Spirolides in Bivalve Mollusk of the Galician (NW Spain) Coast: Interspecific, Spatial, Temporal Variation and Presence of an Isomer of 13-Desmethyl Spirolide C
Abstract
1. Introduction
2. Results
2.1. Toxin Profile
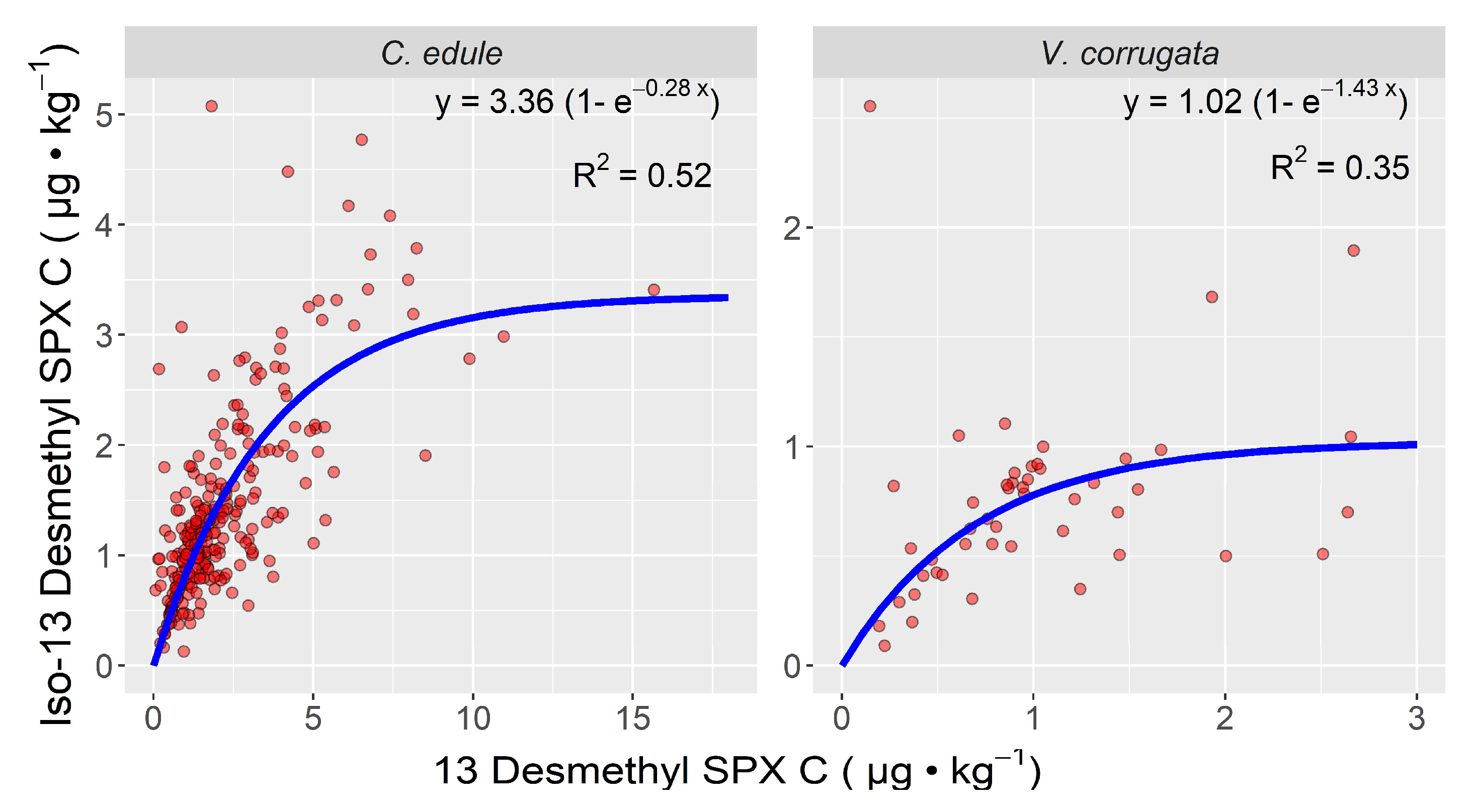
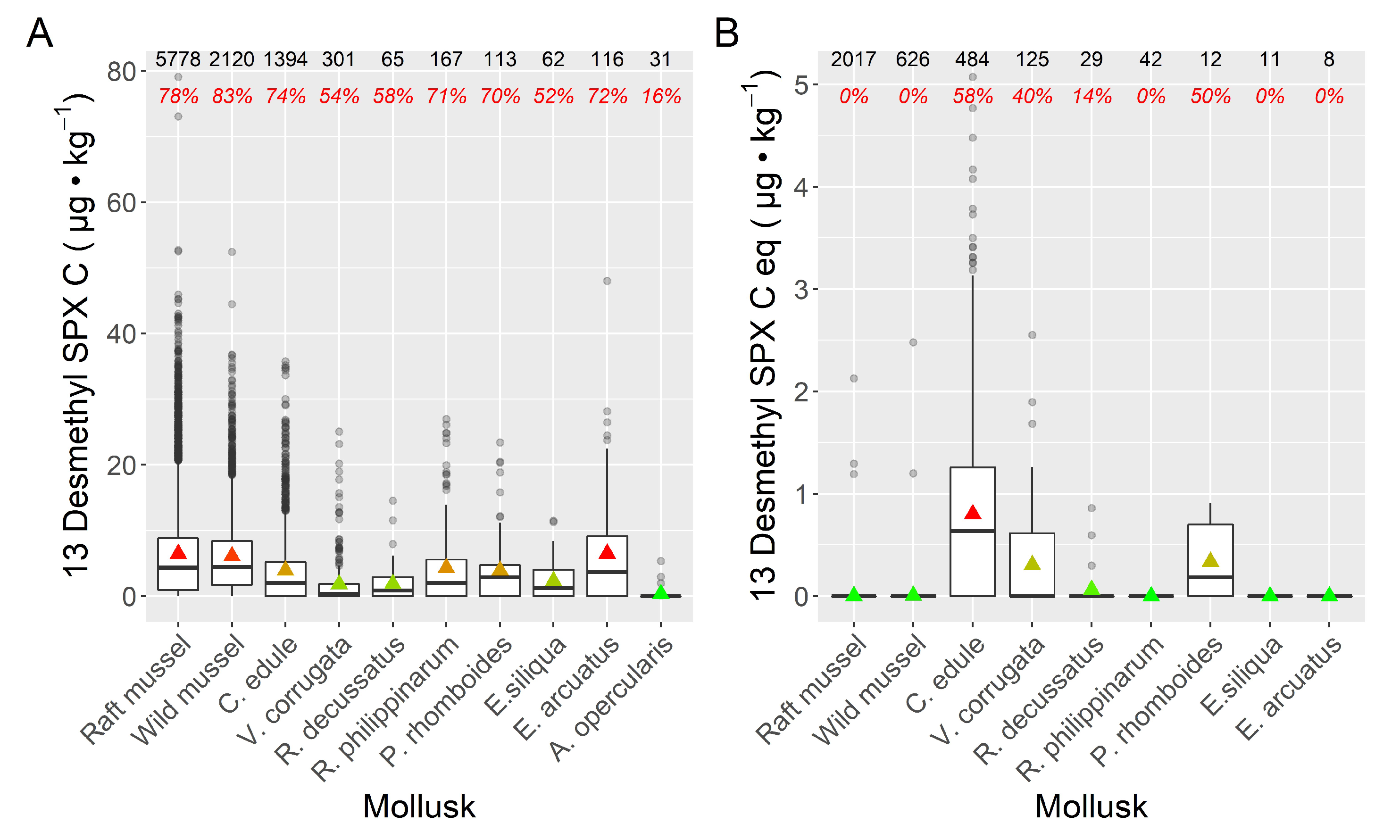
2.2. Inter-Specific Variability
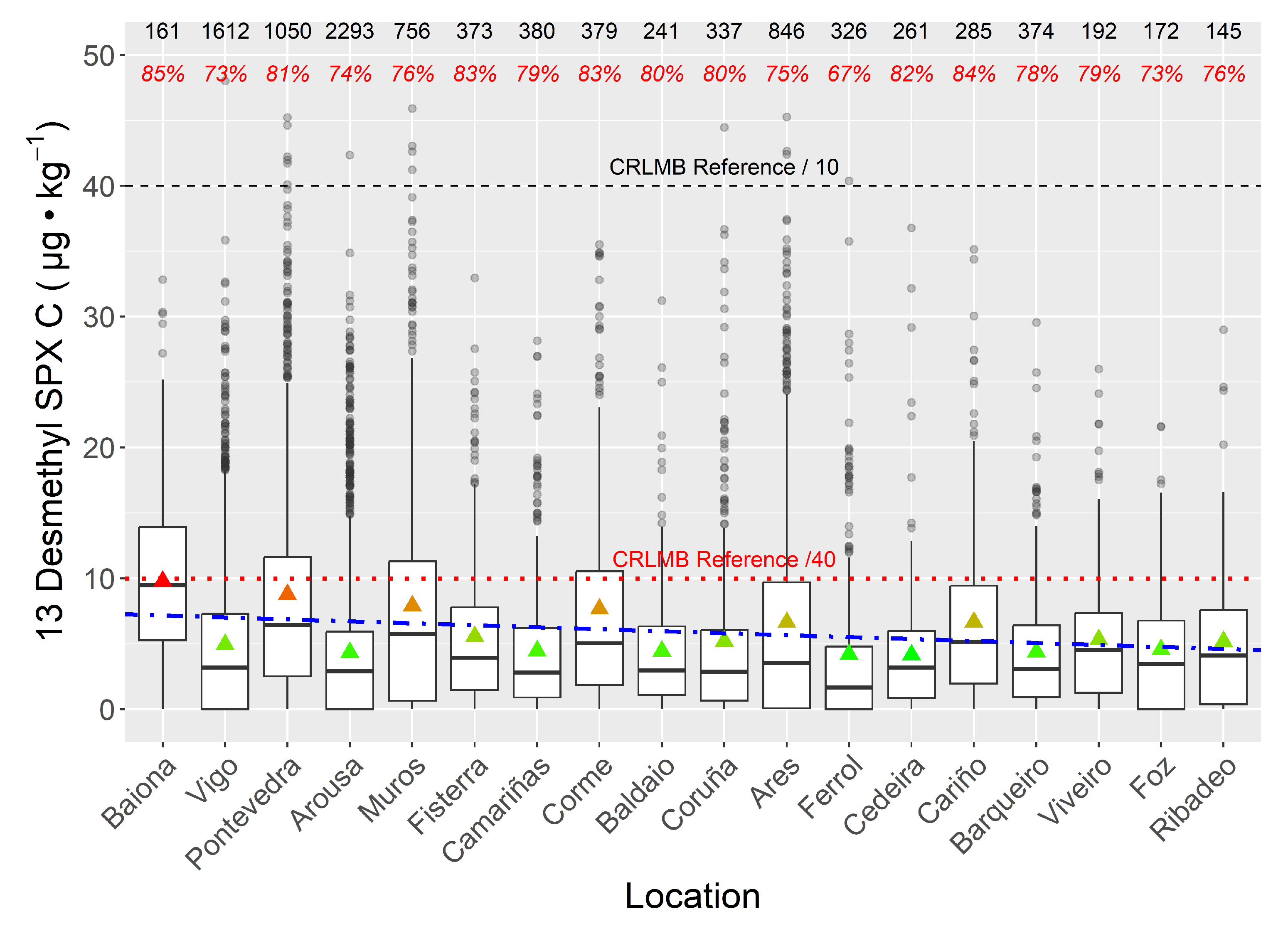
2.3. Spatial Variability
2.4. Temporal Variability
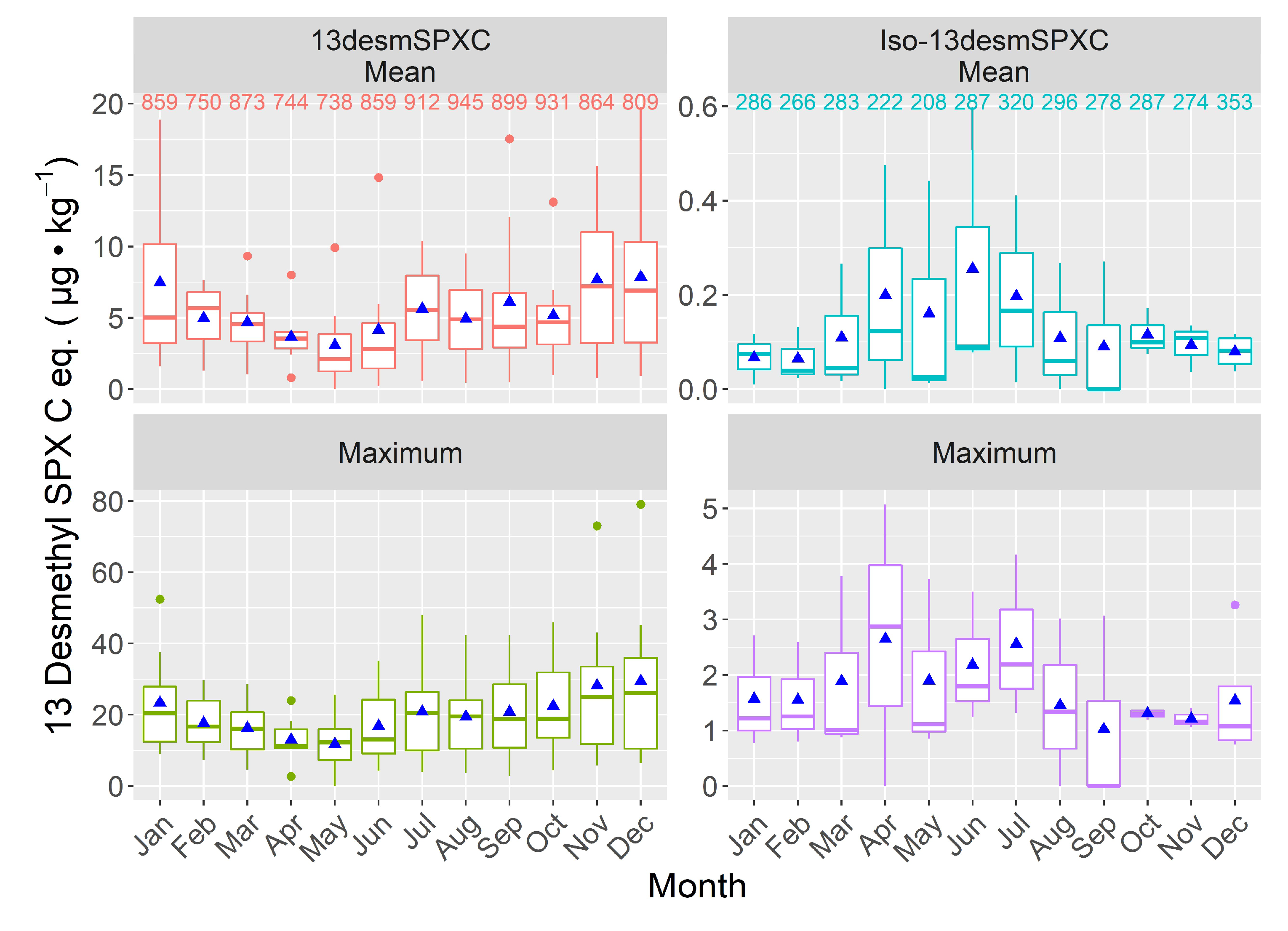
2.4.1. Seasonality
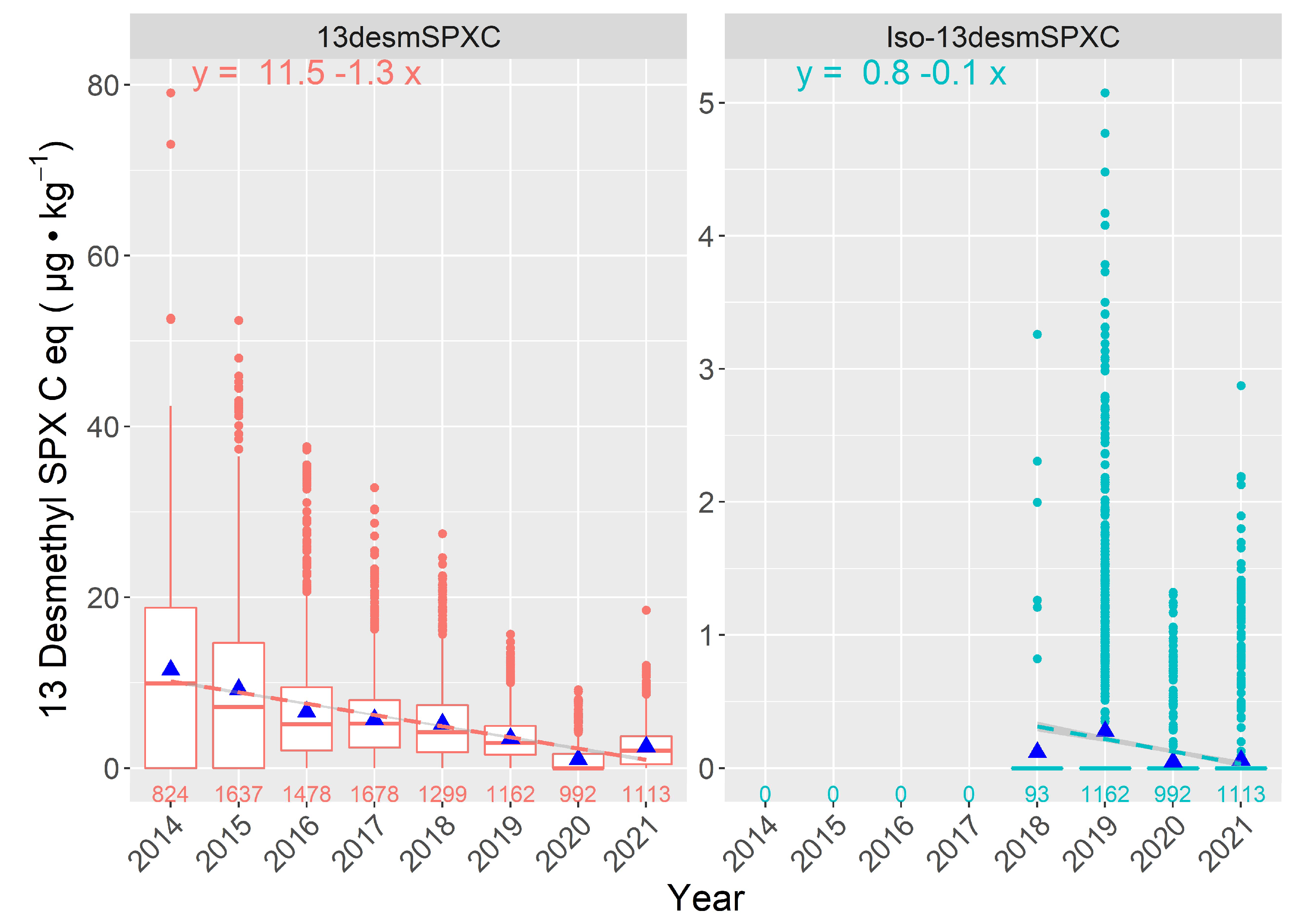
2.4.2. Interannual Variation
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Chemicals, Solvents, and Reference Materials
5.2. Sampling
5.3. Extraction and Sample Preparation
5.4. LC-MS/MS Quantification
5.5. LC-MS2 and LC-MS3
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zurhelle, C.; Nieva, J.; Tillmann, U.; Harder, T.; Krock, B.; Tebben, J. Identification of Novel Gymnodimines and Spirolides from the Marine Dinoflagellate Alexandrium ostenfeldii. Marine Drugs 2018, 16, 446. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Curtis, J.M.; Oshima, Y.; Quilliam, M.A.; Walter, J.A.; Watson-Wright, W.M.; Wright, J.L.C. Spirolides B and D, two novel macrocycles isolated from the digestive glands of shellfish. J. Chem. Soc. Chem. Commun. 1995, 2159–2161. [Google Scholar] [CrossRef]
- John, U.; Cembella, A.; Hummert, C.; Elbrachter, M.; Groben, R.; Medlin, L. Discrimination of the toxigenic dinoflagellates Alexandrium tamarense and A. ostenfeldii in co-occurring natural populations from Scottish coastal waters. Eur. J. Phycol. 2003, 38, 25–40. [Google Scholar] [CrossRef]
- Aasen, J.; Mackinnon, S.L.; Leblanc, P.; Walter, J.A.; Hovgaard, P.; Aune, T.; Quilliam, M.A. Detection and Identification of Spirolides in Norwegian Shellfish and Plankton. Chem. Res. Toxicol. 2005, 18, 509–515. [Google Scholar] [CrossRef]
- Gribble, K.E.; Keafer, B.A.; Quilliam, M.A.; Cembella, A.D.; Kulis, D.M.; Manahan, A.; Anderson, D.M. Distribution and Toxicity of Alexandrium ostenfeldii (Dinophyceae) in the Gulf of Maine, USA. Deep-Sea Res. Part II-Top. Stud. Oceanogr. 2005, 52, 2745–2763. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Magno, S.; Tartaglione, L.; Cangini, M.; Pompei, M.; Guerrini, F.; Boni, L.; Pistocchi, R. Toxin profile of Alexandrium ostenfeldii (Dinophyceae) from the Northern Adriatic Sea revealed by liquid chromatography-mass spectrometry. Toxicon 2006, 47, 597–604. [Google Scholar] [CrossRef]
- Villar González, A.; Rodríguez-Velasco, M.L.; Ben-Gigirey, B.; Botana, L.M. First evidence of spirolides in Spanish shellfish. Toxicon 2006, 48, 1068–1074. [Google Scholar] [CrossRef]
- MacKinnon, S.L.; Walter, J.A.; Quilliam, M.A.; Cembella, A.D.; LeBlanc, P.; Burton, I.W.; Hardstaff, W.R.; Lewis, N.I. Spirolides isolated from Danish strains of the toxigenic dinoflagellate Alexandrium ostenfeldii. J. Nat. Prod. 2006, 69, 983–987. [Google Scholar] [CrossRef]
- Amzil, Z.; Sibat, M.; Royer, F.; Masson, N.; Abadie, E. Report on the First Detection of Pectenotoxin-2, Spirolide-a and Their Derivatives in French Shellfish. Mar. Drugs 2007, 5, 168–179. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on marine biotoxins in shellfish—Cyclic imines (spirolides, gymnodimines, pinnatoxins and pteriatoxins) 1. EFSA J. 2010, 8, 1628. [Google Scholar]
- Touzet, N.; Lacaze, J.P.; Maher, M.; Turrell, E.; Raine, R. Summer dynamics of Alexandrium ostenfeldii (Dinophyceae) and spirolide toxins in Cork Harbour, Ireland. Mar. Ecol. -Prog. Ser. 2011, 425, 21–33. [Google Scholar] [CrossRef]
- Nincevic-Gladan, Z.; Ujevic, I.; Milandri, A.; Marasovic, I.; Ceredi, A.; Pigozzi, S.; Arapov, J.; Skejic, S. Lipophilic Toxin Profile in Mytilus galloprovincialis during Episodes of Diarrhetic Shellfish Poisoning (DSP) in the NE Adriatic Sea in 2006. Molecules 2011, 16, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Uribe, E.; Ávalos, P.; Mariño, C.; Blanco, J. First identification of azaspiracid and spirolides in Mesodesma donacium and Mulinia edulis from Northern Chile. Toxicon 2010, 55, 638–641. [Google Scholar] [CrossRef]
- Paredes-Banda, P.; García-Mendoza, E.; Ponce-Rivas, E.; Blanco, J.; Almazán-Becerril, A.; Galindo-Sánchez, C.; Cembella, A. Association of the Toxigenic Dinoflagellate Alexandrium ostenfeldii with Spirolide Accumulation in Cultured Mussels (Mytilus galloprovincialis) From Northwest Mexico. Front. Mar. Sci. 2018, 5, 491. [Google Scholar] [CrossRef]
- Wu, H.Y.; Guo, M.M.; Tan, Z.J.; Cheng, H.Y.; Li, Z.X.; Zhai, Y.X. Liquid chromatography quadrupole linear ion trap mass spectrometry for multiclass screening and identification of lipophilic marine biotoxins in bivalve mollusks. J. Chromatogr. A 2014, 1358, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Almandoz, G.O.; Montoya, N.G.; Hernando, M.P.; Benavides, H.R.; Carignan, M.O.; Ferrario, M.E. Toxic strains of the Alexandrium ostenfeldii complex in southern South America (Beagle Channel, Argentina). Harmful Algae 2014, 37, 100–109. [Google Scholar] [CrossRef]
- Turner, A.D.; Goya, A.B. Occurrence and profiles of lipophilic toxins in shellfish harvested from Argentina. Toxicon 2015, 102, 32–42. [Google Scholar] [CrossRef]
- Cembella, A.; Quilliam, M.; Lewis, N.; Bauder, A.; Wright, J. Identifying the planktonic origin and distribution of spirolides in coastal Nova Scotian waters. In Harmful Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and Intergovernmental Oceanographic Commission (UNESCO): Santiago de Compostela, Spain, 1998; pp. 481–484. [Google Scholar]
- Cembella, A.D.; Bauder, A.G.; Lewis, N.I.; Quilliam, M.A. Association of the gonyaulacoid dinoflagellate Alexandrium ostenfeldii with spirolide toxins in size-fractionated plankton. J. Plankton Res. 2001, 23, 1413–1419. [Google Scholar] [CrossRef]
- Munday, R.; Quilliam, M.A.; LeBlanc, P.; Lewis, N.; Gallant, P.; Sperker, S.A.; Ewart, H.S.; MacKinnon, S.L. Investigations into the Toxicology of Spirolides, a Group of Marine Phycotoxins. Toxins 2012, 4, 1–14. [Google Scholar] [CrossRef]
- Richard, D.; Arsenault, E.; Cembella, A.; Quilliam, M. Investigations into the toxicology and pharmacology of spirolides, a novel group of shellfish toxins. In Harmful Algal Blooms 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; IOC of UNESCO: Paris, France, 2001; pp. 383–386. [Google Scholar]
- Molgó, J.; Benoit, E.; Aráoz, R.; Zakarian, A.; Iorga, B.I. Spirolides and Cyclic Imines: Toxicological Profile. In Marine and Freshwater Toxins; Springer: Dordrecht, The Netherlands, 2016; pp. 193–217. [Google Scholar] [CrossRef]
- Munday, R. Toxicity of cyclic imines. In Toxins and Biologically Active Compounds from Microalgae; Rossini, G.P., Ed.; CRC Press: Boca Raton, FL, USA, 2014; Volume 2, pp. 283–308. [Google Scholar]
- Cembella, A.D.; Lewis, N.I.; Quilliam, M.A. The marine dinoflagellate Alexandrium ostenfeldii (Dinophyceae) as the causative organism of spirolide shellfish toxins. Phycologia 2000, 39, 67–74. [Google Scholar] [CrossRef]
- Tillmann, U.; Kremp, A.; Tahvanainen, P.; Krock, B. Characterization of spirolide producing Alexandrium ostenfeldii (Dinophyceae) from the western Arctic. Harmful Algae 2014, 39, 259–270. [Google Scholar] [CrossRef]
- Suikkanen, S.; Kremp, A.; Hautala, H.; Krock, B. Paralytic shellfish toxins or spirolides?: The role of environmental and genetic factors in toxin production of the Alexandrium ostenfeldii complex. Harmful Algae 2013, 26, 52–59. [Google Scholar] [CrossRef]
- Van Wagoner, R.M.; Misner, I.; Tomas, C.R.; Wright, J.L.C. Occurrence of 12-methylgymnodimine in a spirolide-producing dinoflagellate Alexandrium peruvianum and the biogenetic implications. Tetrahedron Lett. 2011, 52, 4243–4246. [Google Scholar] [CrossRef]
- Rundberget, T.; Aasen, J.A.B.; Selwood, A.I.; Miles, C.O. Pinnatoxins and spirolides in Norwegian blue mussels and seawater. Toxicon 2011, 58, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Guerrini, F.; Pezzolesi, L.; Pistocchi, R. Characterization of 27-hydroxy-13-desmethyl spirolide C and 27-oxo-13,19-didesmethyl spirolide C. Further insights into the complex Adriatic Alexandrium ostenfeldii toxin profile. Toxicon 2010, 56, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’aversano, C.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Guerrini, F.; Pistocchi, R. Spirolide Toxin Profile of Adriatic Alexandrium ostenfeldii Cultures and Structure Elucidation of 27-Hydroxy-13,19-Didesmethyl Spirolide C. J. Nat. Prod. 2007, 70, 1878–1883. [Google Scholar] [CrossRef]
- Rambla-Alegre, M.; Miles, C.O.; de la Iglesia, P.; Fernández-Tejedor, M.; Jacobs, S.; Sioen, I.; Verbeke, W.; Samdal, I.A.; Sandvik, M.; Barbosa, V.; et al. Occurrence of cyclic imines in European commercial seafood and consumers risk assessment. Environ. Res. 2018, 161, 392–398. [Google Scholar] [CrossRef]
- Blanco, J.; Martin-Morales, E.; Álvarez, G. Stability of okadaic acid and 13-desmethyl spirolide C in seawater and sediment. Mar. Chem. 2018, 207, 21–25. [Google Scholar] [CrossRef]
- Martín-Morales, E.; Mariño, C.; Arévalo, F.; Correa, J.; Moroño, Á.; Blanco, J. A prospective study of cyclic imines in two Galician aquaculture areas. In Proceedings of the Marine and Freshwater Toxins Analysis Fourth Joint Symposium and AOAC Task Force Meeting, Baiona, Spain, 5–9 May 2013; pp. 5–9. [Google Scholar]
- Rossignoli, A.E.; Lamas, J.P.; Mariño, C.; Martín, H.; Blanco, J. Enzymatic Biotransformation of 13-desmethyl Spirolide C by Two Infaunal Mollusk Species: The Limpet Patella vulgata and the Cockle Cerastoderma edule. Toxins 2022, 14, 848. [Google Scholar] [CrossRef]
- Takada, N.; Umemura, N.; Suenaga, K.; Chou, T.; Nagatsu, A.; Haino, T.; Yamada, K.; Uemura, D. Pinnatoxins B and C, the most toxic components in the pinnatoxin series from the Okinawan bivalve Pinna muricata. Tetrahedron Lett. 2001, 42, 3491–3494. [Google Scholar] [CrossRef]
- Uemura, D.; Chou, T.; Haino, T.; Nagatsu, A.; Fukuzawa, S.; Zheng, S.z.; Chen, H.s. Pinnatoxin A: A toxic amphoteric macrocycle from the Okinawan bivalve Pinna muricata. J. Am. Chem. Soc. 1995, 117, 1155–1156. [Google Scholar] [CrossRef]
- García-Altares, M.; Casanova, A.; Bane, V.; Diogene, J.; Furey, A.; de la Iglesia, P. Confirmation of Pinnatoxins and Spirolides in Shellfish and Passive Samplers from Catalonia (Spain) by Liquid Chromatography Coupled with Triple Quadrupole and High-Resolution Hybrid Tandem Mass Spectrometry. Mar. Drugs 2014, 12, 3706–3732. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.M.; Burton, I.W.; Cembella, A.D.; Curtis, J.M.; Quilliam, M.A.; Walter, J.A.; Wright, J.L.C. Characterization of Spirolides A, C, and 13-Desmethyl C, New Marine Toxins Isolated From Toxic Plankton and Contaminated Shellfish. J. Nat. Prod. 2001, 64, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Van De Waal, D.B.; Tillmann, U.; Martens, H.; Krock, B.; van Scheppingen, Y.; John, U. Characterization of multiple isolates from an Alexandrium ostenfeldii bloom in The Netherlands. Harmful Algae 2015, 49, 94–104. [Google Scholar] [CrossRef]
- Qiu, J.; Rafuse, C.; Lewis, N.I.; Li, A.; Meng, F.; Beach, D.G.; McCarron, P. Screening of cyclic imine and paralytic shellfish toxins in isolates of the genus Alexandrium (Dinophyceae) from Atlantic Canada. Harmful Algae 2018, 77, 108–118. [Google Scholar] [CrossRef]
- Cembella, A.D.; Bauder, A.G.; Lewis, N.I.; Quilliam, M.A. Population dynamics and spirolide composition of the toxigenic dinofladellate Alexandrium ostenfeldii in coastal embayments of Nova Scotia. In Harmful Algal Blooms 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; IOC of UNESCO: Paris, France, 2001; pp. 173–176. [Google Scholar]
- Liu, Y.; Yu, R.C.; Kong, F.Z.; Li, C.; Dai, L.; Chen, Z.F.; Geng, H.X.; Zhou, M.J. Contamination status of lipophilic marine toxins in shellfish samples from the Bohai Sea, China. Environ. Pollut. 2019, 249, 171–180. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Du, S.; Lin, Z.R.; Zhou, Y.Y.; Chen, L.Z.; Yu, R.C.; Zhang, L. Occurrence and distribution of lipophilic phycotoxins in a subtropical bay of the South China Sea. Chemosphere 2020, 243, 125352. [Google Scholar] [CrossRef]
- Ji, Y.; Yan, G.W.; Wang, G.X.; Liu, J.W.; Tang, Z.X.; Yan, Y.J.; Qiu, J.B.; Zhang, L.; Pan, W.Y.; Fu, Y.L.; et al. Prevalence and distribution of domoic acid and cyclic imines in bivalve mollusks from Beibu Gulf, China. J. Hazard. Mater. 2022, 423, 127078. [Google Scholar] [CrossRef]
- Touzet, N.; Franco, J.M.; Raine, R. Morphogenetic diversity and biotoxin composition of Alexandrium (Dinophyceae) in Irish coastal waters. Harmful Algae 2008, 7, 782–797. [Google Scholar] [CrossRef]
- Brown, L.; Bresnan, E.; Graham, J.; Lacaze, J.P.; Turrell, E.; Collins, C. Distribution, diversity and toxin composition of the genus Alexandrium (Dinophyceae) in Scottish waters. Eur. J. Phycol. 2010, 45, 375–393. [Google Scholar] [CrossRef]
- Martens, H.; Van De Waal, D.B.; Brandenburg, K.M.; Krock, B.; Tillmann, U. Salinity effects on growth and toxin production in an Alexandrium ostenfeldii (Dinophyceae) isolate from The Netherlands. J. Plankton Res. 2016, 80, 107–115. [Google Scholar] [CrossRef]
- Moreiras, G.; Leao, J.M.; Gago-Martinez, A. Analysis of Cyclic Imines in Mussels (Mytilus galloprovincialis) from Galicia (NW Spain) by LC-MS/MS. Int. J. Environ. Res. Public Health 2020, 17, 281. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Barreiro, A.; Rodriguez, P.; Otero, P.; Azevedo, J.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New invertebrate vectors for PST, spirolides and okadaic acid in the North Atlantic. Mar. Drugs 2013, 11, 1936–1960. [Google Scholar] [CrossRef] [PubMed]
- Picot, C.; Limon, G.; Durand, G.; Wesolek, N.; Parent-Massin, D.; Roudot, A.C. Domoic Acid, Okadaic Acid and Spirolides: Inter-Species Variability in Contamination and Cooking Effects. Food Public Health 2012, 2, 50–57. [Google Scholar] [CrossRef][Green Version]
- Kremp, A.; Hansen, P.J.; Tillmann, U.; Savela, H.; Suikkanen, S.; Voss, D.; Barrera, F.; Jakobsen, H.H.; Krock, B. Distributions of three Alexandrium species and their toxins across a salinity gradient suggest an increasing impact of GDA producing A. pseudogonyaulax in shallow brackish waters of Northern Europe. Harmful Algae 2019, 87, 101622. [Google Scholar] [CrossRef]
- Burson, A.; Matthijs, H.C.P.; de Bruijne, W.; Talens, R.; Hoogenboom, R.; Gerssen, A.; Visser, P.M.; Stomp, M.; Steur, K.; van Scheppingen, Y.; et al. Termination of a toxic Alexandrium bloom with hydrogen peroxide. Harmful Algae 2014, 31, 125–135. [Google Scholar] [CrossRef]
- Borkman, D.G.; Smayda, T.J.; Tomas, C.R.; York, R.; Strangman, W.; Wright, J.L.C. Toxic Alexandrium peruvianum (Balech and de Mendiola) Balech and in Narragansett Bay, Rhode Island (USA). Harmful Algae 2012, 19, 92–100. [Google Scholar] [CrossRef]
- Bacchiocchi, S.; Siracusa, M.; Campacci, D.; Ciriaci, M.; Dubbini, A.; Tavoloni, T.; Stramenga, A.; Gorbi, S.; Piersanti, A. Cyclic Imines (CIs) in Mussels from North-Central Adriatic Sea: First Evidence of Gymnodimine A in Italy. Toxins 2020, 12, 370. [Google Scholar] [CrossRef]
- Kvrgic, K.; Lesic, T.; Aysal, A.I.; Dzafic, N.; Pleadin, J. Cyclic imines in shellfish and ascidians in the northern Adriatic Sea. Food Addit. Contam. Part B-Surveill. 2020, 14, 12–22. [Google Scholar] [CrossRef]
- Ferrer, L.; Revilla, M.; Laza-Martínez, A.; Sagarminaga, Y.; Fontán, A.; Larreta, J.; Zorita, I.; Solaun, O.; Rodríguez, J.G.; Arantzamendi, L.; et al. Occurrence of the toxic dinoflagellate Alexandrium ostenfeldii in the coastal waters of the southeastern Bay of Biscay. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Rodriguez-Cabo, T.; Morono, A.; Arevalo, F.; Correa, J.; Lamas, J.P.; Rossignoli, A.E.; Blanco, J. Paralytic Shellfish Poisoning (PSP) in Mussels from the Eastern Cantabrian Sea: Toxicity, Toxin Profile, and Co-Occurrence with Cyclic Imines. Toxins 2021, 13, 761. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, I.; Lorenzo, M.N.; deCastro, M. Analysis of chlorophyll a concentration along the Galician coast: Seasonal variability and trends. ICES J. Mar. Sci. 2012, 69, 728–738. [Google Scholar] [CrossRef]
- EURLMB. EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Marine Biotoxins in Molluscs by LC-MS/MS. Version 5. 2014. Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/laboratorios/LNRBM/ARCHIVO2EU-Harmonised-SOP-LIPO-LCMSMS_Version5.pdf (accessed on 27 October 2022).
- Regueiro, J.; Rossignoli, A.E.; Álvarez, G.; Blanco, J. Automated on-line solid-phase extraction coupled to liquid chromatography tandem mass spectrometry for determination of lipophilic marine toxins in shellfish. Food Chemistry 2011, 129, 533–540. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: A Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
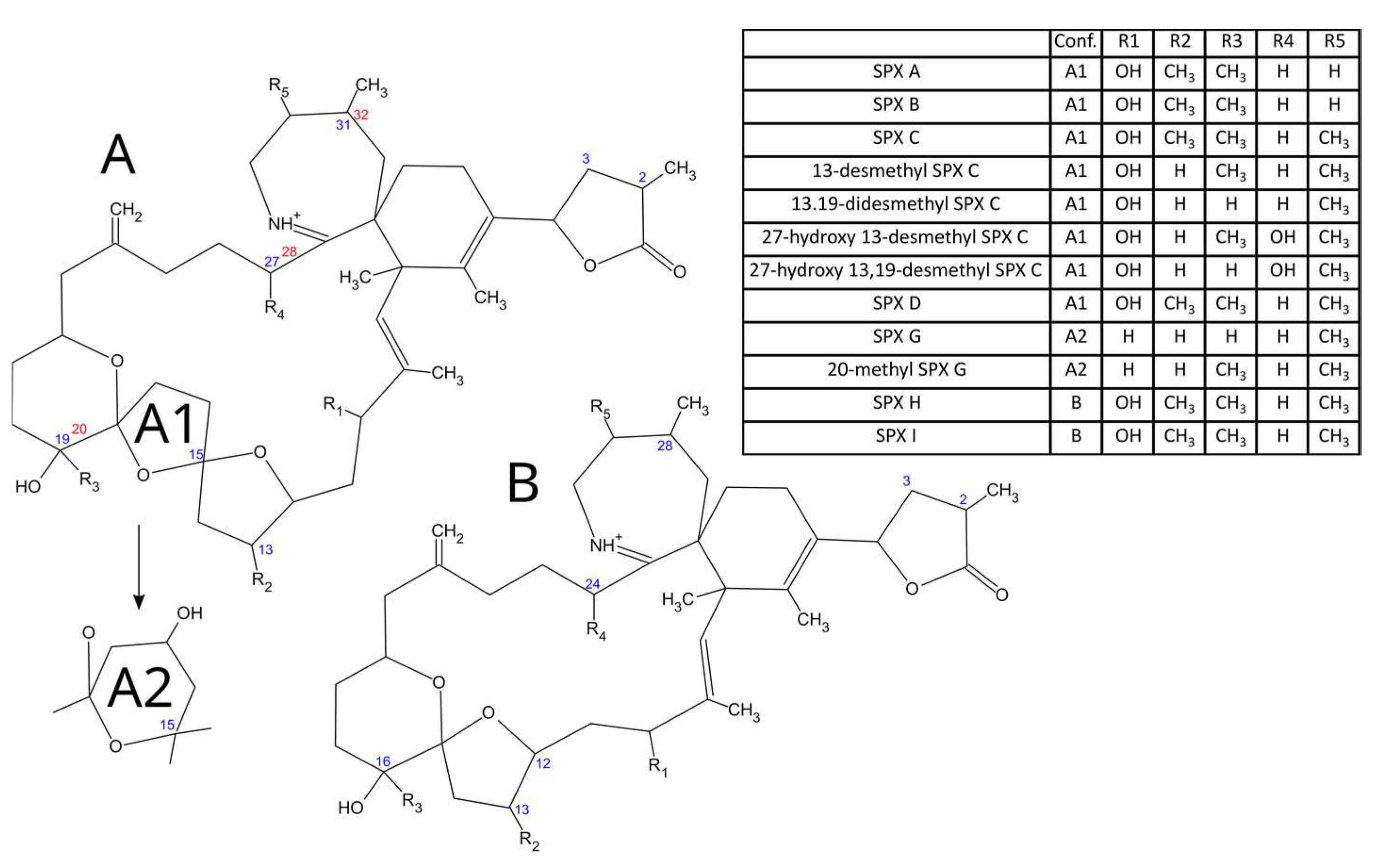




| Spectrum | Collision Energy | Fit | Reverse Fit |
|---|---|---|---|
| 692.5 (MS2) | 60 ± 10 | 91.1 | 87.5 |
| 692.5 > 444.3 (MS3) | 60 ± 10 | 92.8 | 92.6 |
| 692 > 164.2 (MS3) | 60 ± 10 | 89.7 | 84.1 |
| Toxin | MS/MS Transition (m/z) | CE (eV) | |
|---|---|---|---|
| 13desmSPXC and Iso-13desmSPXC | 692.5> | 164.3 | 42 |
| 444.3 | 36 | ||
| 13,19 didesmethyl SPX C | 678.5> | 164 | 42 |
| 430.3 | 36 | ||
| 20-methyl SPX G | 706.5> | 164 | 42 |
| 346.3 | 36 | ||
| Iso 13,19 didesmethyl SPX C | 680> | 164.1 | 42 |
| 432.3 | 42 | ||
| 27 oxo 13,19 didesmethyl SPX C | 692.5> | 178.1 | 42 |
| 444.3 | 42 | ||
| SPX H | 650.5> | 164.1 | 40 |
| 402.3 | 40 | ||
| SPX I | 652.5> | 164.1 | 40 |
| 402.3 | 40 | ||
| SPX A | 692.5> | 150 | 50 |
| 444.3 | 36 | ||
| SPX G | 692.5> | 378.2 | 36 |
| SPX B | 694.4> | 150 | 50 |
| 444.3 | 36 | ||
| 13 desmethyl SPX D | 694.4 | 444.3 | 36 |
| 164 | 50 | ||
| 27 Hydroxy 13,19 desmethyl SPX C | 694.5 | 180.1 | 40 |
| 446.4 | 40 | ||
| SPX C and Iso-SPX C | 706.5> | 164 | 50 |
| 440.3 | 36 | ||
| SPX D | 708.5> | 164 | 50 |
| 458.3 | 36 | ||
| 27-Hydroxy-13-desmethyl SPX C | 708.5 | 180.1 | 40 |
| 460.4 | 40 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, J.; Arévalo, F.; Moroño, Á.; Correa, J.; Rossignoli, A.E.; Lamas, J.P. Spirolides in Bivalve Mollusk of the Galician (NW Spain) Coast: Interspecific, Spatial, Temporal Variation and Presence of an Isomer of 13-Desmethyl Spirolide C. Toxins 2023, 15, 13. https://doi.org/10.3390/toxins15010013
Blanco J, Arévalo F, Moroño Á, Correa J, Rossignoli AE, Lamas JP. Spirolides in Bivalve Mollusk of the Galician (NW Spain) Coast: Interspecific, Spatial, Temporal Variation and Presence of an Isomer of 13-Desmethyl Spirolide C. Toxins. 2023; 15(1):13. https://doi.org/10.3390/toxins15010013
Chicago/Turabian StyleBlanco, Juan, Fabiola Arévalo, Ángeles Moroño, Jorge Correa, Araceli E. Rossignoli, and Juan Pablo Lamas. 2023. "Spirolides in Bivalve Mollusk of the Galician (NW Spain) Coast: Interspecific, Spatial, Temporal Variation and Presence of an Isomer of 13-Desmethyl Spirolide C" Toxins 15, no. 1: 13. https://doi.org/10.3390/toxins15010013
APA StyleBlanco, J., Arévalo, F., Moroño, Á., Correa, J., Rossignoli, A. E., & Lamas, J. P. (2023). Spirolides in Bivalve Mollusk of the Galician (NW Spain) Coast: Interspecific, Spatial, Temporal Variation and Presence of an Isomer of 13-Desmethyl Spirolide C. Toxins, 15(1), 13. https://doi.org/10.3390/toxins15010013







