“Pseudo”-Secondary Treatment Failure Explained via Disease Progression and Effective Botulinum Toxin Therapy: A Pilot Simulation Study
Abstract
:1. Introduction
2. Results
2.1. Examples of Self-Assessment of the First BoNT Injection
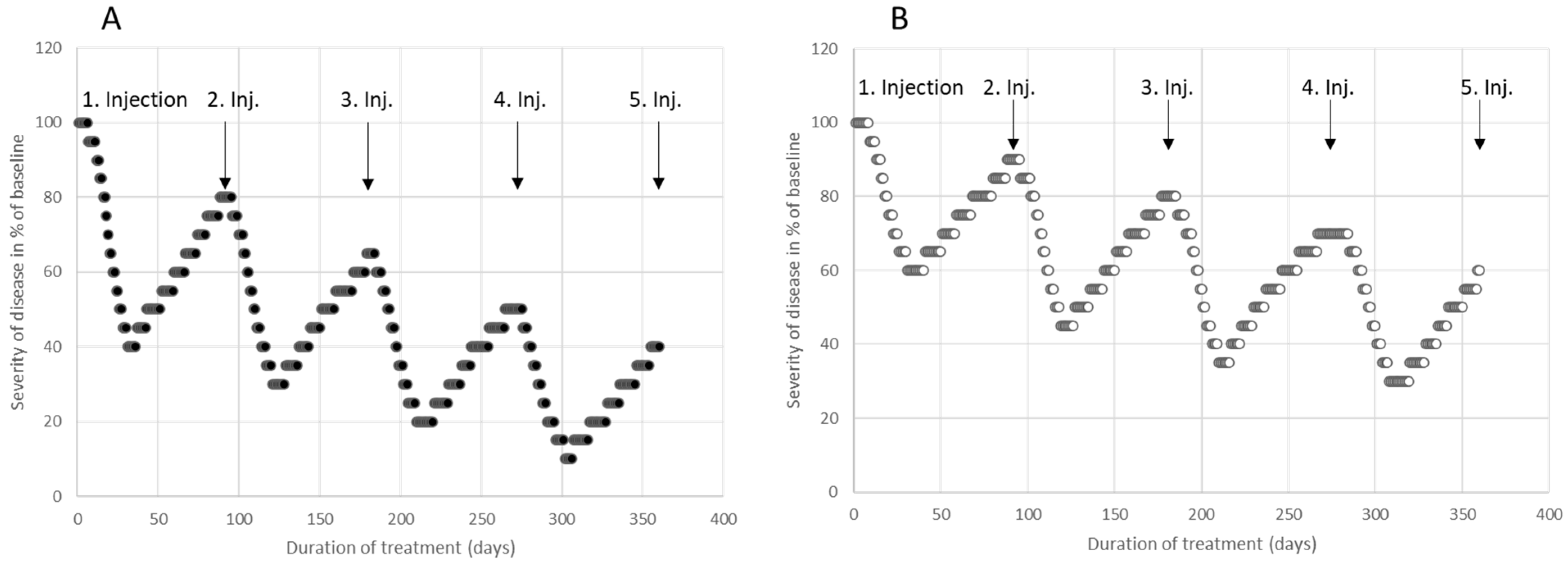
2.2. Examples of Self-Assessment of Four Consecutive BoNT Injections
2.3. Simulation of the Clinical Effect of Consecutive BoNT Injections
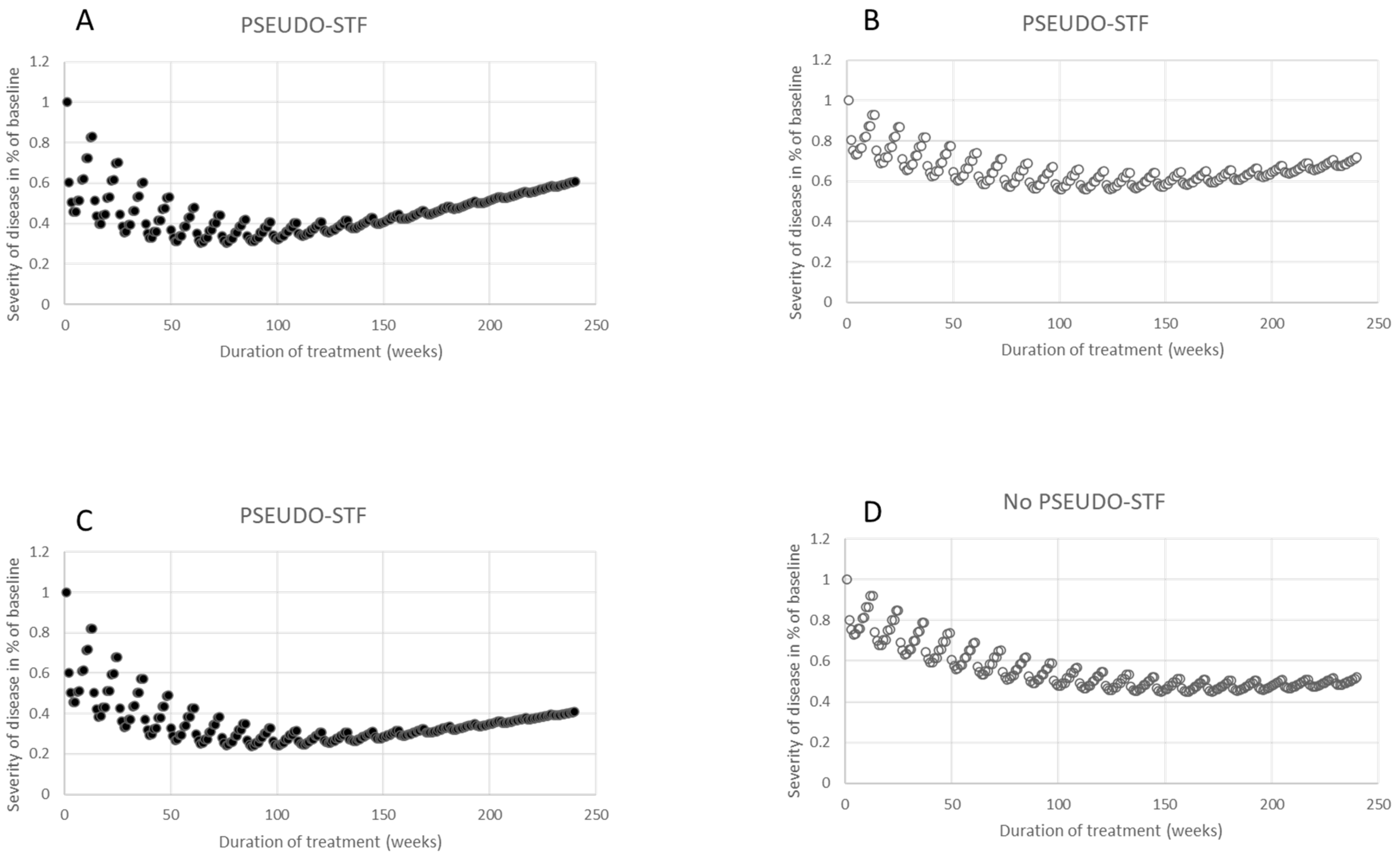
2.4. Simulation of Disease Progression and Effective BoNT Treatment
3. Discussion
3.1. General Aspects of the Choice of Method for the Mathematical Modeling of Therapeutic Outcomes
3.2. Increasing Improvement with Repetitive Injections of BoNT
3.3. Progression of the Disease despite Improvement during BoNT Therapy
3.4. PSEUDO-STF Results from a Good Clinical Response to BoNT and Disease Progression
4. Conclusions
5. Strengths and Limitations of the Simulation Study
6. Materials and Methods
6.1. Self-Assessment Curves
6.2. Simulation
- Twelve data pairs were selected which yielded a simple (x,y) curve, (SI1,p(w)), representing the 12 mean values of remaining severity during the first BoNT injection cycle for week w1, w2, 2026, w12 for a patient, p. Two examples are presented in Figure 1A,B. SI1,p(w1) = 0, since the improvement usually began at the end of the first week after BoNT injection. The value 1-SI1,p(w12) was called qp, and represents the residual improvement after the first injection cycle just before the next injection is performed. In Figure 1A, a SI1,p1(w) curve is presented, with qp1 = 0.2, and in Figure 1B, a SI1,p2(w) curve is displayed, with qp2 = 0.1.
- The simulated remaining severity curve resulted from an iteration process; for the calculation of the remaining disease severity, RSm,p(w), during the mth cycle and week wi, the value RSm−1,p(12) and the SI1,p(w) curve were used, which yielded the formula (I):RSm,p(wi) = RSm−1,p(w12) m− RSm−1,p(w12)*SI1,p(wi), with RS0,p(w12) = 1 and 1 ≤ i ≤ 12. This implies that RS1,p(w1) = 1, since SI1,p(w1) = 0, and that RS1,p(w12) = 1 − q. The value of RSm,p(w12) was (1 − q)m (see the Results section). Therefore, the remaining severity, RSm,p(w12), after m injections can be easily calculated and only depends on q (see Figure 3A,B).
- Then, a linear function (PROm,p(w) curve) was selected, which characterizes the progression of the underlying disease for 240 weeks (=20 BoNT injection cycles = 4.6 years): PROm,p(wi) = 1 + WFp*(m*wi/240), with 1 ≤ m ≤ 20 and 1 ≤ i ≤ 12). WFp is a weighting factor which depends on the patient p.
- D.
- Finally the simulation of the overall clinical effect (CLEFm,p(w) curve) results from the sum of the remaining severity, RSm,p(w), and the progression of the disease, PROm,p(w):CLEFm,p(wi) = RSm,p(wi) + PROm,p(wi) with 1 ≤ m ≤ 20 and w1 ≤ wi ≤ w12.
6.3. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Montecucco, C.; Schiavo, G.; Rossetto, O. The mechanism of action of tetanus and botulinum neurotoxins. Arch. Toxicol. Suppl. 1996, 18, 342–354. [Google Scholar]
- Rossetto, O.; Montecucco, C. Tables of Toxicity of Botulinum and Tetanus Neurotoxins. Toxins 2019, 11, 686. [Google Scholar] [CrossRef]
- Popoff, M.R. Bacterial Toxins, Current Perspectives. Toxins 2020, 12, 570. [Google Scholar] [CrossRef]
- Dong, M.; Masuyer, G.; Stenmark, P. Botulinum and Tetanus Neurotoxins. Annu. Rev. Biochem. 2019, 88, 811–837. [Google Scholar] [CrossRef] [PubMed]
- Arnon, S.S.; Schechter, R.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Hauer, J.; Layton, M.; et al. Botulinum toxin as a biological weapon: Medical and public health management. JAMA 2001, 285, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Botox® U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/103000s5310lbl.pdf (accessed on 23 April 2020).
- Dysport® U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125274s115lbl.pdf (accessed on 23 April 2020).
- Xeomin® U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125360s074lbl.pdf (accessed on 23 April 2020).
- Myobloc® U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/103846s5190lbl.pdf (accessed on 23 April 2020).
- Frevert, J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R&D 2015, 15, 1–9. [Google Scholar]
- Frevert, J.; Dressler, D. Complexing Proteins in Botulinum Toxin Type A Drugs: A Help or a Hindrance? Biologics 2010, 4, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Yokota, K.; Arimitsu, H.; Hwang, H.J.; Sakaguchi, Y.; Cui, J.; Takeshi, K.; Watanabe, T.; Ohyama, T.; Oguma, K. Production of Anti-Neurotoxin Antibody Is Enhanced by Two Subcomponents, HA1 and HA3b, of Clostridium Botulinum Type B 16S Toxin-Haemagglutinin. Microbiology 2005, 151, 3739–3747. [Google Scholar] [CrossRef]
- Kukreja, R.; Chang, T.W.; Cai, S.; Lindo, P.; Riding, S.; Zhou, Y.; Ravichandran, E.; Singh, B.R. Immunological Characterization of the Subunits of Type A Botulinum Neurotoxin and Different Components of Its Associated Proteins. Toxicon 2009, 53, 616–624. [Google Scholar] [CrossRef]
- Hefter, H.; Rosenthal, D.; Jansen, A.; Brauns, R.; Ürer, B.; Bigalke, H.; Hartung, H.P.; Meuth, S.G.; Lee, J.I.; Albrecht, P.; et al. Significantly lower antigenicity of incobotulinumtoxin than abo- or onabotulinumtoxin. J. Neurol. 2023, 270, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Botulinum toxin: State of the art. Mov. Disord. 2017, 32, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Van Kooyk, Y. C-type lectins on dendritic cells: Key modulators for the induction of immune responses. Biochem. Soc. Trans. 2008, 36, 1478–1481. [Google Scholar] [CrossRef]
- Atassi, M.Z.; Dolimbek, B.Z.; Jankovic, J.; Steward, L.E.; Aoki, K.R. Regions of Botulinum Neurotoxin a Light Chain Recognized by Human Anti-Toxin Antibodies from Cervical Dystonia Patients Immunoresistant to Toxin Treatment. The Antigenic Structure of the Active Toxin Recognized by Human Antibodies. Immunobiology 2011, 216, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Deitiker, P.; Jankovic, J.; Atassi, M.Z. The Regions on the Light Chain of Botulinum Neurotoxin Type a Recognized by T Cells from Toxin-Treated Cervical Dystonia Patients. The Complete Human T-Cell Recognition Map of the Toxin Molecule. Immunol. Investig. 2018, 47, 18–39. [Google Scholar] [CrossRef] [PubMed]
- Greene, P.; Fahn, S.; Diamond, B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov. Disord. 1994, 9, 213–217. [Google Scholar] [CrossRef]
- Dressler, D.; Hallet, M. Immunological aspects of Botox®, Dysport® and MyoBloc/NeuroBloc®. Eur. J. Neurol. 2006, 13, 11–15. [Google Scholar] [CrossRef]
- Dressler, D. Clinical presentation and management of antibody-induced failure of botulinum toxin therapy. Mov. Disord. 2004, 19, S92–S100. [Google Scholar] [CrossRef]
- Bellows, S.; Jankovic, J. Immunogenicity associated with botulinum toxin treatment. Toxins 2019, 11, 491. [Google Scholar] [CrossRef]
- Jinnah, H.A.; Goodmann, E.; Rosen, A.R.; Evatt, M.; Freeman, A.; Factor, S. Botulinum toxin treatment failures in cervical dystonia: Causes, management, and outcomes. J. Neurol. 2016, 263, 1188–1194. [Google Scholar] [CrossRef]
- Jinnah, H.A.; Comella, C.L.; Perlmutter, J.; Lungu, C.; Hallett, M. Longitudinal studies of botulinum toxin in cervical dystonia: Why do patients discontinue therapy? Toxicon 2018, 147, 89–95. [Google Scholar] [CrossRef]
- Walter, U.; Mühlenhoff, C.; Benecke, R.; Dressler, D.; Mix, E.; Alt, J.; Wittstock, M.; Dudesek, A.; Storch, A.; Kamm, C. Frequency and risk factors of antibody-induced secondary failure of botulinum neurotoxin therapy. Neurology 2020, 94, e2109–e2120. [Google Scholar] [CrossRef] [PubMed]
- Kranz, G.; Sycha, T.; Voller, B.; Kranz, G.S.; Schnider, P.; Auff, E. Neutralizing antibodies in dystonic patients who still respond well to botulinum toxin type A. Neurology 2008, 70, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Bigalke, H.; Rummel, A. Botulinum neurotoxins: Qualitative and Quantitative analysis using the mouse phrenic nerve hemi-diaphragm assay (MPN). Toxins 2015, 7, 4895–4905. [Google Scholar] [CrossRef]
- Lange, O.; Bigalke, H.; Dengler, R.; Wegner, F.; deGroot, M.; Wohlfarth, K. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: Much ado about nothing? Clin. Neuropharmacol. 2009, 32, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Leodori, G.; Fernandes, R.M.; Bhidayasiri, R.; Marti, M.J.; Colosimo, C.; Ferreira, J.J. Neutralizing Antibody and Botulinum Toxin Therapy: A Systematic Review and Meta-Analysis. Neurotox. Res. 2016, 29, 105–117. [Google Scholar] [CrossRef]
- Hefter, H.; Schomaecker, I.; Schomaecker, M.; Ürer, B.; Brauns, R.; Rosenthal, D.; Albrecht, P.; Samadzadeh, S. Lessons about Botulinum Toxin A Therapy from Cervical Dystonia Patients Drawing the Course of Disease: A Pilot Study. Toxins 2023, 15, 431. [Google Scholar] [CrossRef]
- Meares, R. Natural history of spasmodic torticollis, and effect of surgery. Lancet 1971, 17, 149–151. [Google Scholar] [CrossRef]
- Kessler, K.R.; Skutta, M.; Benecke, R. Long-term treatment of cervical dystonia with botulinum toxin A: Efficacy, safety, and antibody frequency. J. Neurol. 1999, 246, 265–274. [Google Scholar] [CrossRef]
- Hefter, H.; Rosenthal, D.; Moll, M. High botulinum toxin-neutralizing antibody prevalence under long-term cervical dystonia treatment. Mov. Disord. Clin. Pract. 2016, 3, 500–506. [Google Scholar] [CrossRef]
- Albrecht, P.; Jansen, A.; Lee, J.I.; Moll, M.; Ringelstein, M.; Rosenthal, D.; Bigalke, H.; Aktas, O.; Hartung, H.P.; Hefter, H. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology 2019, 92, e48–e54. [Google Scholar] [CrossRef]
- Benecke, R.; Moore, P.; Dressler, D.; Naumann, M. Cervical and axial dystonia. In Handbook of Botulinum Toxin Treatment; Wiley-Blackwell: Hoboken, NJ, USA, 2003; pp. 158–191. [Google Scholar]
- Hefter, H.; Schoemaeker, I.; Schomaecker, M.; Hefter, H. Disease progression of idiopathic cervical dystonia in spite of improvement after botulinum toxin therapy. Front. Neurol. 2020, 11, 588395. [Google Scholar] [CrossRef] [PubMed]
- Greene, P.; Kang, U.J.; Fahn, S. Spread of symptoms in idiopathic torsion dystonia. Mov. Disord. 1995, 10, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.A.; Jinnah, H.A.; Espay, A.J.; Klein, C.; Brüggemann, N.; Barbano, R.L.; Malaty, I.A.; Rodriguez, R.L.; Vidailhet, M.; Roze, E.; et al. Clinical and demographic characteristics related to onset site and spread of cervical dystonia. Mov. Disord. 2016, 31, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Mejia, N.I.; Vuong, K.D.; Jankovic, J. Long-term botulinum toxin efficacy, safety, and immunogenicity. Mov. Disord. 2005, 20, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Baker, M.R.; Chatterjee, S.; Kumar, H. Botulinum Toxin: An Update on Pharmacology and Newer Products in Development. Toxins 2021, 13, 58. [Google Scholar] [CrossRef]
- Turkoglu, A.; Yucesoy, C.A. Simulation of effects of botulinum toxin on muscular mechanics in time course of treatment based on adverse extracellular matrix adaptation. J. Biomech. 2016, 49, 1192–1198. [Google Scholar] [CrossRef]
- Hefter, H.; Moll, M.; Samadzadeh, S. Complete relief of a forearm complex regional pain syndrome after repetitive injections of high doses of incobotulinumtoxin A. Toxins, 2023; currently under review. [Google Scholar]
- Samadzadeh, S.; Brauns, R.; Rosenthal, D.; Hefter, H. The impact of SARS-CoV-2 pandemic lockdown on a botulinum toxin outpatient clinic in Germany. Toxins 2021, 13, 101. [Google Scholar] [CrossRef]
- Isaias, I.U.; Volkmann, J.; Kupsch, A.; Burgunder, J.-M.; Ostrem, J.L.; Alterman, R.L.; Mehdorn, H.M.; Schönecker, T.; Krauss, J.K.; Starr, P.; et al. Factors predicting protracted improvement after pallidal DBS for primary dystonia: The role of age and disease duration. J. Neurol. 2011, 258, 1469–1476. [Google Scholar] [CrossRef]
- Volkmann, J.; Mueller, J.; Deuschl, G.; Kühn, A.A.; Krauss, J.K.; Poewe, W.; Timmermann, L.; Falk, D.; Kupsch, A.; Kivi, A.; et al. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: A randomized, sham-controlled trial. Lancet Neurol. 2014, 13, 875–884. [Google Scholar] [CrossRef]
- Tsui, J.K.; Eisen, A.; Stoessl, A.J.; Calne, S.; Calne, D.B. Double-blind study of botulinum toxin in spasmodic torticollis. Lancet 1986, 2, 245–247. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefter, H.; Rosenthal, D.; Samadzadeh, S. “Pseudo”-Secondary Treatment Failure Explained via Disease Progression and Effective Botulinum Toxin Therapy: A Pilot Simulation Study. Toxins 2023, 15, 618. https://doi.org/10.3390/toxins15100618
Hefter H, Rosenthal D, Samadzadeh S. “Pseudo”-Secondary Treatment Failure Explained via Disease Progression and Effective Botulinum Toxin Therapy: A Pilot Simulation Study. Toxins. 2023; 15(10):618. https://doi.org/10.3390/toxins15100618
Chicago/Turabian StyleHefter, Harald, Dietmar Rosenthal, and Sara Samadzadeh. 2023. "“Pseudo”-Secondary Treatment Failure Explained via Disease Progression and Effective Botulinum Toxin Therapy: A Pilot Simulation Study" Toxins 15, no. 10: 618. https://doi.org/10.3390/toxins15100618
APA StyleHefter, H., Rosenthal, D., & Samadzadeh, S. (2023). “Pseudo”-Secondary Treatment Failure Explained via Disease Progression and Effective Botulinum Toxin Therapy: A Pilot Simulation Study. Toxins, 15(10), 618. https://doi.org/10.3390/toxins15100618




