Botulism in Spain: Epidemiology and Outcomes of Antitoxin Treatment, 1997–2019
Abstract
1. Introduction
2. Results
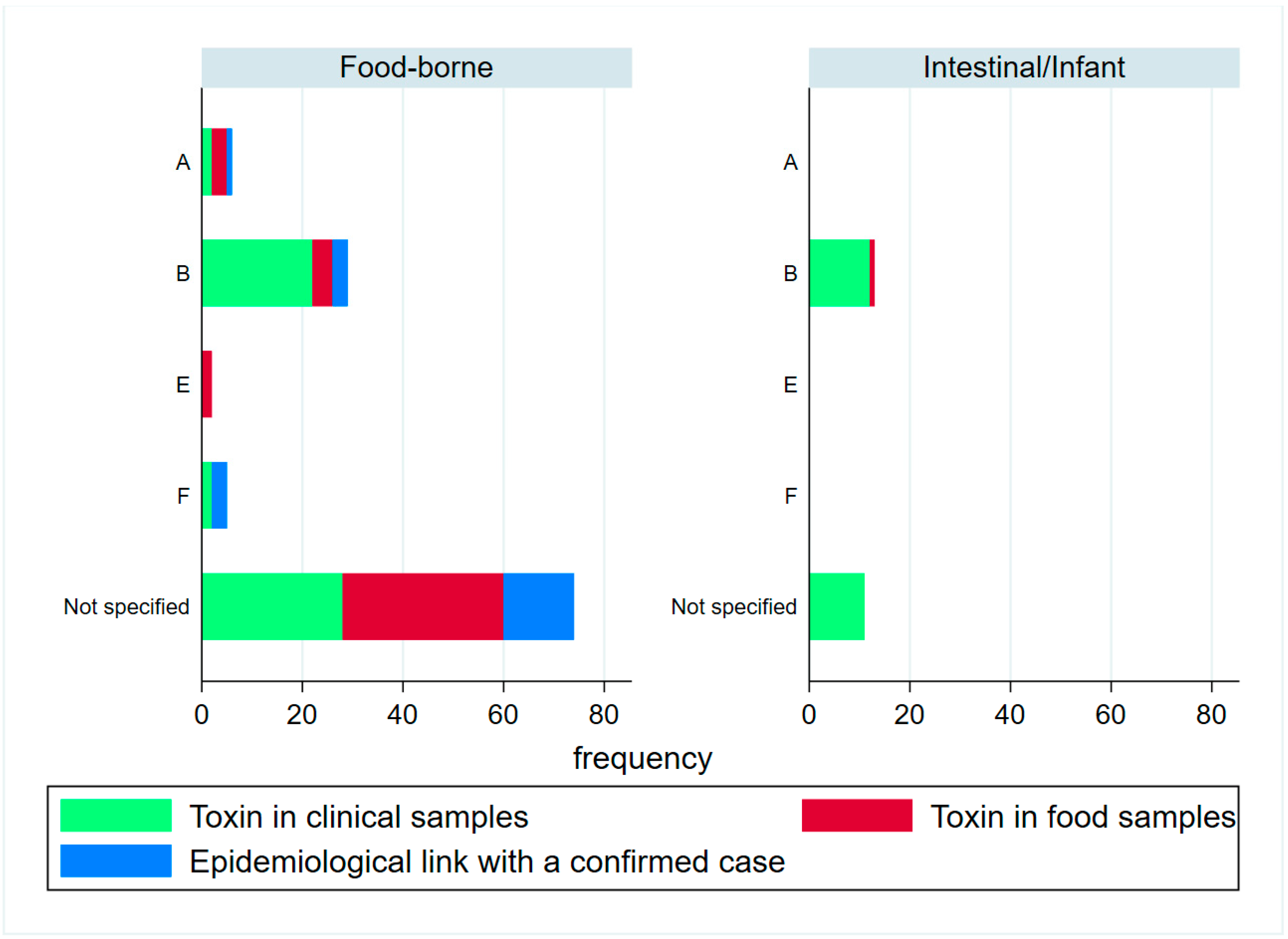
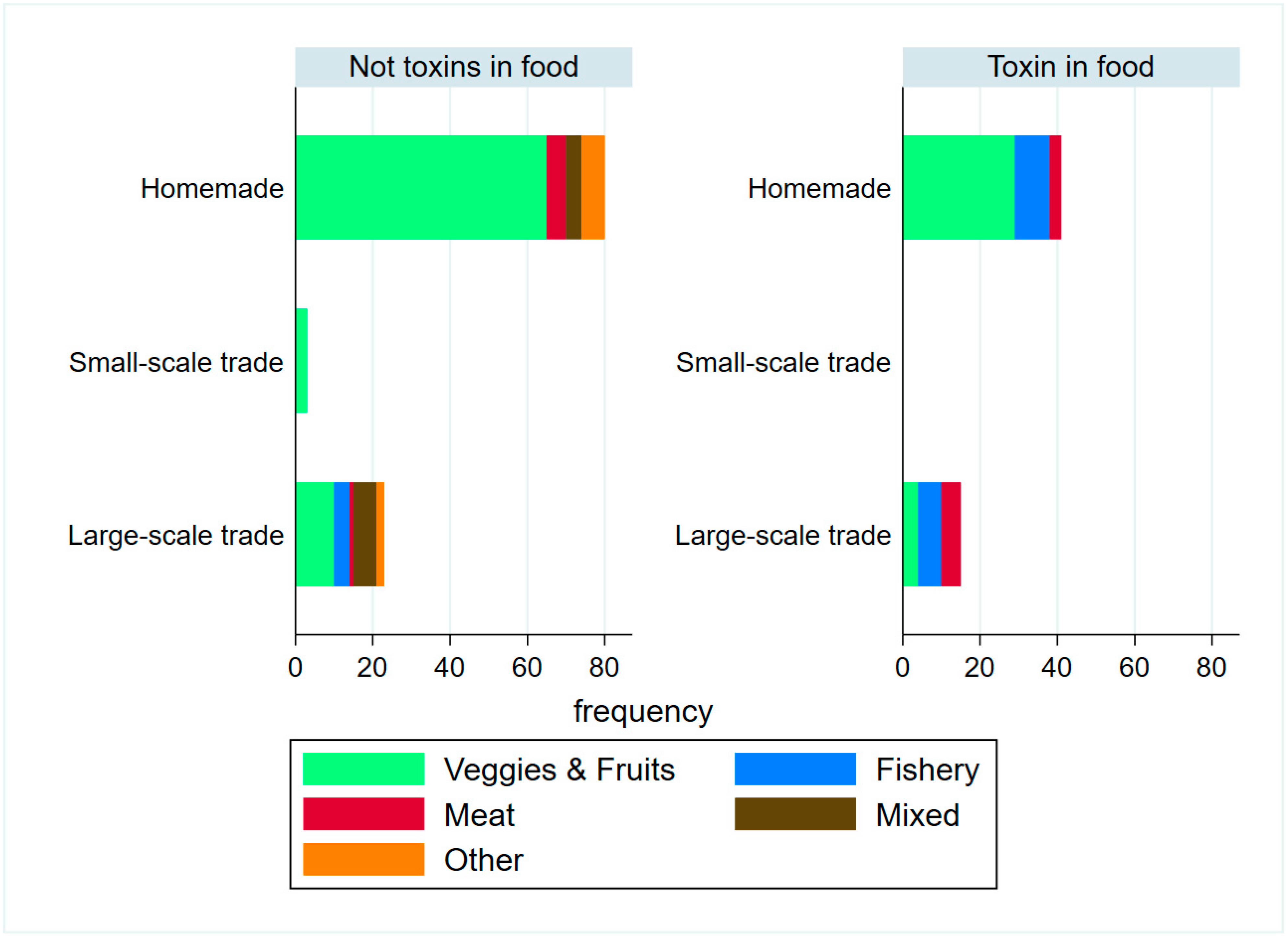
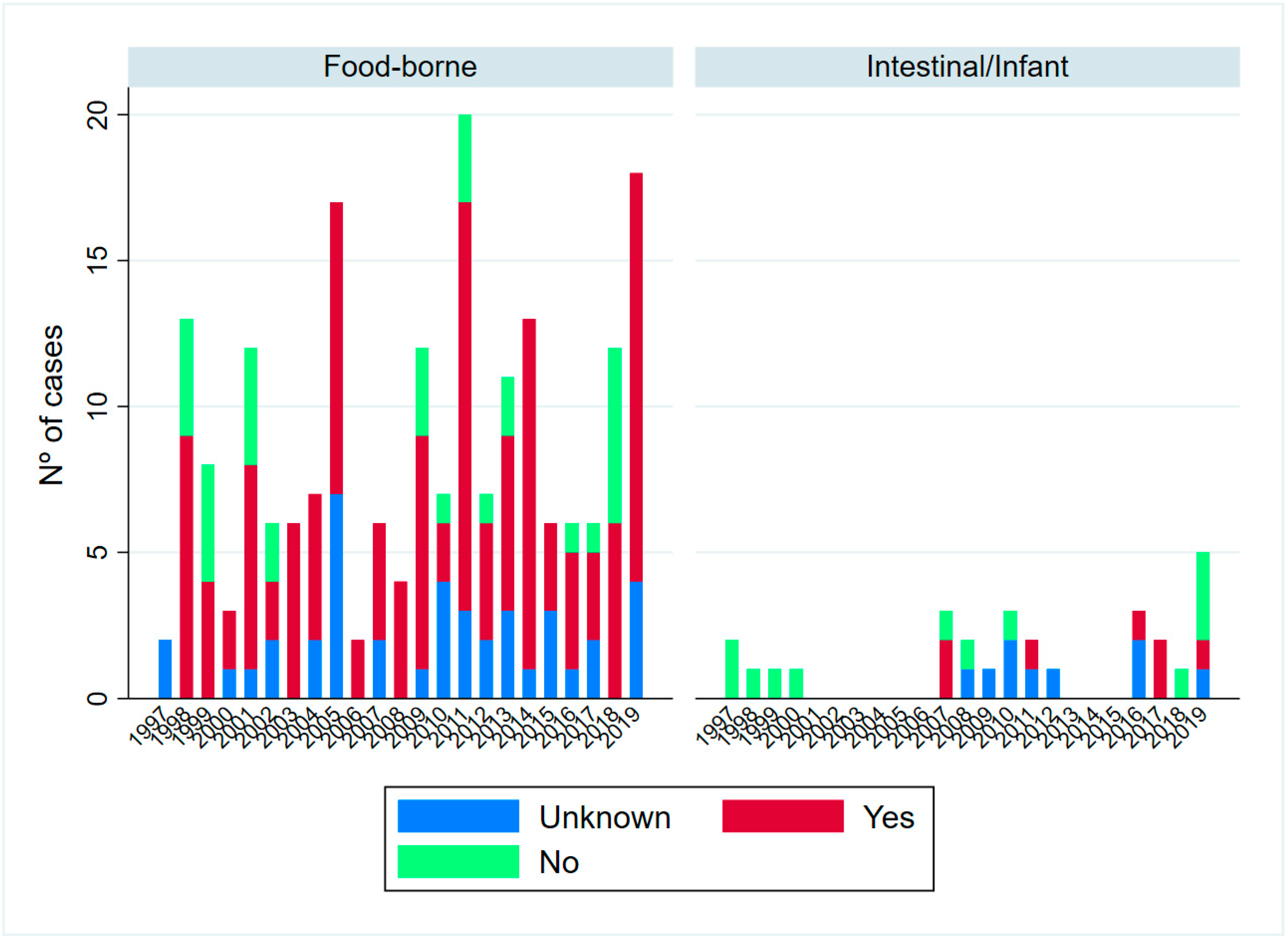
2.1. Epidemiological Description of Cases
2.2. Antitoxin Treatment
3. Discussion
4. Conclusions
5. Material and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azarnia Tehran, D.; Pirazzini, M.; Leka, O.; Mattarei, A.; Lista, F.; Binz, T.; Rossetto, O.; Montecucco, C. Hsp90 is involved in the entry of clostridial neurotoxins into the cytosol of nerve terminals: Hsp90 chaperones the L chain refolding of CNTs. Cell. Microbiol. 2017, 19, e12647. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Zanetti, G.; Megighian, A.; Scorzeto, M.; Fillo, S.; Shone, C.C.; Binz, T.; Rossetto, O.; Lista, F.; Montecucco, C. Thioredoxin and Its Reductase Are Present on Synaptic Vesicles, and Their Inhibition Prevents the Paralysis Induced by Botulinum Neurotoxins. Cell Rep. 2014, 8, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Pirazzini, M.; Lista, F.; Montecucco, C. The role of the single interchains disulfide bond in tetanus and botulinum neurotoxins and the development of antitetanus and antibotulism drugs. Cell. Microbiol. 2019, 21, e13037. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Saberi, F.A. Botulinum Toxin: Mechanisms of Action. Eur. Neurol. 2005, 53, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J. Botulism. Clin. Infect. Dis. 2005, 41, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.W.; Smith, T.J.; Anniballi, F.; Austin, J.W.; Bano, L.; Bradshaw, M.; Cuervo, P.; Cheng, L.W.; Derman, Y.; Dorner, B.G.; et al. Historical Perspectives and Guidelines for Botulinum Neurotoxin Subtype Nomenclature. Toxins 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Tehran, D.; Pirazzini, M. Novel Botulinum Neurotoxins: Exploring Underneath the Iceberg Tip. Toxins 2018, 10, 190. [Google Scholar] [CrossRef]
- Hatheway, C. Clostridium botulinum and Other Clostridia that Produce Botulinum Neurotoxin. In Clostridium Botulinum, 1st ed.; CRC Press: New York, NY, USA, 1992; pp. 3–20. [Google Scholar]
- Harvey, S.M.; Sturgeon, J.; Dassey, D.E. Botulism Due to Clostridium baratii Type F Toxin. J. Clin. Microbiol. 2002, 40, 2260–2262. [Google Scholar] [CrossRef]
- Zornetta, I.; Tehran, D.A.; Arrigoni, G.; Anniballi, F.; Bano, L.; Leka, O.; Zanotti, G.; Binz, T.; Montecucco, C. The first non Clostridial botulinum-like toxin cleaves VAMP within the juxtamembrane domain. Sci. Rep. 2016, 6, 30257. [Google Scholar] [CrossRef]
- Zhang, S.; Masuyer, G.; Zhang, J.; Shen, Y.; Lundin, D.; Henriksson, L.; Miyashita, S.-I.; Martínez-Carranza, M.; Dong, M.; Stenmark, P. Identification and characterization of a novel botulinum neurotoxin. Nat. Commun. 2017, 8, 14130. [Google Scholar] [CrossRef]
- Mansfield, M.; Wentz, T.G.; Zhang, S.; Lee, E.J.; Dong, M.; Sharma, S.K.; Doxey, A.C. Bioinformatic discovery of a toxin family in Chryseobacterium piperi with sequence similarity to botulinum neurotoxins. Sci. Rep. 2019, 9, 1634. [Google Scholar] [CrossRef] [PubMed]
- Brunt, J.; Carter, A.T.; Stringer, S.C.; Peck, M.W. Identification of a novel botulinum neurotoxin gene cluster in Enterococcus. FEBS Lett. 2018, 592, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, N.; Johnson, E.; Pillai, S.; Hodge, D.; Stanker, L.; Wentz, T.; Singh, B.; Venkateswaran, K.; McNutt, P.; Adler, M.; et al. Botulinum Neurotoxin Detection Methods for Public Health Response and Surveillance. Front. Bioeng. Biotechnol. 2018, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Chertow, D.S.; Tan, E.T.; Maslanka, S.E.; Schulte, J.; Bresnitz, E.A.; Weisman, R.S.; Bernstein, J.; Marcus, S.M.; Kumar, S.; Malecki, J.; et al. Botulism in 4 Adults Following Cosmetic Injections With an Unlicensed, Highly Concentrated Botulinum Preparation. JAMA 2006, 296, 2476. [Google Scholar] [CrossRef]
- Lonati, D.; Schicchi, A.; Crevani, M.; Buscaglia, E.; Scaravaggi, G.; Maida, F.; Cirronis, M.; Petrolini, V.M.; Locatelli, C.A. Foodborne Botulism: Clinical Diagnosis and Medical Treatment. Toxins 2020, 12, 509. [Google Scholar] [CrossRef]
- World Health Organization. Botulism. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/botulism (accessed on 15 December 2021).
- Aesan—Agencia Española de Seguridad Alimentaria y Nutrición. Available online: http://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/subdetalle/Botulismo.htm (accessed on 15 December 2021).
- Comunidad de Madrid Consejería de Sanidad. Conservas Caseras: Evitar el Botulismo. Available online: https://www.comunidad.madrid/servicios/salud/conservas-caseras-evitar-botulismo (accessed on 30 September 2022).
- Rioja Salud. ¡Ojo! El Botulismo se Conserva en Casa. Available online: https://www.riojasalud.es/salud-publica-consumo/seguridad-alimentaria/ojo-el-botulismo-se-conserva-en-casa (accessed on 30 September 2022).
- Anniballi, F.; Auricchio, B.; Fiore, A.; Lonati, D.; Locatelli, C.A.; Lista, F.; Fillo, S.; Mandarino, G.; De Medici, D. Botulism in Italy, 1986 to 2015. Eurosurveillance 2017, 22, 30550. [Google Scholar] [CrossRef]
- Woodruff, B.A.; Griffin, P.M.; McCroskey, L.M.; Smart, J.F.; Wainwright, R.B.; Bryant, R.G.; Hutwagner, L.C.; Hatheway, C.L. Clinical and laboratory comparison of botulism from toxin types A, B, and E in the United States, 1975–1988. J. Infect. Dis. 1992, 166, 1281–1286. [Google Scholar] [CrossRef]
- Yu, P.A.; Lin, N.H.; Mahon, B.E.; Sobel, J.; Yu, Y.; Mody, R.K.; Gu, W.; Clements, J.; Kim, H.J.; Rao, A.K. Safety and Improved Clinical Outcomes in Patients Treated With New Equine-Derived Heptavalent Botulinum Antitoxin. Clin. Infect. Dis. 2018, 66 (Suppl. 1), S57–S64. [Google Scholar] [CrossRef]
- Research C for BE and BAT (Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G)—(Equine). FDA, 20 December 2019. Available online: https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/bat-botulism-antitoxin-heptavalent-b-c-d-e-f-g-equine (accessed on 22 March 2022).
- Centro Nacional de Epidemiología. Instituto de Salud Carlos III. Protocolo de Vigilancia de Botulismo. Protocolos de la Red Nacional de Vigilancia Epidemiológica (RENAVE). 2022. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/PROTOCOLOS/Protocolo%20Vigilancia%20botulismo_RENAVE_v1.pdf (accessed on 1 November 2022).
- Arnon, S.S.; Schechter, R.; Maslanka, S.E.; Jewell, N.P.; Hatheway, C.L. Human Botulism Immune Globulin for the Treatment of Infant Botulism. N. Engl. J. Med. 2006, 354, 462–471. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Botulism. In ECDC Annual Epidemiological Report for 2015; ECDC: Stockholm, Sweden, 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/botulism-annual-epidemiological-report-2015 (accessed on 9 March 2022).
- Disease Data from ECDC Surveillance Atlas—Botulism. European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/botulism/surveillance/atlas (accessed on 9 March 2022).
- Ministerio de Sanidad y Consumo. Real Decreto 2210/1995, de 28 de Diciembre, Por el Que se Crea la Red Nacional de Vigilancia Epidemiológica. Sect. 1, Real Decreto 2210/24 January 1995; Ministerio de Sanidad y Consumo: Madrid, Spain, 1996; pp. 2153–2158. Available online: https://www.boe.es/eli/es/rd/1995/12/28/2210 (accessed on 12 March 2022).
- Lindström, M.; Korkeala, H. Laboratory Diagnostics of Botulism. Clin. Microbiol. Rev. 2006, 19, 298–314. [Google Scholar] [CrossRef]
- Rao, A.K.; Sobel, J.; Chatham-Stephens, K.; Luquez, C. Clinical Guidelines for Diagnosis and Treatment of Botulism, 2021. MMWR Recomm. Rep. 2021, 70, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, S.; Nolla, J.; Valdezate, S.; Tortajada, C.; Vargas-Leguas, H.; Parron, I.; Saez-Nieto, J.A.; Portana, S.; Carrasco, G.; Moguel, E.; et al. Short report: Two simultaneous botulism outbreaks in Barcelona: Clostridium baratii and Clostridium botulinum. Epidemiol. Infect. 2013, 141, 1993–1995. [Google Scholar] [CrossRef] [PubMed]
- Souayah, N.; Mehyar, L.S.; Khan, H.M.; Yacoub, H.A.; A Al-Qudah, Z.A.A.-K.; Nasar, A.; Sheikh, Z.B.; Maybodi, L.; Qureshi, A.I. Trends in outcome and hospitalization charges of adult patients admitted with botulism in the United States. Neuroepidemiology 2012, 38, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Rasetti-Escargueil, C.; Lemichez, E.; Popoff, M.R. Human Botulism in France, 1875–2016. Toxins 2020, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Lúquez, C.; Edwards, L.; Griffin, C.; Sobel, J. Foodborne Botulism Outbreaks in the United States, 2001–2017. Front. Microbiol. 2021, 12, 1982. [Google Scholar] [CrossRef]
- National Botulism Surveillance Summary, 2018|Botulism|CDC. 2021. Available online: https://www.cdc.gov/botulism/surv/2018/index.html (accessed on 24 March 2022).
- Espelund, M.; Klaveness, D. Botulism outbreaks in natural environments—An update. Front. Microbiol. 2014, 5, 287. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control; European Food Safety Authority. Type E Botulism Associated with Fish Product Consumption—Germany and Spain, 21 December 2016; ECDC: Stockholm, Sweden, 2016. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/01-12-2016-RRA-Botulism-Germany%2C%20Spain.pdf (accessed on 24 March 2022).
- Rasetti-Escargueil, C.; Lemichez, E.; Popoff, M.R. Public Health Risk Associated with Botulism as Foodborne Zoonoses. Toxins 2019, 12, 17. [Google Scholar] [CrossRef]
- Sobel, J.; Malavet, M.; John, S. Outbreak of clinically mild botulism type E illness from home-salted fish in patients presenting with predominantly gastrointestinal symptoms. Clin. Infect. Dis. 2007, 45, e14–e16. [Google Scholar] [CrossRef]
- Aureli, P.; Franciosa, G.; Fenicia, L. Infant botulism and honey in Europe: A commentary. Pediatr. Infect. Dis. J. 2002, 21, 866–868. [Google Scholar] [CrossRef]
- Infant Botulism: Information for Clinicians|Botulism|CDC. 2021. Available online: https://www.cdc.gov/botulism/infant-botulism.html (accessed on 24 February 2022).
- Welcome to the Infant Botulism Treatment and Prevention Program. Available online: https://www.infantbotulism.org/physician/patient.php (accessed on 28 February 2022).
- Monsalve, J.C.; Alcolea, A.N.S. Botulismo infantil. An. Esp. Pediatr. 1999, 51, 572–573. [Google Scholar]
- Mohd Tamrin, M.I. The dilemma of diagnosing wound botulism in an infant: A rare case of paralysis with topical application of honey. Int. J. Infect. Dis. 2020, 95, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.; Luquez, C.; Dejong, L.; Fernandez, R. Presence of Clostridium botulinum spores in Matricaria chamomilla (chamomile) and its relationship with infant botulism. Int. J. Food Microbiol. 2008, 121, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.S.; Parrera, G.S.; Astacio, H.; Sahota, H.; Anderson, D.M.; Hall, C.; Babinchak, T. Safety and Clinical Outcomes of an Equine-derived Heptavalent Botulinum Antitoxin Treatment for Confirmed or Suspected Botulism in the United States. Clin. Infect. Dis. 2020, 70, 1950–1957. [Google Scholar] [CrossRef]
- Anderson, D.M.; Kumar, V.R.; Arper, D.L.; Kruger, E.; Bilir, S.P.; Richardson, J.S. Cost savings associated with timely treatment of botulism with botulism antitoxin heptavalent product. PLoS ONE 2019, 14, e0224700. [Google Scholar] [CrossRef] [PubMed]
- Laín Terés, N.; Supino, M.; Álvarez, N.V.; Julián-Jiménez, A. Botulismo. In Manejo de Infecciones en Urgencias, 3rd ed.; Editorial Médica Panamericana, S.A.: Madrid, Spain, 2019; pp. 659–665. [Google Scholar]
- Mensa, J.; Soriano, A.; García-Sánchez, J.E.; Marco, F.; Letang, E.; Llinares, P.; López-Suñé, E.; Barberán, J. Guía de Terapéutica Antimicrobiana 2019; Antares: Barcelona, Spain, 2019. [Google Scholar]
- Rasetti-Escargueil, C.; Lemichez, E.; Popoff, M.R. Toxemia in Human Naturally Acquired Botulism. Toxins 2020, 12, 716. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Pesa y Alimentación G de E. Base de Datos de Consumo en Hogares. Consumo y Tendencias en Alimentación. Available online: https://www.mapa.gob.es/app/consumo-en-hogares/consulta.asp (accessed on 30 June 2022).
- Tejada García, M.; Guindel Jiménez, C. Tratamiento con antitoxina botulínica en dos casos de botulismo alimentario. Farm. Hosp. 2010, 34, 47–48. [Google Scholar] [CrossRef] [PubMed]
- O’Horo, J.C.; Harper, E.P.; El Rafei, A.; Ali, R.; DeSimone, D.C.; Sakusic, A.; Abu Saleh, O.M.; Marcelin, J.R.; Tan, E.M.; Rao, A.K.; et al. Efficacy of Antitoxin Therapy in Treating Patients With Foodborne Botulism: A Systematic Review and Meta-analysis of Cases, 1923–2016. Clin. Infect. Dis. 2018, 66 (Suppl. 1), S43–S56. [Google Scholar] [CrossRef]
- Schussler, E.; Sobel, J.; Hsu, J.; Yu, P.; Meaney-Delman, D.; Grammer, L.C., III; Nowak-Węgrzyn, A. Workgroup Report by the Joint Task Force Involving American Academy of Allergy, Asthma & Immunology (AAAAI); Food Allergy, Anaphylaxis, Dermatology and Drug Allergy (FADDA) (Adverse Reactions to Foods Committee and Adverse Reactions to Drugs, Biologicals, and Latex Committee); and the Centers for Disease Control and Prevention Botulism Clinical Treatment Guidelines Workgroup—Allergic Reactions to Botulinum Antitoxin: A Systematic Review. Clin. Infect. Dis. 2018, 66 (Suppl. 1), S65–S72. [Google Scholar]
- Laso, E.L.; Navero, J.P.; Aguirre, J.R.; González, M.M.; García, M.M.; Aranzana, M.C.; de la Rosa, I.I. Botulismo del lactante. An. Pediatría 2008, 68, 499–502. [Google Scholar] [CrossRef]
- Cagan, E.; Peker, E.; Dogan, M.; Caksen, H. Infant botulism. Eurasian J. Med. 2010, 42, 92–94. [Google Scholar] [CrossRef]
- Oppdatert, P. Botulisme. Folkehelseinstituttet. Available online: https://www.fhi.no/nettpub/smittevernveilederen/sykdommer-a-a/botulisme---veileder-for-helseperso/ (accessed on 28 February 2022).
- Chalk, C.H.; Benstead, T.J.; Pound, J.D.; Keezer, M.R. Medical treatment for botulism. Cochrane Database Syst. Rev. 2019, 4, CD008123. [Google Scholar] [CrossRef] [PubMed]
- López-Laso, E.; Roncero-Sánchez-Cano, I.; Arce-Portillo, E.; Ley-Martos, M.; Aguirre-Rodríguez, J.; García-Ron, A.; Mora-Navarro, D.; Méndez-García, M.; Camino-León, R. Infant botulism in Andalusia (Southern Spain). Eur. J. Paediatr. Neurol. 2014, 18, 321–326. [Google Scholar] [CrossRef] [PubMed]
- The European Commission. Commission Implementing Decision (EU) 2018/945 of 22 June 2018 on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions. Off. J. Eur. Union 2018, 61, 1–74. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0945 (accessed on 24 September 2022).

| Case Category | Case Definition |
|---|---|
| Possible ^ | Those meeting clinical criteria and a request for laboratory diagnosis. Clinical criteria in food-borne cases comprise at least one of the following two: bilateral cranial nerve impairment and/or peripheral symmetric paralysis. Clinical criteria in intestinal/infant cases comprise at least one of the following six: constipation, lethargy, difficulty in sucking or feeding, ptosis; dysphagia and/or general muscle weakness. |
| Probable ᵟ | Those meeting the clinical criteria and had been exposed to a contaminated food (confirmed by the laboratory) or had an epidemiological link with a confirmed case (related to consumption of the same food as a confirmed case). To detect the botulinum toxin food, the standard mouse bioassay (SMB) was used. The isolation of toxin-producing clostridia in food was used for infant botulism. |
| Confirmed ᵟ | Those meeting the clinical criteria and with a positive result in the clinical samples. SMB was performed on feces as the preferred sample [30], but standard culture methods and a described multiplex PCR method able to detect A, B, E and F neurotoxin genes in feces, were also carried out [30]. |
| Outbreak | ≥2 cases (independent of their category) with an epidemiological link. |
| Total | Confirmed | Probable | Possible | ||
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| Age group and sex | 232 | 77 (33.19) | 63 (27.16) | 92 (39.66) | |
| <1 year (F) | 12 | 11 (91.67) | 0 | 1 (8.33) | |
| <1 year (M) | 18 | 13 (72.22) | 1 (5.56) | 4 (22.22) | |
| 1–14 years (F) | 3 | 2 (66.67) | 1 (33.33) | 0 | |
| 1–14 years (M) | 8 | 2 (25) | 1 (12.5) | 5 (62.5) | |
| 15–24 years (F) | 8 | 1 (12.5) | 5 (62.5) | 2 (25) | |
| 15–24 years (M) | 7 | 1 (14.29) | 2 (28.57) | 4 (57.14) | |
| 25–44 years (F) | 15 | 3 (20) | 7 (46.67) | 5 (33.33) | |
| 25–44 years (M) | 32 | 7 (21.88) | 12 (29.27) | 18 (43.9) | |
| 45–64 years (F) | 31 | 8 (25.81) | 14 (45.16) | 9 (29.03) | |
| 45–64 years (M) | 41 | 11 (26.81) | 12 (29.27) | 18 (43.9) | |
| 65–80 years (F) | 30 | 5 (16.67) | 5 (16.67) | 20 (66.67) | |
| 65–80 years (M) | 22 | 10 (45.45) | 3 (13.64) | 9 (40.91) | |
| >80 years (F) | 3 | 2 (66.67) | 0 | 1 (33.33) | |
| >80 years (M) | 2 | 1 (50) | 0 | 1 (50) | |
| p-value | <0.001 | ||||
| Category | |||||
| Food-borne | 204 | 54 (26.47) | 62 (30.39) | 88 (43.14) | |
| Intestinal/infant | 28 | 23 (82.14) | 1 (3.57) | 4 (14.29) | |
| p-value | <0.001 | ||||
| Outbreak-related | |||||
| No | 115 | 49 (42.61) | 5 (4.35) | 61 (53.04) | |
| Yes | 117 | 28 (23.93) | 58 (49.57) | 31 (26.50) | |
| p-value | <0.001 | ||||
| Antitoxin administration | |||||
| Yes | 133 | 40 (30.08) | 31 (23.31) | 62 (46.62) | |
| No | 58 | 24 (41.38) | 14 (24.14) | 20 (34.48) | |
| Unknown | 41 | 13 (31.71) | 18 (43.90) | 10 (24.39) | |
| p-value | 0.025 |
| Food-Borne Botulism | Infant Botulism | |
|---|---|---|
| ≤48 h between onset of symptoms and admission | 45.20% | 44% |
| Mean hospitalization length (days) | 25.56 (SD: 53.7) | 21.19 (SD: 10.6) |
| Median hospitalization length (days) | 14 (IQR:18) | 19.5 (IQR:17) |
| Adjusted Analysis (Cox Regression) | Crude Analysis (Kruskal–Wallis) | ||||
|---|---|---|---|---|---|
| Hazard Ratio | 95%CI | p-Value | χ² (df) with Ties | p-Value | |
| Antitoxin administration category (hours after onset of symptoms) | |||||
| ≤48 h | 1 | - | - | 5.940 (2) | 0.0513 |
| >48 h | 0.446 | 0.219–0.909 | 0.026 | 6.219 (1) | 0.0126 |
| No antitoxin | 0.264 | 0.116–0.601 | 0.002 | 2.695 (1) | 0.1007 |
| Age | 0.988 | 0.978–0.998 | 0.022 | ||
| Sex | |||||
| Male | 1 | - | - | 0.8998 (1) | 0.8998 |
| Female | 1.436 | 0.887–2.327 | 0.141 | ||
| Case classification | |||||
| Possible | 1 | - | - | 10.473 (2) | 0.0053 |
| Probable | 0.783 | 0.461–1.330 | 0.365 | 0.093 (1) | 0.7605 |
| Confirmed | 0.495 | 0.281–0.869 | 0.014 | 6.903 (1) | 0.0086 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñuelas, M.; Guerrero-Vadillo, M.; Valdezate, S.; Zamora, M.J.; Leon-Gomez, I.; Flores-Cuéllar, Á.; Carrasco, G.; Díaz-García, O.; Varela, C. Botulism in Spain: Epidemiology and Outcomes of Antitoxin Treatment, 1997–2019. Toxins 2023, 15, 2. https://doi.org/10.3390/toxins15010002
Peñuelas M, Guerrero-Vadillo M, Valdezate S, Zamora MJ, Leon-Gomez I, Flores-Cuéllar Á, Carrasco G, Díaz-García O, Varela C. Botulism in Spain: Epidemiology and Outcomes of Antitoxin Treatment, 1997–2019. Toxins. 2023; 15(1):2. https://doi.org/10.3390/toxins15010002
Chicago/Turabian StylePeñuelas, Marina, María Guerrero-Vadillo, Sylvia Valdezate, María Jesús Zamora, Inmaculada Leon-Gomez, Ángeles Flores-Cuéllar, Gema Carrasco, Oliva Díaz-García, and Carmen Varela. 2023. "Botulism in Spain: Epidemiology and Outcomes of Antitoxin Treatment, 1997–2019" Toxins 15, no. 1: 2. https://doi.org/10.3390/toxins15010002
APA StylePeñuelas, M., Guerrero-Vadillo, M., Valdezate, S., Zamora, M. J., Leon-Gomez, I., Flores-Cuéllar, Á., Carrasco, G., Díaz-García, O., & Varela, C. (2023). Botulism in Spain: Epidemiology and Outcomes of Antitoxin Treatment, 1997–2019. Toxins, 15(1), 2. https://doi.org/10.3390/toxins15010002





