Canadian Physicians’ Use of Intramuscular Botulinum Toxin Injections for Shoulder Spasticity: A National Cross-Sectional Survey
Abstract
1. Introduction
2. Results
2.1. Participant Criteria
2.2. Clinicians’ Demographics
2.3. Patient Profiles and Interventions
2.4. Intervention Techniques and Timing
2.5. Dosing
2.6. Patient Outcomes
2.7. Barriers to Treatment
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Design
5.2. Survey Design
5.3. CANOSC
5.4. Participant Recruitment
5.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Pandyan, A.D.; Gregoric, M.; Barnes, M.P.; Wood, D.; Van Wijck, F.; Burridge, J.; Hermens, H.; Johnson, G.R. Spasticity: Clinical perceptions, neurological realities and meaningful measurement. Disabil. Rehabil. 2005, 27, 2–6. [Google Scholar] [CrossRef]
- Wissel, J.; Schelosky, L.D.; Scott, J.; Christe, W.; Faiss, J.H.; Mueller, J. Early development of spasticity following stroke: A prospective, observational trial. J. Neurol. 2010, 257, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Hefter, H.; Jost, W.H.; Reissig, A.; Zakine, B.; Bakheit, A.M.; Wissel, J. Classification of posture in poststroke upper limb spasticity: A potential decision tool for botulinum toxin A treatment? Int. J. Rehabil. Research. Int. Z. Rehabilitationsforschung. Rev. Int. Rech. Readapt. 2012, 35, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Teasell, R.; Foley, N.; Pereira, S.; Sequeira, K.; Miller, T. Evidence to practice: Botulinum toxin in the treatment of spasticity post stroke. Top. Stroke Rehabil. 2012, 19, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.P.; Pinto, D.; Gorayeb, M.; Jacinto, J. Analysis of a 15-years’ experience in including shoulder muscles, when treating upper-limb spasticity post-stroke with botulinum toxin type A. Top. Stroke Rehabil. 2018, 25, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Doussoulin, A.; Rivas, C.; Bacco, J.; Sepúlveda, P.; Carvallo, G.; Gajardo, C.; Soto, A.; Rivas, R. Prevalence of Spasticity and Postural Patterns in the Upper Extremity Post Stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2020, 29, 105253. [Google Scholar] [CrossRef]
- Teasell, R.; Salbach, N.M.; Foley, N.; Mountain, A.; Cameron, J.I.; Jong A de Acerra, N.E.; Bastasi, D.; Carter, S.L.; Fung, J.; Halabi, M.-L.; et al. Canadian Stroke Best Practice Recommendations: Rehabilitation, Recovery, and Community Participation following Stroke. Part One: Rehabilitation and Recovery Following Stroke; 6th Edition Update 2019. Int. J. Stroke 2020, 15, 763–788. [Google Scholar] [CrossRef]
- Jacinto, J.; Camões-Barbosa, A.; Carda, S.; Hoad, D.; Wissel, J. A practical guide to botulinum neurotoxin treatment of shoulder spasticity 1: Anatomy, physiology, and goal setting. Front. Neurol. 2022, 13, 1004629. [Google Scholar] [CrossRef]
- Van Kuijk, A.A.; Geurts, A.C.; Bevaart, B.J.; van Limbeek, J. Treatment of upper extremity spasticity in stroke patients by focal neuronal or neuromuscular blockade: A systematic review of talhe literature. J. Rehabil. Med. 2002, 34, 51–61. [Google Scholar] [CrossRef]
- Teasell, R.; Salbach, N.; Acerra, N.; Bastasi, D.; Carter, S.; Fung, J.; Halabi, M.; Harris, J.; Kim, E.; Noland, A.; et al. 5.2. Range of Motion and Spasticity in the Shoulder, Arm and Hand. Canadian Stroke Best Practices. 19 December 2019. Available online: https://www.strokebestpractices.ca/recommendations/stroke-rehabilitation/range-of-motion-and-spasticity-in-the-shoulder-arm-and-hand (accessed on 23 August 2022).
- Kong, H.; Neo, J.; Chua, K.S. A randomized controlled study of botulinum toxin A in the treatment of hemiplegic shoulder pain associated with spasticity. Clin. Rehabil. 2016, 21, 28–35. [Google Scholar] [CrossRef]
- Marciniak, C.M.; Harvey, R.L.; Gagnon, C.M.; Duraski, S.A.; Denby, F.A.; McCarty, S.; Bravi, L.A.; Polo, K.M.; Fierstein, K.M. Does botulinum toxin type A decrease pain and lessen disability in hemiplegic survivors of stroke with shoulder pain and spasticity?: A randomized, double-blind, placebo-controlled trial. Am. J. Phys. Med. Rehabil. 2012, 91, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Jia, L. Ultrasound-Guided BoNT-A (Botulinum Toxin A) Injection Into the Subscapularis for Hemiplegic Shoulder Pain: A Randomized, Double-Blind, Placebo-Controlled Trial. Stroke 2021, 52, 3759–3767. [Google Scholar] [CrossRef] [PubMed]
- Yelnik, A.P.; Colle, F.M.; Bonan, I.V.; Vicaut, E. Treatment of shoulder pain in spastic hemiplegia by reducing spasticity of the subscapular muscle: A randomised, double blind, placebo controlled study of botulinum toxin A. J. Neurol. Neurosurg. Psychiatry 2007, 78, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Kassam, A.; Phadke, C.; Ismail, F.; Boulias, C. Physician Preferences for Botulinum Toxin Injections in Anticoagulated Patients with Spasticity. Can. J. Neurol. Sci./J. Can. Sci. Neurol. 2016, 43, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.J.; Connell, L.A. A survey of the current practice of intramuscular Botulinum toxin injections for hemiplegic shoulder pain in the UK. Disabil. Rehabil. 2019, 41, 720–726. [Google Scholar] [CrossRef]
- Nalysnyk, L.; Papapetropoulos, S.; Rotella, P.; Simeone, J.C.; Alter, K.E.; Esquenazi, A. OnabotulinumtoxinA muscle injection patterns in adult spasticity: A systematic literature review. BMC Neurol. 2013, 13, 118. [Google Scholar] [CrossRef]
- Gomes, A.; Mello, F.F.; Cocicov Neto, J.; Benedeti, M.C.; Modolo, L.; Riberto, M. Can the positions of the spastic upper limb in stroke survivors help muscle choice for botulinum toxin injections? Arq. Neuro-Psiquiatr. 2019, 77, 568–573. [Google Scholar] [CrossRef]
- Jost, W.H.; Hefter, H.; Reissig, A.; Kollewe, K.; Wissel, J. Efficacy and safety of botulinum toxin type A (Dysport) for the treatment of post-stroke arm spasticity: Results of the German-Austrian open-label post-marketing surveillance prospective study. J. Neurol. Sci. 2014, 337, 86–90. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Wissel, J.; Bensmail, D.; Scheschonka, A.; Flatau-Baqué, B.; Simon, O.; Althaus, M.; Simpson, D.M. Post hoc analysis of the improvement in shoulder spasticity and safety observed following treatment with incobotulinumtoxinA. J. Rehabil. Med. 2020, 52, jrm00028. [Google Scholar] [CrossRef]
- Chan, A.K.; Finlayson, H.; Mills, P.B. Does the method of botulinum neurotoxin injection for limb spasticity affect outcomes? A systematic review. Clin. Rehabil. 2017, 31, 713–721. [Google Scholar] [CrossRef]
- Picelli, A.; Tamburin, S.; Bonetti, P.; Fontana, C.; Barausse, M.; Dambruoso, F.; Gajofatto, F.; Santilli, V.; Smania, N. Botulinum toxin type A injection into the gastrocnemius muscle for spastic equinus in adults with stroke: A randomized controlled trial comparing manual needle placement, electrical stimulation and ultrasonography-guided injection techniques. Am. J. Phys. Med. Rehabil. 2012, 91, 957–964. [Google Scholar] [CrossRef]
- Liang, K.E.; Ngo, P.V.; Winston, P. Access to Focal Spasticity Care: A Cross Canada Survey of Physiatrists. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2020, 47, 834–838. [Google Scholar] [CrossRef]
- Choi, J.G.; Shin, J.H.; Kim, B.R. Botulinum Toxin A Injection into the Subscapularis Muscle to Treat Intractable Hemiplegic Shoulder Pain. Ann. Rehabil. Med. 2016, 40, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Gonnade, N.; Lokhande, V.; Ajij, M.; Gaur, A.; Shukla, K. Phenol Versus Botulinum Toxin A Injection in Ambulatory Cerebral Palsy Spastic Diplegia: A Comparative Study. J. Pediatr. Neurosci. 2017, 12, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Woo, J.; Mas, M.F. Early Use of Phenol Neurolysis Likely Reduces the Total Amount of Botulinum Toxin in Management of Post-Stroke Spasticity. Front. Rehabil. Sci. 2021, 2, 729178. [Google Scholar] [CrossRef] [PubMed]
- Canadian Medical Association. Physical Medicine and Rehabilitation Profile-CMA. Physical Medicine and Rehabilitation Profile. December 2019. Available online: https://www.cma.ca/sites/default/files/2019-01/physical-med-rehab-e.pdf (accessed on 5 October 2022).
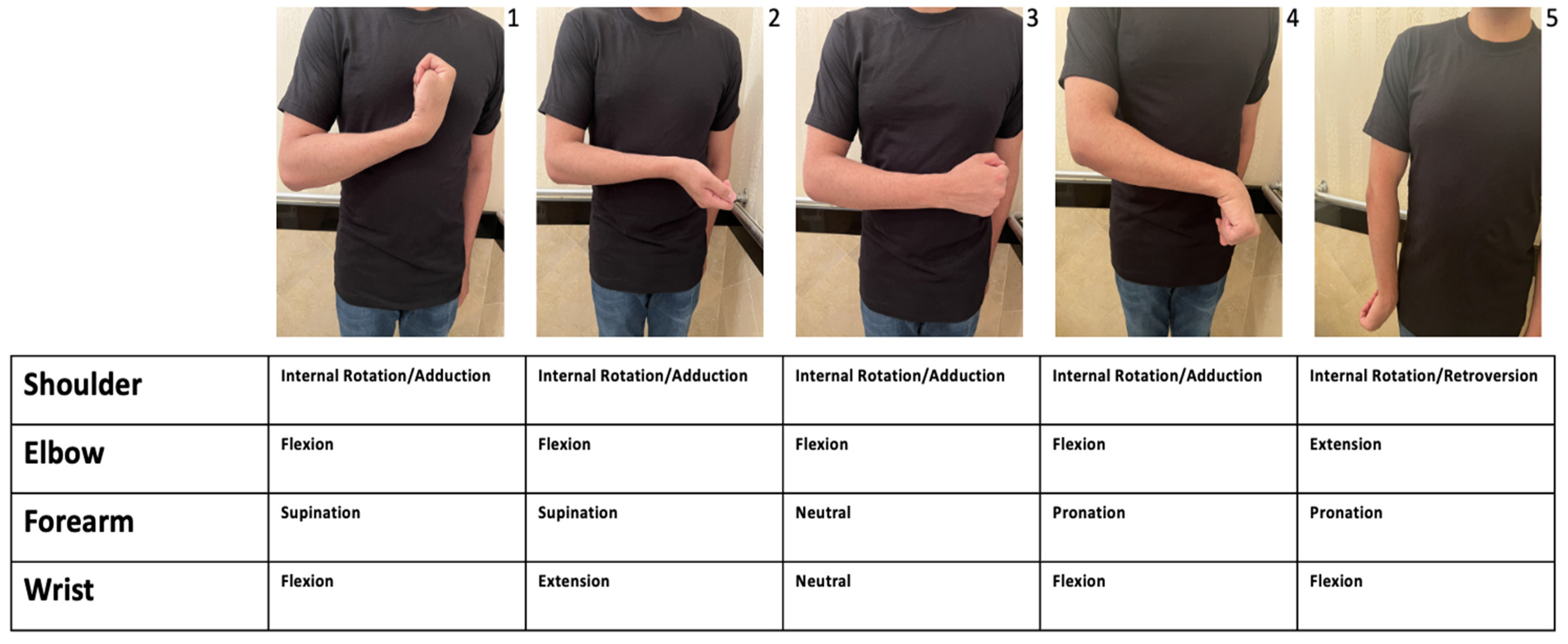


| % (n) | ||
|---|---|---|
| Location | British Columbia Ontario Quebec Alberta Saskatchewan Newfoundland and Labrador New Brunswick | 33.33% (18) 24.07% (13) 20.37% (11) 12.96% (7) 3.70% (2) 3.70% (2) 1.85% (1) |
| Speciality | PM&R Neurology PM&R and Pediatrics | 90.74% (49) 7.41% (4) 1.85% (1) |
| Setting for Spasticity Assessment and Management | Outpatient clinic Rehabilitation unit Acute care setting Other | 88.89% (48) 74.07% (40) 55.56% (30) 7.41% (4) |
| Number of Years Injecting BoNT-A | 0–9 10–19 20–29 >30 | 44.44% (24) 37.04% (20) 14.81% (8) 3.6% (2) |
| % (n) | ||
|---|---|---|
| Patient’s Treated | Adults only Both children and adults Children only | 63.67% (36) 31.48% (17) 1.85% (1) |
| Service provided to the spasticity population of: | Stroke | 96.30% (52) |
| Cerebral Palsy | 83.33% (45) | |
| Spinal Cord Injury | 81.48% (44) | |
| Traumatic Brain Injury | 79.63% (43) | |
| Multiple Sclerosis | 77.78% (42) | |
| Other and idiopathic (e.g., Hereditary spastic paraparesis, ALS) | 77.07% (40) | |
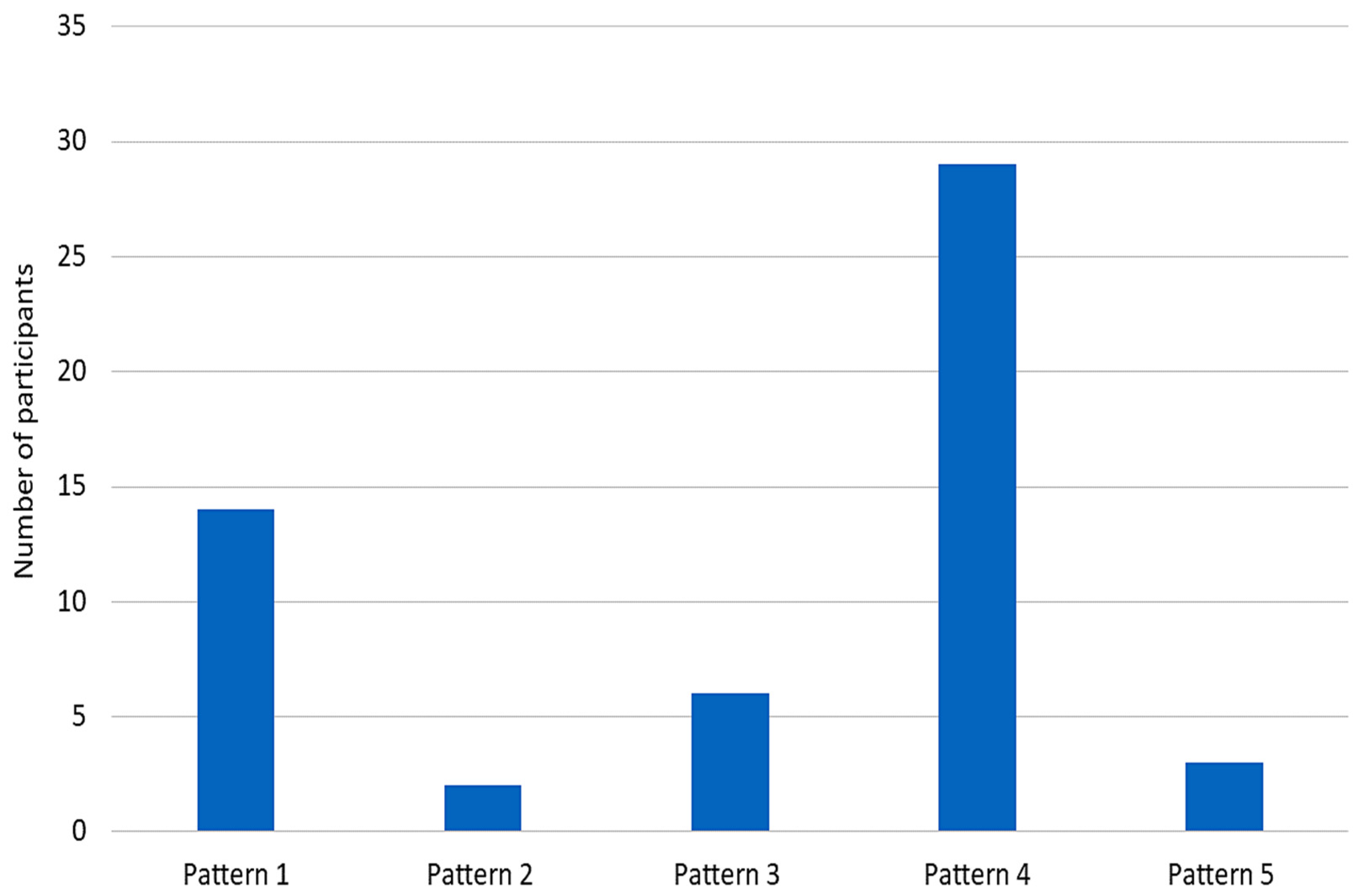
| When considering post stroke spasticity (PSS), how often do you observe that the shoulder is held in the internally rotated and adducted position? | 50–100% 0–50% | 92.59% (50) 7.41% (4) |
| When considering other causes of spasticity, including MS, SCI, and CP, how often do you observe that the shoulder is held in the internally rotated and adducted position? | 50–100% 0–50% | 53.70% (29) 46.29% (25) |
| When considering upper extremity PSS, how often do you identify problematic spasticity that requires management with BoNT-A in the shoulder as part of your plan? | Always/Often Sometimes Seldom | 81.48% (44) 16.67% (9) 1.85% (1) |
| When considering other causes of upper limb spasticity with problematic adduction and internal rotation, including MS, SCI, and CP, how often do you identify problematic spasticity that requires management with BoNT-A in the shoulder as part of your plan? | Always/Often Sometimes Seldom Never | 46.29% (25) 38.89% (21) 12.96% (7) 1.85% (1) |
| If you suspect contracture causing shoulder internal rotation and/or adduction, have you referred such patients for surgical release? | Always Often Sometimes Seldom Never Not Applicable | 1.92% (1) 9.62% (5) 15.38% (8) 15.38% (8) 44.23% (23) 13.46% (7) |
| % (n) | ||
|---|---|---|
| Do you inject the muscles of the shoulder girdle with BoNT-A for spasticity management? | Yes | 100% (54) |
| How often do you include shoulder muscles in your first round of management with BoNT-A if adduction and/or internal rotation spasticity is identified? | Always Often Sometimes Seldom | 25.93% (14) 53.70% (29) 16.67% (9) 3.70% (2) |
| What is the minimum Modified Ashworth Scale you will inject BoNT-A for the shoulder? | 0 1 1+ 2 3 | 1.92% (1) 5.77% (3) 57.69% (30) 23.08% (12) 11.54% (6) |
| What target muscle localization methods do you use for BoNT-A injection around the shoulder girdle? | Electromyography Ultrasound Electrical stimulation Anatomical landmarks only Other | 78.84% (41) 59.62% (31) 48.08% (25) 32.69% (17) 1.92% (1) |
| Do you ever grasp the wad (muscle belly) of the pectoralis muscles or latisimus dorsi in your hand when you inject to avoid going too deep? | Yes No | 88.46% (46) 11.53% (6) |
| List the number of lung punctures that have occurred with shoulder muscle injections that caused a pneumothorax for you personally? | 0 1 2 | 94.23% (49) 3.85% (2) 1.92% (1) |
| Do you use phenol or alcohol for shoulder adduction or internal rotation spasticity? | Yes No | 5.77% (3) 94.23% (49) |
| Which nerves do you target? | Lateral Pectoral Nerve Medial Pectoral Nerve Subscapular Nerve | 66.67% (2) 66.67% (2) 33.33% (1) |
| Botox Onabutulinum Toxin A | Dysport Abobotulinum Toxin A | Xeomin Incobotulinum Toxin A | |
|---|---|---|---|
| Pectoralis Major | 10–200 | 50–500 | 25–200 |
| Pectoralis Minor | 10–100 | 50–150 | 10–75 |
| Subscapularis | 15–150 | 50–400 | 25–150 |
| Latissimus Dorsi | 15–200 | 40–500 | 25–200 |
| Teres Major | 10–100 | 50–300 | 20–100 |
| Deltoid | 10–200 | 50–300 | 20–200 |
| % (n) | ||
|---|---|---|
| When you inject the shoulder muscles with BoNT-A for adduction and internal rotation, what are your goals? | Achieve a patient or caregiver goal such as using the Goal Attainment Scale | 96.15% (50) |
| Reduce pain | 94.23% (49) | |
| Increase range of motion | 86.53% (45) | |
| Reduce spasticity as measured with the Modified Ashworth Scale or similar | 44.23% (23) | |
| Other | 9.6% (5) |
| What Improved Outcomes Have You Observed to the Use of BoNT-A Injections on Patients with Shoulder Spasticity? |
|---|
| Improved pain, ROM and function of shoulder and arm. |
| Reduced pain, improved function, increased comfort, facilitated cares & reduced skin breakdown. |
| Better ease of dressing and hygiene. |
| Reduction in pain, increased range of motion active and passively & easier care for caregivers. |
| Increase ROM decrease pain ease motion for caregivers. |
| Able to dress self, decreased caregiver burden & less pain. |
| Pain reduction, improved passive participation in ADL & improved caregiver satisfaction. |
| Lots. All goals set in collaboration with patient/caregiver. Active and passive. |
| What Complications Have You Observed with the Use of BoNT-A Injections on Patients with Shoulder Spasticity? |
|---|
| None |
| Increased short term discomfort |
| Minimal |
| Weakness with reduced function |
| % (n) | ||
|---|---|---|
| What are barriers to the use of BoNT-A for patients with shoulder spasticity as compared to other upper extremity regions? | No barriers | 38.46% (20) |
| Financial—patient resources | 36.54% (19) | |
| Lack of interdisciplinary care | 26.92% (14) | |
| Patient does not want to have BoNT-A treatment | 17.31% (9) | |
| Financial—physician/clinic resources | 15.38% (8) | |
| Clinic not equipped with necessary equipment | 13.46% (7) | |
| Risk of adverse events | 11.54% (6) | |
| Clinician time constraints (e.g., clinical practice too busy) | 9.62% (5) | |
| Other | 9.62% (5) | |
| Off-Label | 5.77% (3) | |
| Lack of evidence in the literature | 3.85% (2) | |
| Lack of effectiveness from clinical experience | 0% (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassam, F.; Lim, B.; Afroz, S.; Boissonnault, È.; Reebye, R.; Finlayson, H.; Winston, P. Canadian Physicians’ Use of Intramuscular Botulinum Toxin Injections for Shoulder Spasticity: A National Cross-Sectional Survey. Toxins 2023, 15, 58. https://doi.org/10.3390/toxins15010058
Kassam F, Lim B, Afroz S, Boissonnault È, Reebye R, Finlayson H, Winston P. Canadian Physicians’ Use of Intramuscular Botulinum Toxin Injections for Shoulder Spasticity: A National Cross-Sectional Survey. Toxins. 2023; 15(1):58. https://doi.org/10.3390/toxins15010058
Chicago/Turabian StyleKassam, Farris, Brendan Lim, Sadia Afroz, Ève Boissonnault, Rajiv Reebye, Heather Finlayson, and Paul Winston. 2023. "Canadian Physicians’ Use of Intramuscular Botulinum Toxin Injections for Shoulder Spasticity: A National Cross-Sectional Survey" Toxins 15, no. 1: 58. https://doi.org/10.3390/toxins15010058
APA StyleKassam, F., Lim, B., Afroz, S., Boissonnault, È., Reebye, R., Finlayson, H., & Winston, P. (2023). Canadian Physicians’ Use of Intramuscular Botulinum Toxin Injections for Shoulder Spasticity: A National Cross-Sectional Survey. Toxins, 15(1), 58. https://doi.org/10.3390/toxins15010058





