Cold Finger: Raynaud Phenomenon Following Snakebite Envenoming by Nikolsky’s Viper (Vipera berus nikolskii)
Abstract
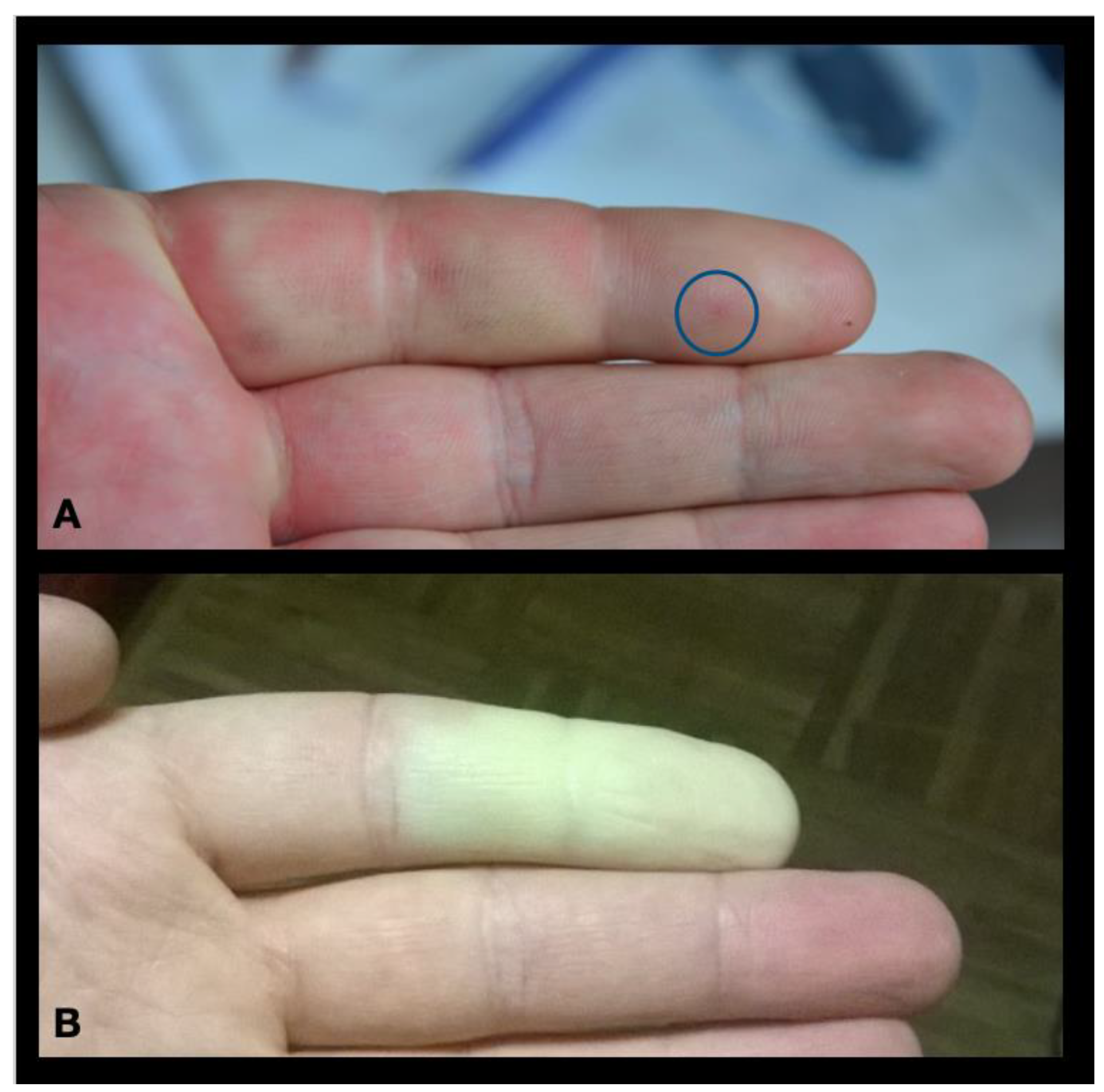
:1. Case Report
2. Discussion
2.1. Raynaud Phenomenon
2.2. Venom-Induced Raynaud Phenomenon in English-Language Medical Literature
2.3. Vipera berus Venom
2.4. Raynaud Phenomenon, Snakebite and Occupational Medicine
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Raynaud, A.G.M. De Asclepiade Bithyno, Medico Ac Philosopho, Thesim Proponehat Facultati Litterarum Parisiensi; Didier: Paris, France, 1862. [Google Scholar]
- Wigley, F.M.; Flavahan, N.A. Raynaud’s Phenomenon. N. Engl. J. Med. 2016, 375, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Flavahan, N.A. A Vascular Mechanistic Approach to Understanding Raynaud Phenomenon. Nat. Rev. Rheumatol. 2015, 11, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, S.A.; Ratnatunga, N. Severe Systemic Effects of Merrem’s Hump-Nosed Viper Bite. Ceylon Med. J. 1999, 44, 169–170. [Google Scholar] [PubMed]
- Sathyanathan, V.P.; Mathew, M.T. Raynaud’s Phenomenon and Gangrene Following Snake Envenomation. J. Assoc. Physicians India 1993, 41, 122–123. [Google Scholar] [PubMed]
- Binnetoglu, F.K.; Kizildag, B.; Topaloglu, N.; Kasapcopur, O. Severe Digital Necrosis in a 4-Year-Old Boy: Primary Raynaud’s or Jellyfish Sting. Case Rep. 2013, 2013, bcr2013201478. [Google Scholar] [CrossRef] [PubMed]
- El Khatib, H.A.; Al Basti, H.B. Finger Ischemia Following a Jelly Fish Sting: A Surgical Approach. Qatar Med. J. 2000, 2000. [Google Scholar] [CrossRef]
- Tamkun, M.M.; Hessinger, D.A. Isolation and Partial Characterization of a Hemolytic and Toxic Protein from the Nematocyst Venom of the Portuguese Man-of-War, Physalia Physalis. Biochim. Biophys. Acta (BBA) Protein Struct. 1981, 667, 87–98. [Google Scholar] [CrossRef]
- Mayser, P.; Dreyer, F.; Repp, H. Persistent Skin Reaction and Raynaud Phenomenon after a Sting by Echiichthys Draco (Great Weever Fish). Hautarzt 2003, 54, 633–637. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, G. Adverse Events Associated with the Clinical Use of Bee Venom: A Review. Toxins 2022, 14, 562. [Google Scholar] [CrossRef]
- Herrick, A.L. Raynaud’s Phenomenon. J. Scleroderma Relat. Disord. 2019, 4, 89–101. [Google Scholar] [CrossRef]
- Smith, V.; Herrick, A.L.; Ingegnoli, F.; Damjanov, N.; De Angelis, R.; Denton, C.P.; Distler, O.; Espejo, K.; Foeldvari, I.; Frech, T.; et al. Standardisation of Nailfold Capillaroscopy for the Assessment of Patients with Raynaud’s Phenomenon and Systemic Sclerosis. Autoimmun Rev. 2020, 19, 102458. [Google Scholar] [CrossRef] [PubMed]
- Greaney, J.L.; Alexander, L.M.; Kenney, W.L. Sympathetic Control of Reflex Cutaneous Vasoconstriction in Human Aging. J. Appl. Physiol. 2015, 119, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, I.; Nawaz, Y.; Nawaz, E.; Manan, M.R.; Mahmood, A. Raynaud’s Phenomenon: Reviewing the Pathophysiology and Management Strategies. Cureus 2022, 14, e21681. [Google Scholar] [CrossRef] [PubMed]
- Fardoun, M.M.; Nassif, J.; Issa, K.; Baydoun, E.; Eid, A.H. Raynaud’s Phenomenon: A Brief Review of the Underlying Mechanisms. Front. Pharmacol. 2016, 7, 438. [Google Scholar] [CrossRef] [PubMed]
- Zinenko, O.; Tovstukha, I.; Korniyenko, Y. PLA2 Inhibitor Varespladib as an Alternative to the Antivenom Treatment for Bites from Nikolsky’s Viper Vipera Berus Nikolskii. Toxins 2020, 12, 356. [Google Scholar] [CrossRef]
- Nițescu, G.V.; Ulmeanu, C.E.; Crăciun, M.-D.; Ciucă, A.M.; Ulici, A.; Ghira, I.; Lonati, D. Neurotoxicity and Other Clinical Manifestations of a Common European Adder (Vipera berus) Bite in Romania. Toxins 2022, 14, 500. [Google Scholar] [CrossRef]
- Logonder, U.; Kriaj, I.; Rowan, E.G.; Harris, J.B. Neurotoxicity of Ammodytoxin A in the Envenoming Bites of Vipera Ammodytes Ammodytes. J. Neuropathol. Exp. Neurol. 2008, 67, 1011–1019. [Google Scholar] [CrossRef]
- Weinelt, W.; Sattler, R.W.; Mebs, D. Persistent Paresis of the Facialis Muscle after European Adder (Vipera Berus) Bite on the Forehead. Toxicon 2002, 40, 1627–1629. [Google Scholar] [CrossRef]
- Taylor, M.S. Cold Weather Injuries during Peacetime Military Training. Mil. Med. 1992, 157, 602–604. [Google Scholar] [CrossRef]
- Medical Standards for Military Service: Appointment, Enlistment, or Induction. DoD Instruction 6130.03, Volume 1 DoDI 6130.03-V1, March 30, 2018 Change 4, November 16, 2022. Available online: https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF?ver=7fhqacc0jGX_R9_1iexudA%3D%3D (accessed on 13 September 2023).
- Cheok, L.J.; Ooi, C.K. Post-Traumatic Raynaud’s Phenomenon: A Case Report. J. Emerg. Med. 2017, 52, e237–e238. [Google Scholar] [CrossRef]
- Frech, T.M.; Murtaugh, M.A.; Amuan, M.; Pugh, M.J. The Frequency of Raynaud’s Phenomenon, Very Early Diagnosis of Systemic Sclerosis, and Systemic Sclerosis in a Large Veteran Health Administration Database. BMC Rheumatol. 2021, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Pauling, J.D.; Hughes, M.; Pope, J.E. Raynaud’s Phenomenon-an Update on Diagnosis, Classification and Management. Clin. Rheumatol. 2019, 38, 3317–3330. [Google Scholar] [CrossRef] [PubMed]
- Abouyannis, M.; Esmail, H.; Hamaluba, M.; Ngama, M.; Mwangudzah, H.; Mumba, N.; Yeri, B.K.; Mwalukore, S.; Alphan, H.J.; Aggarwal, D.; et al. A Global Core Outcome Measurement Set for Snakebite Clinical Trials. Lancet Glob. Health 2023, 11, e296–e300. [Google Scholar] [CrossRef] [PubMed]
- Tupetz, A.; Phillips, A.J.; Kelly, P.E.; Barcenas, L.K.; Lavonas, E.J.; Vissoci, J.R.N.; Gerardo, C.J. Contextualizing the Impact of Snakebite Envenoming on Patients: A Qualitative Content Analysis of Patient-Specific Functional Scale Activities Using the International Classification of Functioning, Disability and Health. Int. J. Environ. Res. Public Health 2021, 18, 9608. [Google Scholar] [CrossRef] [PubMed]
- Gerardo, C.J.; Vissoci, J.R.N.; Evans, C.S.; Simel, D.L.; Lavonas, E.J. Does This Patient Have a Severe Snake Envenomation? JAMA Surg. 2019, 154, 346. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Vaiyapuri, R.; Ashokan, R.; Ramasamy, K.; Nattamaisundar, K.; Jeyaraj, A.; Chandran, V.; Gajjeraman, P.; Baksh, M.F.; Gibbins, J.M.; et al. Snakebite and Its Socio-Economic Impact on the Rural Population of Tamil Nadu, India. PLoS ONE 2013, 8, e80090. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, S.; Kallakuri, S.; Kaur, A.; Devarapalli, S.; Daniel, M. Mental Health Conditions after Snakebite: A Scoping Review. BMJ Glob. Health 2020, 5, e004131. [Google Scholar] [CrossRef]
- Kasturiratne, A.; Lalloo, D.G.; Janaka de Silva, H. Chronic Health Effects and Cost of Snakebite. Toxicon X 2021, 9–10, 100074. [Google Scholar] [CrossRef] [PubMed]
- Waiddyanatha, S.; Silva, A.; Weerakoon, K.; Siribaddana, S.; Isbister, G.K. Long-Term Health Effects Perceived by Snakebite Patients in Rural Sri Lanka: A Cohort Study. PLoS Negl. Trop. Dis. 2022, 16, e0010723. [Google Scholar] [CrossRef]
- Kowtoniuk, R.A.; Liu, Y.E.; Jeter, J.P. Cutaneous Cold Weather Injuries in the US Military. Cutis 2021, 108, 181–184. [Google Scholar] [CrossRef]


| Venom | Comments |
|---|---|
| Cnidaria [6,7] | Physalia sp. (Man O’ War) venom contains the hemolytic protein physalitoxin a [8], which composes 28% of its protein content. It is thought to be the main contributor to the venom’s lethality and hemolytic activity. |
| Fish [9] | The lesser and greater weever fish (Echiichthys draco) are venomous species primarily found in Atlantic and North-Sea waters with sporadic occurrence throughout the Mediterranean Sea. |
| Swarming Hymenoptera (Bees, wasps, ants) | Reported from therapeutic use of bee sting and reported fire ant envenoming [10]. |
| Snakebite? | Reported in association with acute embolic, necrotic or terminal events [4,5]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinenko, O.; Durkin, D.M.; Carter, R.W.; Ritter, B.; Lewin, M.R. Cold Finger: Raynaud Phenomenon Following Snakebite Envenoming by Nikolsky’s Viper (Vipera berus nikolskii). Toxins 2023, 15, 598. https://doi.org/10.3390/toxins15100598
Zinenko O, Durkin DM, Carter RW, Ritter B, Lewin MR. Cold Finger: Raynaud Phenomenon Following Snakebite Envenoming by Nikolsky’s Viper (Vipera berus nikolskii). Toxins. 2023; 15(10):598. https://doi.org/10.3390/toxins15100598
Chicago/Turabian StyleZinenko, Oleksandr, Daniela M. Durkin, Rebecca W. Carter, Brandi Ritter, and Matthew R. Lewin. 2023. "Cold Finger: Raynaud Phenomenon Following Snakebite Envenoming by Nikolsky’s Viper (Vipera berus nikolskii)" Toxins 15, no. 10: 598. https://doi.org/10.3390/toxins15100598
APA StyleZinenko, O., Durkin, D. M., Carter, R. W., Ritter, B., & Lewin, M. R. (2023). Cold Finger: Raynaud Phenomenon Following Snakebite Envenoming by Nikolsky’s Viper (Vipera berus nikolskii). Toxins, 15(10), 598. https://doi.org/10.3390/toxins15100598





