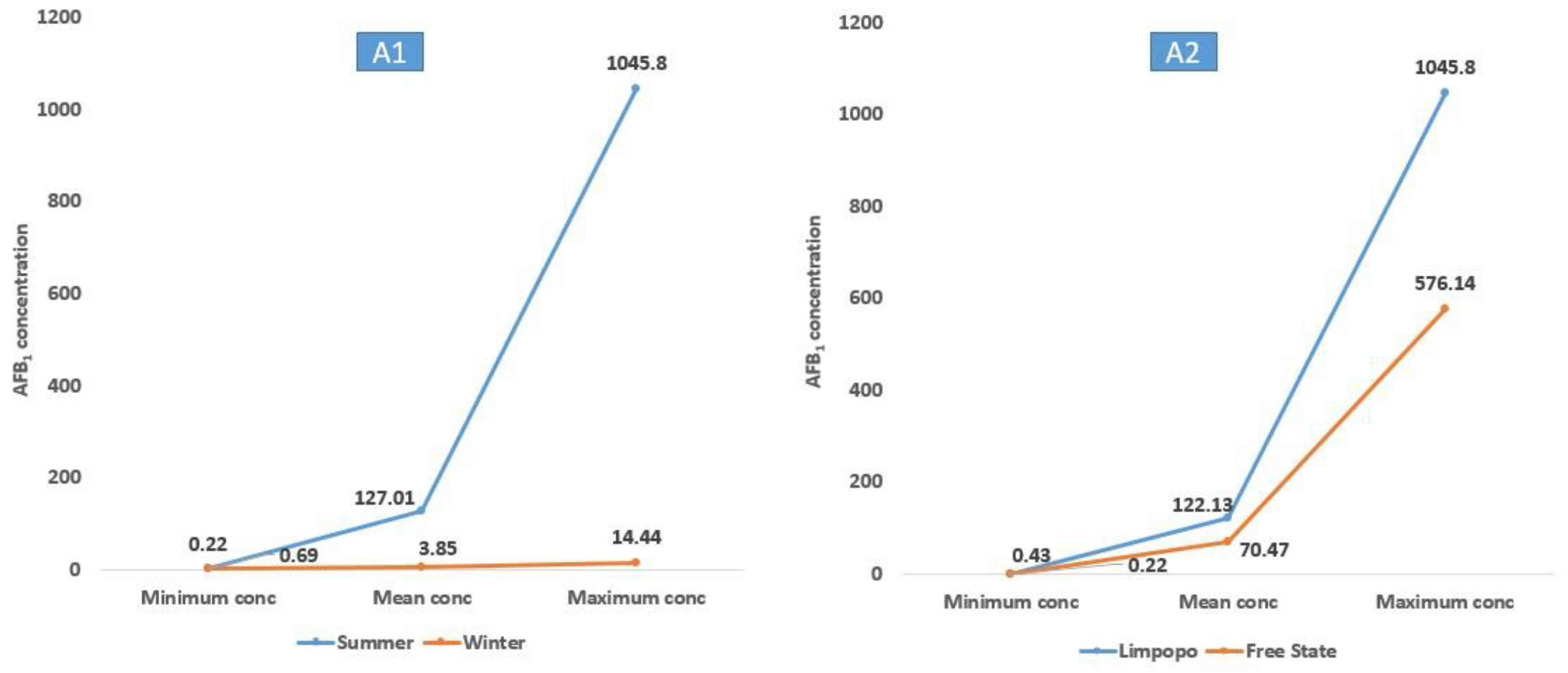
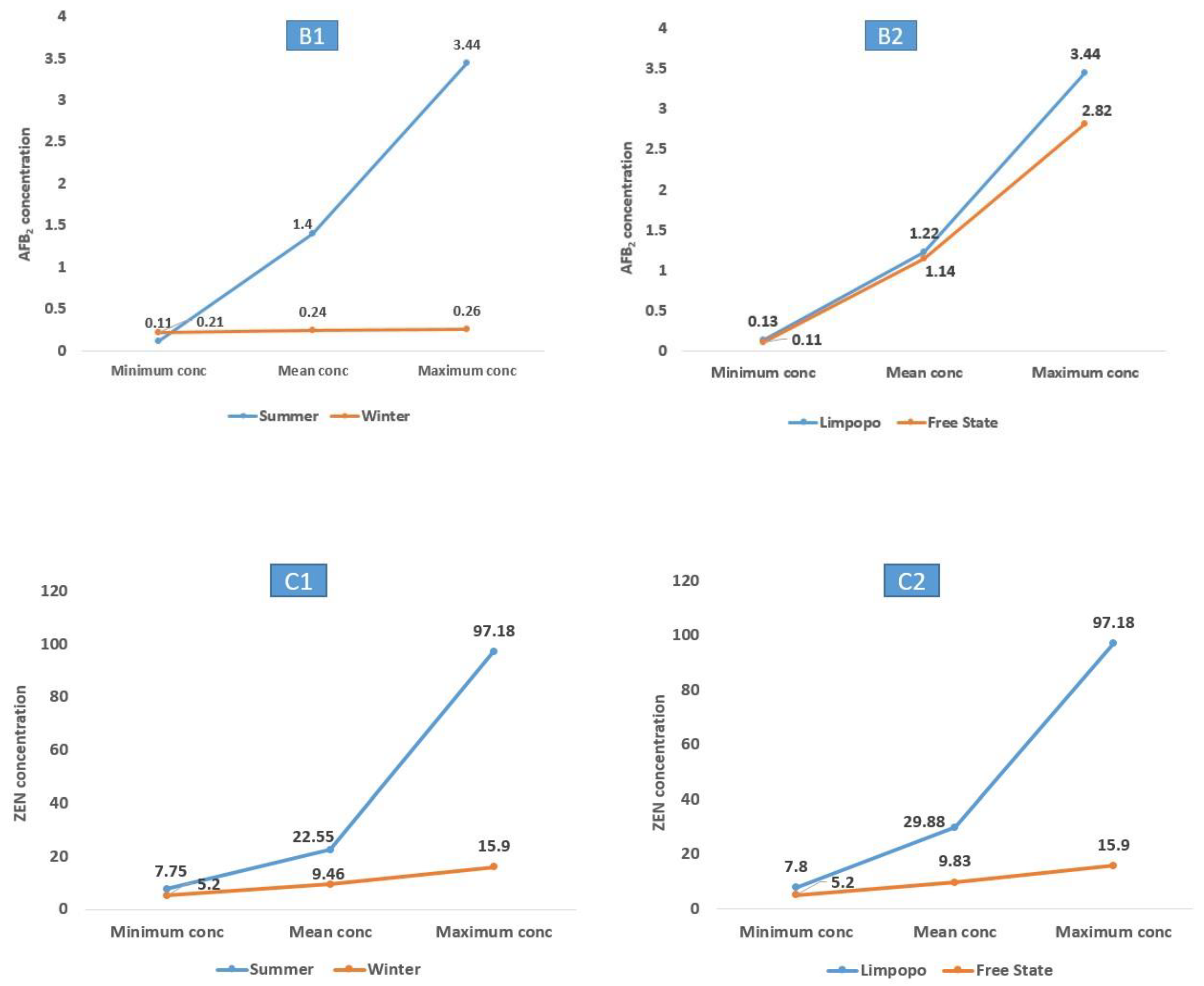
2.3. Seasonal and Geographical Impacts on Mycotoxigenicity of Aspergillus and Fusarium Species
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Mycotoxin Standards
5.3. Fungal Strains and Inoculation
5.4. Multi-Mycotoxin Extraction
5.5. LC-MS/MS Apparatus and Condition
5.6. Method Validation
5.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Agar Preparations
Appendix A.1. Czapek Concentrate
Appendix A.2. Czapek Yeast Extract Agar (CYA)
References
- Kralik, G.; Kralik, Z.; Grcevic, M.; Hanžek, D. Quality of chicken meat. In Animal Husbandry and Nutrition, 1st ed.; Yücel, B., Taskin, T., Eds.; Intech Open: London, UK, 2018. [Google Scholar]
- Gonçalves, B.L.; Corassin, C.H.; Oliveira, C.A.F. de Mycotoxicoses in dairy cattle: A review. Asian J. Anim. Vet. Adv. 2015, 10, 752–760. [Google Scholar] [CrossRef]
- Nkya, R.; Kessy, B.M.; Lyimo, Z.C.; Msangi, B.S.J.; Turuka, F.; Mtenga, K. Constraints on smallholder market oriented dairy systems in the north eastern coastal region of Tanzania. Trop. Anim. Health Prod. 2007, 39, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; VanLeeuwen, J.A.; Shepelo, G.; Gitau, G.K.; Wichtel, J.; Kamunde, C.; Uehlinger, F. Randomized controlled trial on impacts of dairy meal feeding interventions on early lactation milk production in smallholder dairy farms of Central Kenya. Prev. Vet. Med. 2016, 125, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kemboi, D.C.; Antonissen, G.; Ochieng, P.E.; Croubels, S.; Okoth, S.; Kangethe, E.K.; Faas, J.; Lindahl, J.F.; Gathumbi, J.K. A review of the impact of mycotoxins on dairy cattle health: Challenges for food safety and dairy production in sub-Saharan Africa. Toxins 2020, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Changwa, R.; De Boevre, M.; De Saeger, S.; Njobeh, P.B. Feed-Based Multi-Mycotoxin Occurrence in Smallholder Dairy Farming Systems of South Africa: The Case of Limpopo and Free State. Toxins 2021, 13, 166. [Google Scholar] [CrossRef]
- Atanda, O.; Makun, H.A.; Ogara, I.M.; Edema, M.; Idahor, K.O.; Eshiett, M.E.; Oluwabamiwo, B.F. Fungal and mycotoxin contamination of nigerian foods and feeds. In Mycotoxin and Food Safety in Developing Countries; BoD—Books on Demand: Norderstedt, Germany, 2013; Volume 68, pp. 1455–1458. [Google Scholar] [CrossRef]
- Aasa, A.O.; Fru, F.F.; Adelusi, O.A.; Oyeyinka, S.A.; Njobeh, P.B. A review of toxigenic fungi and mycotoxins in feeds and food commodities in West Africa. World Mycotoxin J. 2022, 1–16. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Ceremuga, M.; Stela, M.; Saluk-bijak, J.; Siadkowski, A.; Bijak, M. Molecular Aspects of Mycotoxins—A Serious Problem for Human Health. Int. J. Mol. Sci. 2020, 21, 8187. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Fink-Gremmels, J.; van der Merwe, D. Mycotoxins in the food chain: Contamination of foods of animal origin. In Chemical Hazards in Foods of Animals Origins; ECVPH Food Safety Assurance and Veterinary Public Health; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; pp. 241–261. [Google Scholar]
- Claudious, G. Isolation Of Aspergillus Flavus from Dairy Cattle Feed And Assessment Of Aflatoxin M1 In Milk From Small Dairy Farms Around Harare, Zimbabwe. Adv. Microbiol. Res. 2019, 3, 1–7. [Google Scholar] [CrossRef]
- Izzo, L.; Mikušová, P.; Lombardi, S.; Sulyok, M.; Ritieni, A. Analysis of Mycotoxin and Secondary Metabolites in Commercial and Traditional Slovak Cheese Samples. Toxins 2022, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.S.; Cunha, S.C.; Fernandes, J.O. Prevalent mycotoxins in animal feed: Occurrence and analytical methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Iha, M.H.; Barbosa, C.B.; Okada, I.A.; Trucksess, M.W. Aflatoxin M 1 in milk and distribution and stability of aflatoxin M 1 during production and storage of yoghurt and cheese. Food Control 2013, 29, 1–6. [Google Scholar] [CrossRef]
- Djekic, I.; Petrovic, J.; Jovetic, M.; Redzepovic-Djordjevic, A.; Stulic, M.; Lorenzo, J.M.; Iammarino, M.; Tomasevic, I. Aflatoxins in milk and dairy products: Occurrence and exposure assessment for the serbian population. Appl. Sci. 2020, 10, 7420. [Google Scholar] [CrossRef]
- Álvarez-Días, F.; Torres-Parga, B.; Valdivia-Flores, A.G.; Quezada-Tristán, T.; Alejos-De La Fuente, J.I.; Sosa-Ramírez, J.; Rangel-Muñoz, E.J. Aspergillus flavus and Total Aflatoxins Occurrence in Dairy Feed and Aflatoxin M1 in Bovine Milk in Aguascalientes, Mexico. Toxins 2022, 14, 292. [Google Scholar] [CrossRef]
- Omotayo, O.P.; Omotayo, A.O.; Babalola, O.O.; Mwanza, M. Comparative study of aflatoxin contamination of winter and summer ginger from the North West Province of South Africa. Toxicol. Reports 2019, 6, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, P.E.; Scippo, M.L.; Kemboi, D.C.; Croubels, S.; Okoth, S.; Kang’ethe, E.K.; Doupovec, B.; Gathumbi, K.; Lindahl, J.F.; Antonissen, G. Mycotoxins in poultry feed and feed ingredients from Sub-Saharan Africa and their impact on the production of broiler and layer chickens: A review. Toxins 2021, 13, 633. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Zhou, X.; Liu, M.; Zang, H.; Liu, X.; Shan, A.; Feng, X. Alleviation of oral exposure to aflatoxin b1-induced renal dysfunction, oxidative stress, and cell apoptosis in mice kidney by curcumin. Antioxidants 2022, 11, 1082. [Google Scholar] [CrossRef]
- Gbashi, S.; Madala, N.E.; De Saeger, S.; De Boevre, M.; Adekoya, I.; Adebo, O.; Njobeh, P.B. The Socio-Economic Impact of Mycotoxin Contamination in Africa; Intech Open: London, UK, 2018; pp. 1–22. [Google Scholar] [CrossRef]
- Barral, B.; Chillet, M.; Doizy, A.; Grassi, M.; Ragot, L.; Mathieu, L.; Durand, N.; Rose, L.J.; Viljoen, A.; Schorr-galindo, S. Pineapple Fruitlet Core Rot. Toxins 2020, 12, 339. [Google Scholar] [CrossRef]
- Aasa, A.O.; Adelusi, O.A.; Fru, F.F.; Areo, O.M.; Njobeh, P.B. Preliminary screening of toxigenic fungi and mycotoxin contamination: A case of agricultural products in Ivory Coast. Food Chem. Adv. 2022, 1, 100132. [Google Scholar] [CrossRef]
- Ghiasian, S.A.; Maghsood, A.H. Occurrence of aflatoxigenic fungi in cow feeds during the summer and winter season in Hamadan, Iran. Afr. J. Microbiol. Res. 2011, 5, 516–521. [Google Scholar] [CrossRef]
- Tangni, K.; Wambacq, E.; Bastiaanse, H.; Haesaert, G.; Pussemier, L.; De, J.; Foucart, F. Survey of fungal diversity in silages supplied to dairy cattle in Belgium over a two-year period. J. Anim. Sci. Adv. 2017, 7, 1861–1873. [Google Scholar] [CrossRef]
- González-Jartín, J.M.; Ferreiroa, V.; Rodríguez-Cañás, I.; Alfonso, A.; Sainz, M.J.; Aguín, O.; Vieytes, M.R.; Gomes, A.; Ramos, I.; Botana, L. Occurrence of mycotoxins and mycotoxigenic fungi in silage from the north of Portugal at feed-out. Int. J. Food Microbiol. 2022, 365, 109556. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Blanco, M.; Ramos, A.J.; Sanchis, V.; Marín, S. Mycotoxins occurrence and fungal populations in different types of silages for dairy cows in Spain. Fungal Biol. 2021, 125, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, C.S.; Dutton, M.F. A survey of South African silage for fungi and mycotoxins. Afr. J. Agric. Res. 2013, 8, 4299–4307. [Google Scholar] [CrossRef]
- Iheanacho, H.E.; Njobeh, P.B.; Dutton, F.M.; Steenkamp, P.A.; Steenkamp, L.; Daru, B.H.; Makun, A.H. Morphological and molecular identification of filamentous Aspergillus flavus and Aspergillus parasiticus isolated from compound feeds in South Africa. Food Microbiol. 2014, 44, 180–184. [Google Scholar] [CrossRef]
- Adelusi, O.A.; Gbashi, S.; Adebiyi, J.A.; Makhuvele, R.; Aasa, A.O.; Oladeji, O.M.; Khoza, M.; Okoth, S.; Njobeh, P.B. Seasonal diversity and occurrence of filamentous fungi in smallholder dairy cattle feeds and feedstuffs in South Africa. J. Fungi 2022, 8, 1192. [Google Scholar] [CrossRef]
- Munoz-Solano, B.; Gonzalez-Penas, E. Mycotoxin determination in animal feed: An LC-FLD method for simultaneous quantification of aflatoxins, ochratoxins and zearelanone in this Matrix. Toxins 2020, 12, 374. [Google Scholar] [CrossRef]
- Awapak, D.; Petchkongkaew, A.; Sulyok, M.; Krska, R. Co-occurrence and toxicological relevance of secondary metabolites in dairy cow feed from Thailand. Food Addit. Contam. Part A 2021, 38, 1013–1027. [Google Scholar] [CrossRef]
- Njobeh, P.B.; Dutton, M.F.; Åberg, A.T.; Haggblom, P. Estimation of multi-mycotoxin contamination in South African compound feeds. Toxins 2012, 4, 836–848. [Google Scholar] [CrossRef]
- Alam, S.; Shah, H.U.; Khan, H.; Magan, N. Effect of substrate, season, and agroecological zone on mycoflora and aflatoxin contamination of poultry feed from Khyber Pakhtunkhwa, PakistanThe. Mycopathologia 2012, 174, 341–349. [Google Scholar] [CrossRef]
- Abdou, K.; Hassan, A.A.; Hassan, N.E.; El-hamed, R.A. Seasonal Variation in Prevalence of Mycotoxins in Feed and Feedstuffs at Beni-Suef Governorate in Egypt. Eur. J. Acad. Essays 2017, 4, 99–109. [Google Scholar]
- Changwa, R.; Abia, W.; Msagati, T.; Nyoni, H.; Ndleve, K.; Njobeh, P. Multi-mycotoxin occurrence in dairy cattle feeds from the gauteng province of South Africa: A pilot study using UHPLC-QTOF-MS/MS. Toxins 2018, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Chilaka, C.A.; de Kock, S.; Phoku, J.Z.; Mwanza, M.; Egbuta, M.A.; Dutton, M.F. Fungal and mycotoxin contamination of South African commercial maize. J. Food Agric. Environ. 2012, 10, 296–303. [Google Scholar]
- European Commission. Commission Regulation (EC) No 401/2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, 24, 1–42. [Google Scholar]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Bandyopadhyay, R.; Müller, J.; Vanlauwe, B. Mycotoxins in Sub-Saharan Africa: Present situation, socio-economic impact, awareness, and outlook. Food Control 2017, 72, 110–122. [Google Scholar] [CrossRef]
- Lasram, S.; Hamdi, Z.; Chenenaoui, S.; Mliki, A.; Ghorbel, A. Comparative study of toxigenic potential of Aspergillus flavus and Aspergillus niger isolated from Barley as affected by temperature, water activity and carbon source. J. Stored Prod. Res. 2016, 69, 58–64. [Google Scholar] [CrossRef]
- Yogendrarajah, P.; Devlieghere, F.; Njumbe Ediage, E.; Jacxsens, L.; De Meulenaer, B.; De Saeger, S. Toxigenic potentiality of Aspergillus flavus and Aspergillus parasiticus strains isolated from black pepper assessed by an LC-MS/MS based multi-mycotoxin method. Food Microbiol. 2015, 52, 185–196. [Google Scholar] [CrossRef]
- Yazid, S.N.E.; Ng, W.J.; Selamat, J.; Ismail, S.I.; Samsudin, N.I.P. Diversity and toxigenicity of mycobiota in grain corn: A case study at pioneer grain corn plantations in Terengganu, Malaysia. Agriculture 2021, 11, 237. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Balasubramanian, B.; Park, S.; Jha, R.; Andretta, I.; Bakare, A.G.; Kim, I.H. Ochratoxin A: Carryover from animal feed into livestock and the mitigation strategies. Anim. Nutr. 2021, 7, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Passamani, F.R.F.; Hernandes, T.; Lopes, N.A.; Bastos, S.C.; Santiago, W.D.; Cardoso, M.D.G.; Batist, L.R. Effect of temperature, water activity, and pH on growth and production of ochratoxin A by Aspergillus niger and Aspergillus carbonarius from Brazilian grapes. J. Food Prot. 2014, 77, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Hu, C.; Nie, P.; Zhai, H.; Zhang, S.; Li, N.; Lv, Y.; Hu, Y. Insights into the Underlying mechanism of ochratoxin a production in Aspergillus niger CBS 513.88 using different carbon sources. Toxins 2022, 14, 551. [Google Scholar] [CrossRef] [PubMed]
- Susca, A.; Proctor, R.H.; Morelli, M.; Haidukowski, M.; Gallo, A.; Logrieco, A.F.; Moretti, A. Variation in fumonisin and ochratoxin production associated with differences in biosynthetic gene content in Aspergillus niger and A. welwitschiae isolates from multiple crop and geographic origins. Front. Microbiol. 2016, 7, 1412. [Google Scholar] [CrossRef]
- Esteban, A.; Abarca, M.L.; Bragulat, M.R.; Cabañes, F.J. Effects of temperature and incubation time on production of ochratoxin a by black aspergilli. Res. Microbiol. 2004, 155, 861–866. [Google Scholar] [CrossRef]
- Munitz, M.S.; Resnik, S.L.; Pacin, A.; Salas, P.M.; Gonzalez, H.H.L.; Montti, M.I.T.; Drunday, V.; Guillin, E.A. Mycotoxigenic potential of fungi isolated from freshly harvested Argentinean blueberries. Mycotoxin Res. 2014, 30, 221–229. [Google Scholar] [CrossRef]
- Njobeh, P.B.; Dutton, M.F.; Koch, S.H.; Chuturgoon, A.; Stoev, S.; Seifert, K. Contamination with storage fungi of human food from Cameroon. Int. J. Food Microbiol. 2009, 135, 193–198. [Google Scholar] [CrossRef]
- Sultan, Y.; Magan, N. Mycotoxigenic fungi in peanuts from different geographical regions of Egypt. Mycotoxin Res. 2010, 26, 133–140. [Google Scholar] [CrossRef]
- Mngadi, P.T.; Govinden, R.; Odhav, B. Co-occurring mycotoxins in animal feeds. African J. Biotechnol. 2008, 7, 2239–2243. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, H.; Neng, J.; Gao, J.; Yang, B.; Liu, Y. The influence of NaCl and glucose content on growth and ochratoxin a production by aspergillus ochraceus, aspergillus carbonarius and penicillium nordicum. Toxins 2020, 12, 515. [Google Scholar] [CrossRef]
- O’Callaghan, J.; Stapleton, P.C.; Dobson, A.D.W. Ochratoxin A biosynthetic genes in Aspergillus ochraceus are differentially regulated by pH and nutritional stimuli. Fungal Genet. Biol. 2006, 43, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.; Cavaglieri, L.; Ribeiro, J.; Keller, K.; Alonso, V.; Chiacchiera, S.; Dalcero, A.; Lopes, C. Mycobiota and naturally-occurring ochratoxin A in dairy cattle feed from Rio de Janeiro State, Brazil. World Mycotoxin J. 2008, 1, 195–201. [Google Scholar] [CrossRef]
- Adekoya, I.; Njobeh, P.; Obadina, A.; Landschoot, S.; Audenaert, K.; Okoth, S.; De Boevre, M.; De Saeger, S. Investigation of the metabolic profile and toxigenic variability of fungal species occurring in fermented foods and beverage from nigeria and South Africa using UPLC-MS/MS. Toxins 2019, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Phoku, J.Z.; Barnard, T.G.; Potgieter, N.; Dutton, M.F. Mycotoxigenic potentials of the genera: Aspergillus, Fusarium and Penicillium isolated from houseflies (Musca domestica L.). Acta Trop. 2017, 168, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Barros, G.; Zanon, M.S.A.; Palazzini, J.M.; Haidukowski, M.; Pascale, M.; Chulze, S. Trichothecenes and zearalenone production by Fusarium equiseti and Fusarium semitectum species isolated from Argentinean soybean. Food Addit. Contam. Part A 2012, 29, 1436–1442. [Google Scholar] [CrossRef]
- Devegowda, G.; Raju, M.; Afzali, N.; Swamy, H. Mycotoxin picture worldwide: Novel solutions for their counteraction. In Biotechnology in the Feed Industry, Proceedings of Alltech’s 14th Annual Symposium, Nottingham, 24 February 1998, UK; Lyons, T.P., Jacques, K.A., Eds.; Feed Compounder: Whitland, UK, 1998; pp. 241–255. [Google Scholar]
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. A review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Mohale, S.; Medina, A.; Rodríguez, A. Mycotoxigenic fungi and mycotoxins associated with stored maize from different regions of Lesotho. Mycotoxin Resaerch 2013, 29, 209–219. [Google Scholar] [CrossRef]
- Phan, L.T.K.; Tran, T.M.; De Boevre, M.; Jacxsens, L.; Eeckhout, M.; De Saeger, S. Impact of Season, Region, and Traditional Agricultural Practices on Aflatoxins and Fumonisins Contamination in the Rice Chain in the Mekong Delta, Vietnam. Toxins 2021, 13, 667. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and food spoilage; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Adebajo, L.O.; Idowu, A.A.; Adesanya, O.O. Mycoflora, and mycotoxins production in Nigerian corn and corn-based snacks. Mycopathologia 1994, 126, 183–192. [Google Scholar] [CrossRef]
- Matumba, L.; Sulyok, M.; Njoroge, S.; Njumbe Ediage, E.; Van Poucke, C.; De Saeger, S.; Krska, R. Uncommon occurrence ratios of aflatoxin B1, B2, G1, and G2 in maize and groundnuts from Malawi. Mycotoxin Res. 2015, 31, 57–62. [Google Scholar] [CrossRef]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; El Khoury, A. Mycotoxins: Factors influencing production and control strategies. AIMS Agric. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Muga, F.C.; Marenya, M.O.; Workneh, T.S. Effect of temperature, relative humidity and moisture on aflatoxin contamination of stored maize kernels. Bulg. J. Agric. Sci. 2019, 25, 271–277. [Google Scholar]
- Phokane, S.; Flett, B.C.; Ncube, E.; Rheeder, J.P.; Rose, L.J. Agricultural practices and their potential role in mycotoxin contamination of maize and groundnut subsistence farming. S. Afr. J. Sci. 2019, 115, 2–7. [Google Scholar] [CrossRef]
- Gbashi, S.; Madala, N.E.; De Saeger, S.; De Boevre, M.; Njobeh, P.B. Numerical optimization of temperature-time degradation of multiple mycotoxins. Food Chem. Toxicol. 2019, 125, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Tebele, S.; Gbashi, S.; Adebo, O.; Changwa, R.; Naidu, K.; Njobeh, P. Quantification of multi-mycotoxin in cereals (maize, maize porridge, sorghum and wheat) from Limpopo Province of South Africa. Food Addit. Contam. Part A 2020, 37, 1922–1938. [Google Scholar] [CrossRef] [PubMed]
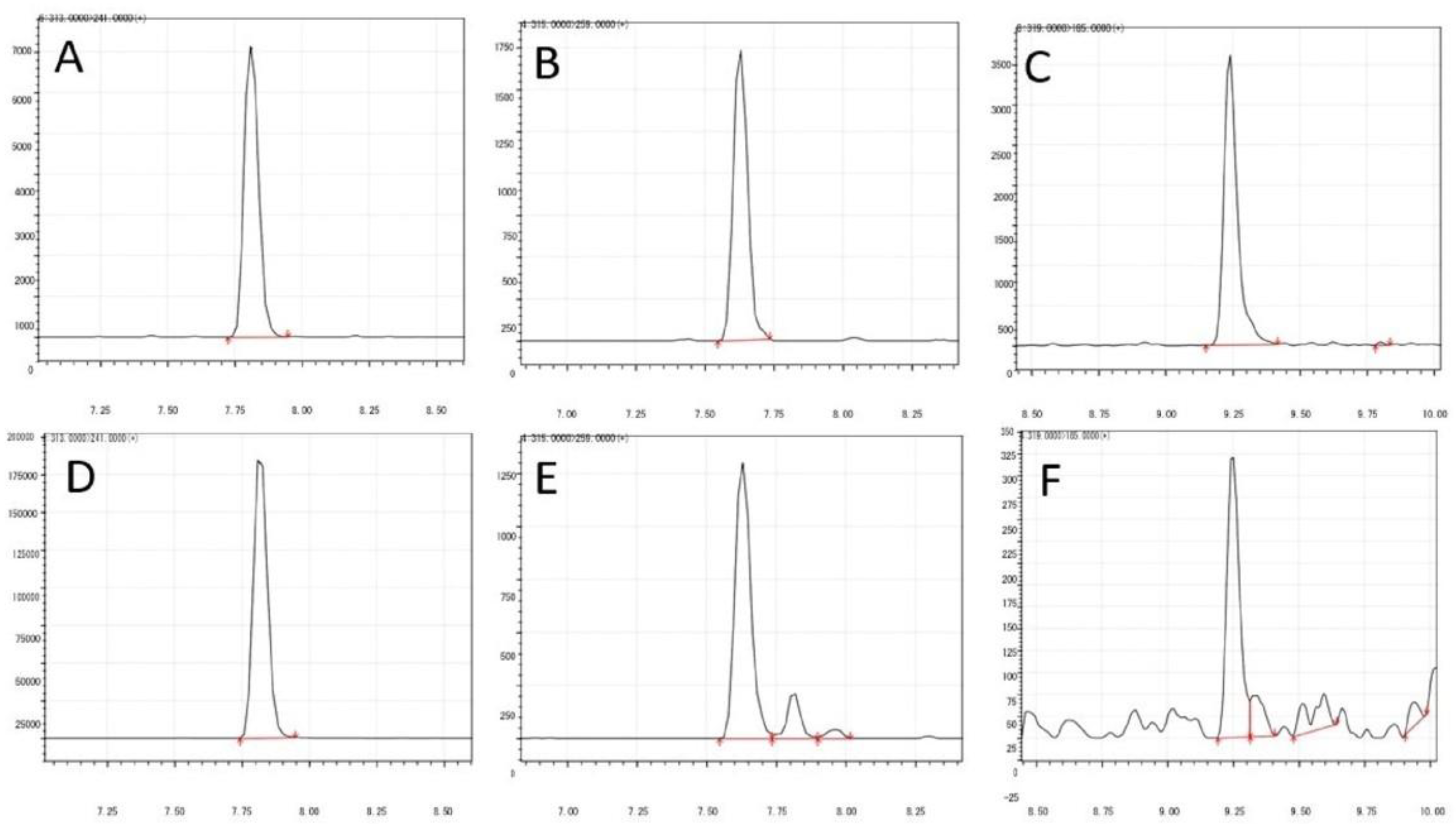
| Mycotoxins | Calibration Points | Ret. Time (min) | R2 | Slope | % Recovery | LOD (µg/kg) | LOQ (µg/kg) |
|---|---|---|---|---|---|---|---|
| OTA | 25, 50, 250, 500 | 9.30 | 0.9987 | 1864.73 | 98.3 | 0.01 | 0.04 |
| AFB1 | 0.5, 1, 50, 250 | 7.84 | 0.9986 | 657.99 | 80.9 | 0.04 | 0.14 |
| AFB2 | 0.5, 1, 100, 250 | 7.64 | 0.9988 | 965.93 | 101.9 | 0.02 | 0.07 |
| AFG1 | 1, 10, 50, 500 | 7.45 | 0.9995 | 748.77 | 90.3 | 0.06 | 0.19 |
| AFG2 | 1, 10, 25, 250 | 7.25 | 0.9994 | 420.65 | 93.3 | 0.05 | 0.17 |
| ZEN | 0.5, 25, 50, 250 | 7.75 | 0.9966 | 11.09 | 92.9 | 0.74 | 2.24 |
| DON | 1, 10, 100, 250 | 4.90 | 0.9970 | 5.45 | 71.4 | 4.42 | 13.40 |
| Summer | Winter | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Isolated Species | Accession No | No of Iso. spp. | No of Tox. spp. | Toxin Produced (Range: µg/kg) | Mean (µg/kg) | No of Iso. spp. | No of Tox. spp. | Toxin Produced (Range: µg/kg) | Mean (µg/kg) |
| Aspergillus | |||||||||
| A. Flavus | ON988996 | 20 | 18 9 | AFB1 (0.22–1045.80) AFB2 (0.11–3.44) | 127.01 1.40 | 9 | 6 2 | AFB1 (0.69–14.44) AFB2 (0.21–0.26) | 3.85 0.24 |
| A. fumigatus | ON988172 | 15 | - | - | - | 15 | - | - | - |
| A. niger | ON988183 | 14 | - | - | - | 8 | - | - | - |
| A. ochraceus | ON988182 | 1 | - | - | - | 1 | - | - | - |
| Fusarium. | - | ||||||||
| F. chlamydosporum | ON993228 | 7 | - | - | - | 4 | - | - | |
| F. equiseti | ON991743 | 3 | 3 | ZEN (8.69–97.18) | 41.64 | 4 | 2 | ZEN (7.64–9.08) | 8.36 |
| F. oxysporum | ON991521 | 8 | 5 | ZEN (7.75–16.29) | 11.09 | 3 | 2 | ZEN (5.20–15.90) | 10.55 |
| Total | 68 | 35 | 44 | 12 | |||||
| Free State | Limpopo | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Isolated Species | Accession No | No of Iso. spp. | No of Tox. spp. | Toxin Produced (Range: µg/kg) | Mean (µg/kg) | No of Iso. Spp. | No of Tox. spp. | Toxin Produced (Range: µg/kg) | Mean (µg/kg) |
| Aspergillus | |||||||||
| A. Flavus | ON988996 | 16 | 12 5 | AFB1 (0.22–576.14) AFB2 (0.11–2.82) | 70.47 1.14 | 13 | 12 6 | AFB1 (0.43–1045.80) AFB2 (0.13–3.44) | 122.13 1.22 |
| A. fumigatus | ON988172 | 16 | - | - | - | 14 | - | - | - |
| A. niger | ON988183 | 12 | - | - | - | 10 | - | - | - |
| A. ochraceus | ON988182 | 2 | - | - | - | - | - | - | - |
| Fusarium | - | ||||||||
| F. chlamydosporum | ON993228 | 8 | - | - | - | 3 | - | - | |
| F. equiseti | ON991743 | 2 | 2 | ZEN (7.64–8.69) | 8.12 | 5 | 3 | ZEN (9.08–97.18) | 41.77 |
| F. oxysporum | ON991521 | 8 | 5 | ZEN (5.20–15.90) | 10.49 | 3 | 2 | ZEN (7.80–16.29) | 12.05 |
| Total | 64 | 24 | 48 | 23 | |||||
| Mycotoxins | Statistics a | df1 | df2 | p-Value |
|---|---|---|---|---|
| AFB1 | 11.363 | 1 | 53.109 | 0.001 |
| AFB2 | 23.893 | 1 | 26.265 | 0.001 |
| ZEN | 5.813 | 1 | 24.898 | 0.024 |
| Mycotoxins | Statistics a | df1 | df2 | p-Value |
|---|---|---|---|---|
| AFB1 | 0.840 | 1 | 54.639 | 0.363 |
| AFB2 | 0.035 | 1 | 29.230 | 0.852 |
| ZEN | 4.148 | 1 | 14.359 | 0.061 |
| S/No | Mycotoxin | Products Ion (m/z) | Precursor Ion (m/z) | Q1 Pre Bias (V) | Collision Energy (CE) | Q3 Pre Bias (V) |
|---|---|---|---|---|---|---|
| 1 | OTA | 239 | 403.8 | −15 | −27 | −24 |
| 221 | −12 | −38 | −21 | |||
| 2 | AFB2 | 259.10 | 315 | −22 | −31 | −25 |
| 287 | −23 | −26 | −30 | |||
| 3 | AFB1 | 241 | 313 | −22 | −41 | −23 |
| 285.1 | −22 | −24 | −29 | |||
| 4 | AFG2 | 245.1 | 331 | −12 | −32 | −24 |
| 313 | −12 | −24 | −20 | |||
| 5 | AFG1 | 243 | 329 | −12 | −28 | −23 |
| 313.1 | −16 | −24 | −14 | |||
| 6 | ZEN | 185 | 319.1 | −12 | −27 | −30 |
| 187.1 | −15 | −21 | −19 | |||
| 7 | DON | 231 | 297.10 | −21 | −13 | −26 |
| 249.10 | −14 | −12 | −25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adelusi, O.A.; Gbashi, S.; Adebo, J.A.; Aasa, A.O.; Oladeji, O.M.; Kah, G.; Adebo, O.A.; Changwa, R.; Njobeh, P.B. Seasonal and Geographical Impact on the Mycotoxigenicity of Aspergillus and Fusarium Species Isolated from Smallholder Dairy Cattle Feeds and Feedstuffs in Free State and Limpopo Provinces of South Africa. Toxins 2023, 15, 128. https://doi.org/10.3390/toxins15020128
Adelusi OA, Gbashi S, Adebo JA, Aasa AO, Oladeji OM, Kah G, Adebo OA, Changwa R, Njobeh PB. Seasonal and Geographical Impact on the Mycotoxigenicity of Aspergillus and Fusarium Species Isolated from Smallholder Dairy Cattle Feeds and Feedstuffs in Free State and Limpopo Provinces of South Africa. Toxins. 2023; 15(2):128. https://doi.org/10.3390/toxins15020128
Chicago/Turabian StyleAdelusi, Oluwasola Abayomi, Sefater Gbashi, Janet Adeyinka Adebo, Adeola Oluwakemi Aasa, Oluwaseun Mary Oladeji, Glory Kah, Oluwafemi Ayodeji Adebo, Rumbidzai Changwa, and Patrick Berka Njobeh. 2023. "Seasonal and Geographical Impact on the Mycotoxigenicity of Aspergillus and Fusarium Species Isolated from Smallholder Dairy Cattle Feeds and Feedstuffs in Free State and Limpopo Provinces of South Africa" Toxins 15, no. 2: 128. https://doi.org/10.3390/toxins15020128
APA StyleAdelusi, O. A., Gbashi, S., Adebo, J. A., Aasa, A. O., Oladeji, O. M., Kah, G., Adebo, O. A., Changwa, R., & Njobeh, P. B. (2023). Seasonal and Geographical Impact on the Mycotoxigenicity of Aspergillus and Fusarium Species Isolated from Smallholder Dairy Cattle Feeds and Feedstuffs in Free State and Limpopo Provinces of South Africa. Toxins, 15(2), 128. https://doi.org/10.3390/toxins15020128





