The Effectiveness of Facet Joint Injection with Steroid and Botulinum Toxin in Severe Lumbar Central Spinal Stenosis: A Randomized Controlled Trial
Abstract
1. Introduction
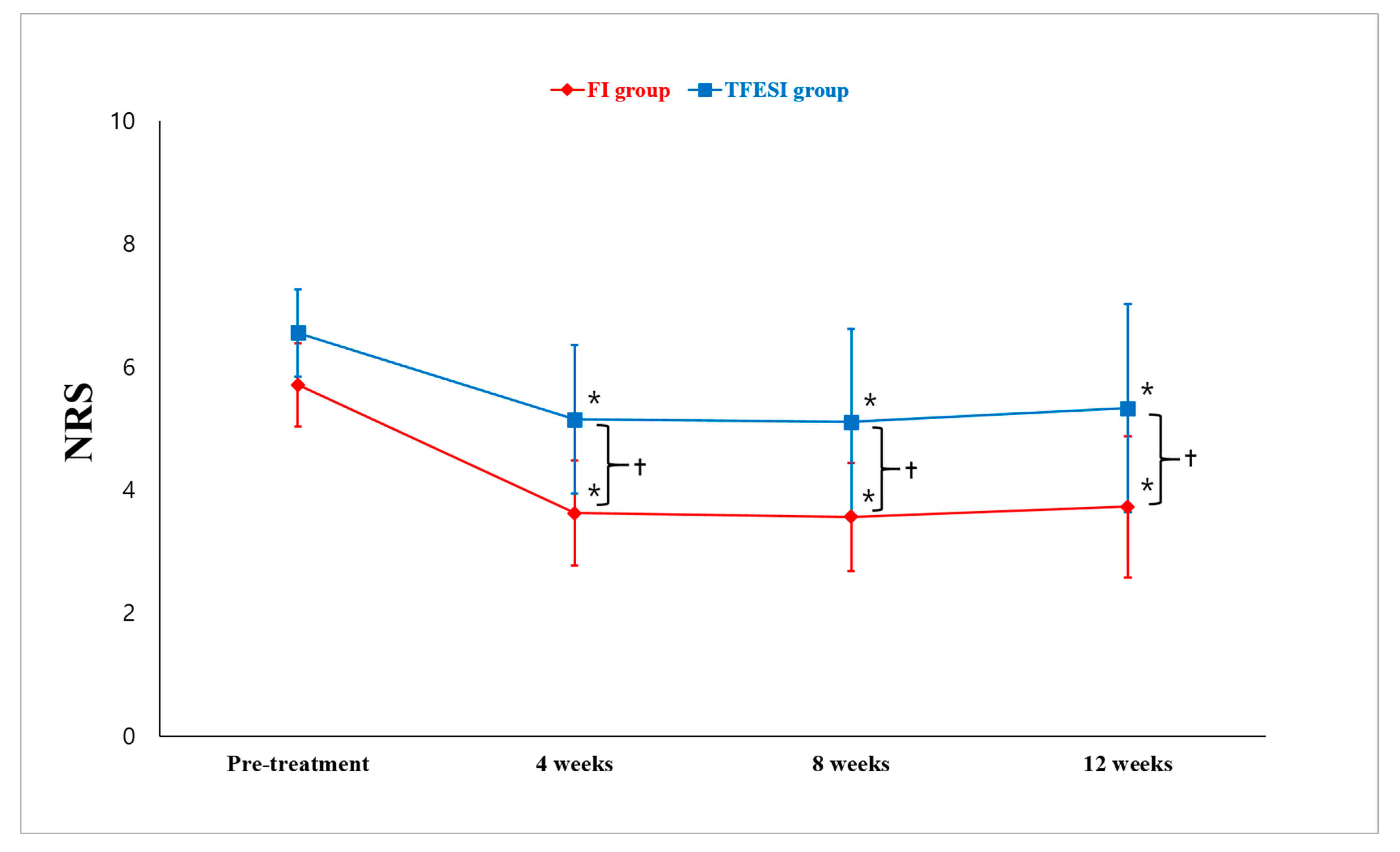
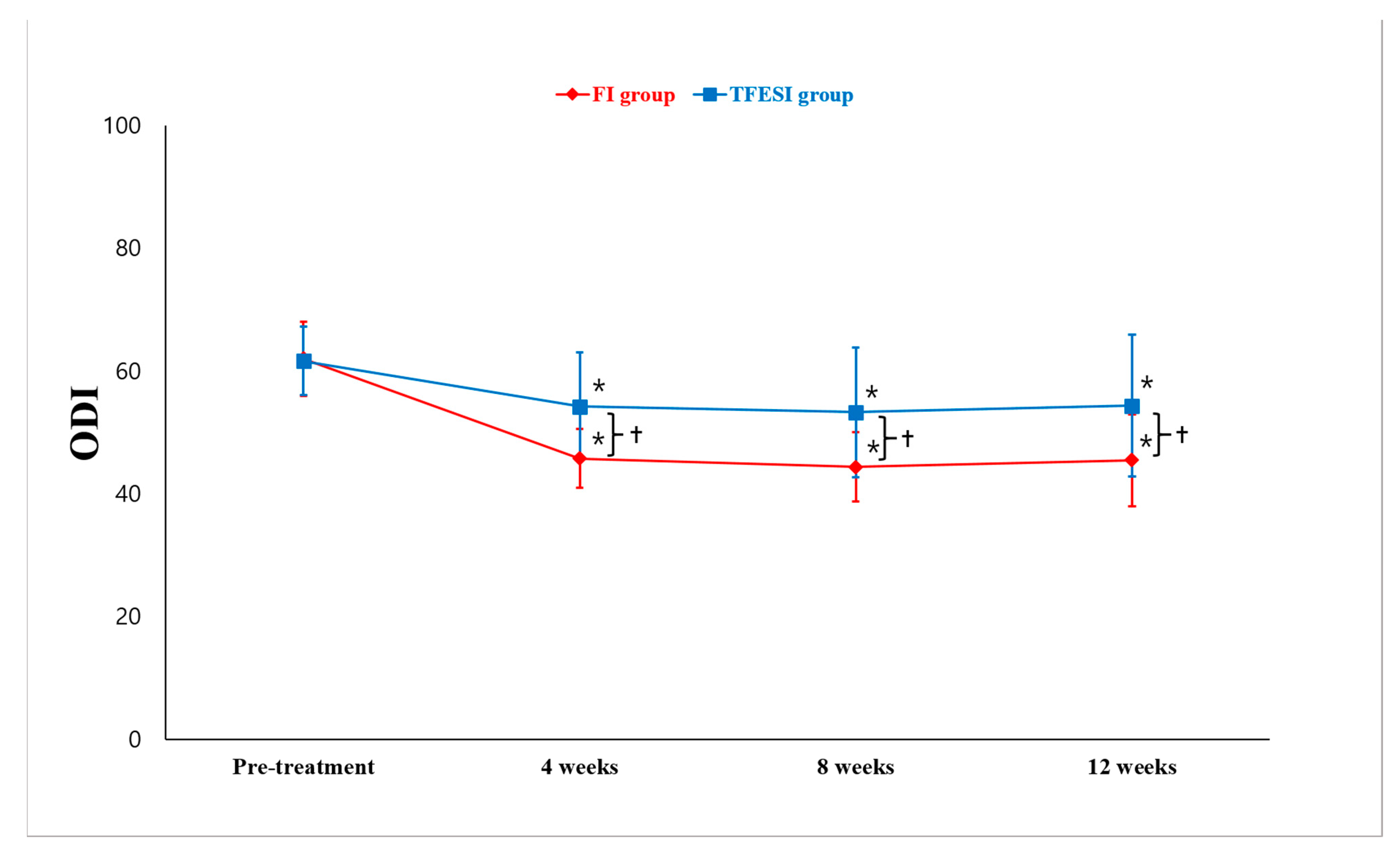
2. Results
3. Discussion
4. Materials and Methods
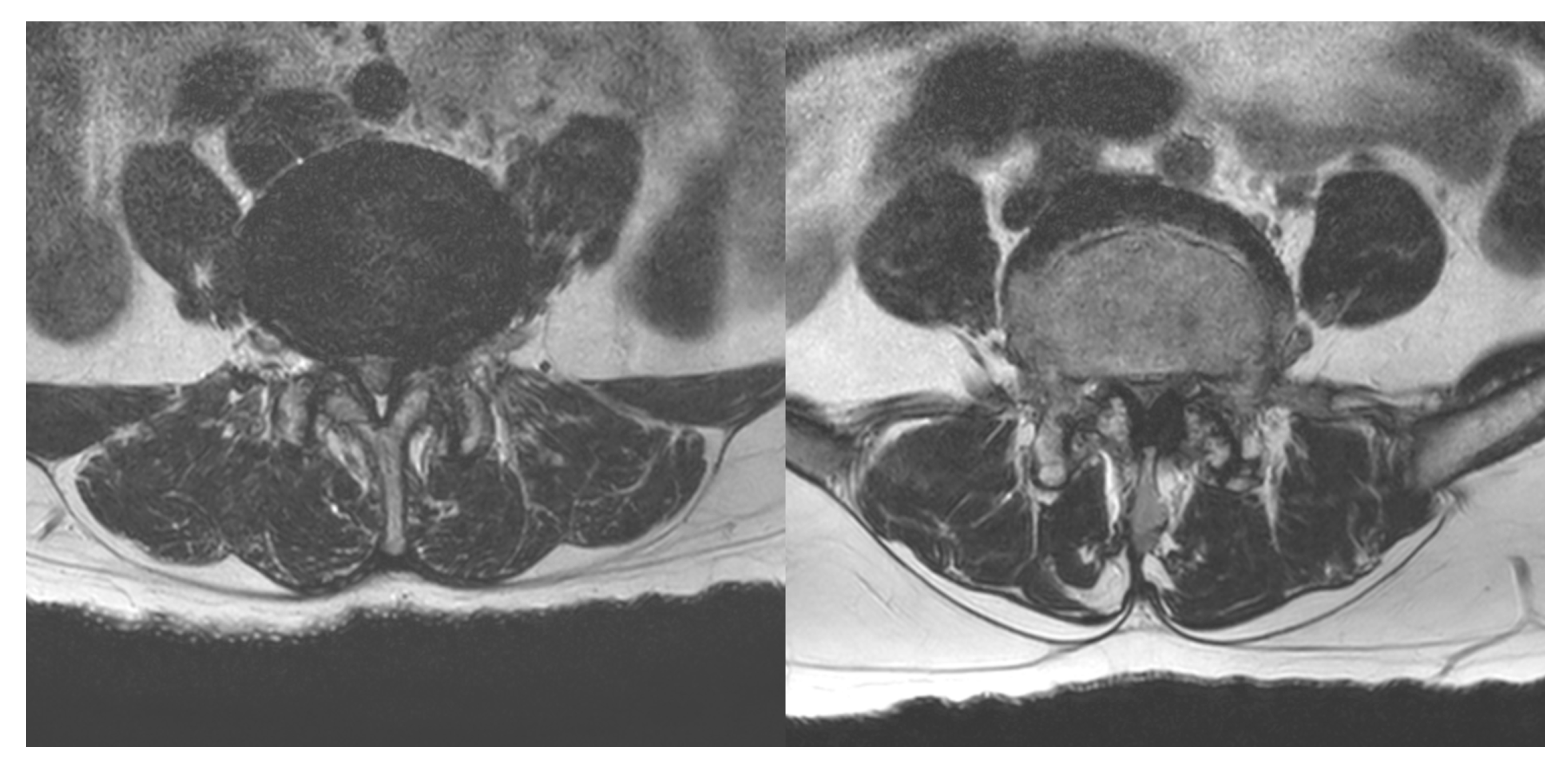
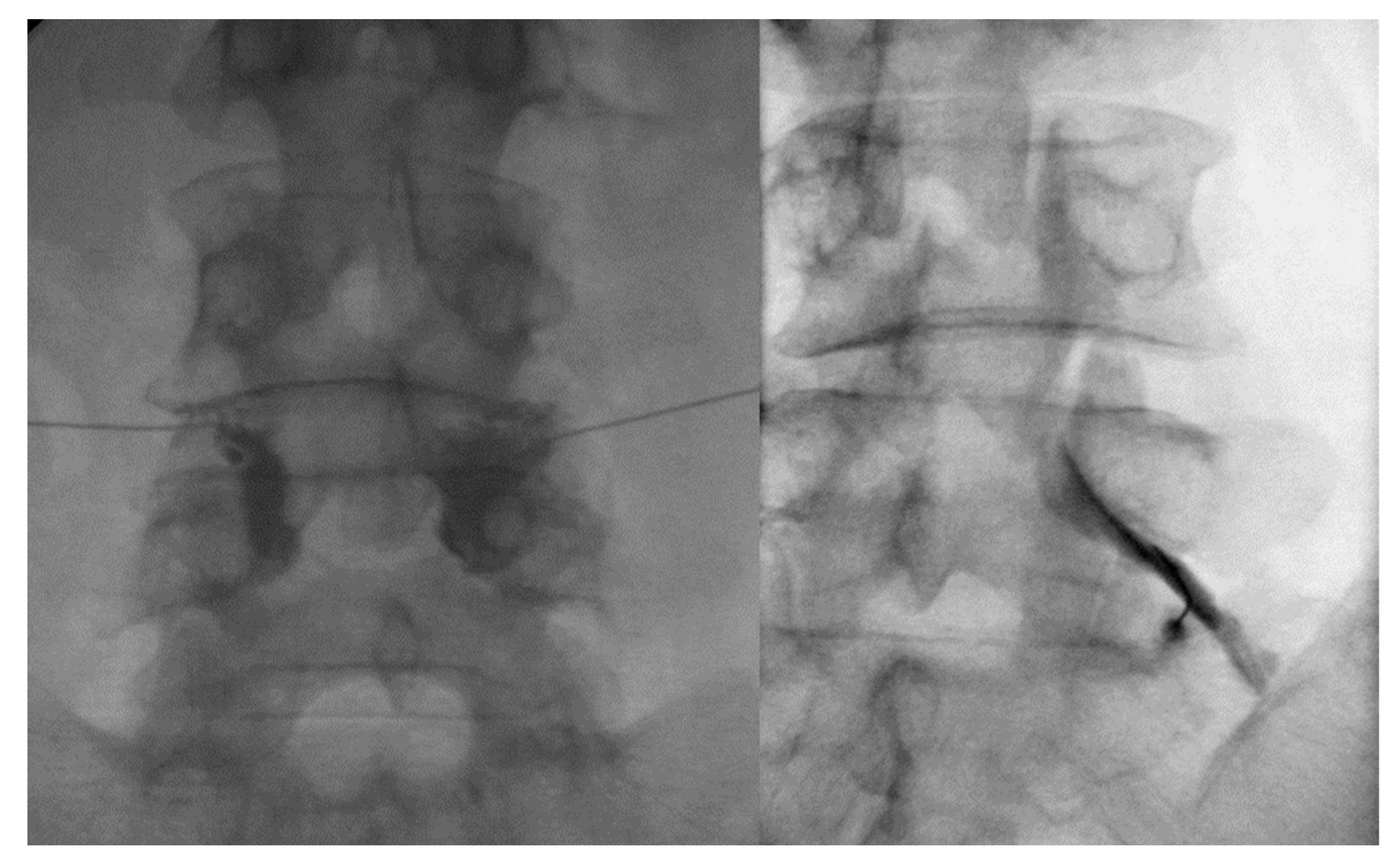
4.1. Facet Joint Injection Procedure
4.2. TFESI Procedure
4.3. Outcomes
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalichman, L.; Cole, R.; Kim, D.H.; Li, L.; Suri, P.; Guermazi, A.; Hunter, D.J. Spinal stenosis prevalence and association with symptoms: The Framingham Study. Spine J. 2009, 9, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Haig, A.J.; Tomkins, C.C. Diagnosis and management of lumbar spinal stenosis. JAMA 2010, 303, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.J.; Chang, M.C. Effectiveness of orthoses for treatment in patients with spinal pain. Yeungnam Univ. J. Med. 2020, 37, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Do, K.H.; Kim, T.H.; Chang, M.C. Effects of interlaminar epidural steroid injection in patients with moderate to severe lumbar central spinal stenosis: A prospective study. Ann. Palliat. Med. 2020, 9, 163–168. [Google Scholar] [CrossRef]
- Macedo, L.G.; Hum, A.; Kuleba, L.; Mo, J.; Truong, L.; Yeung, M.; Battié, M.C. Physical therapy interventions for degenerative lumbar spinal stenosis: A systematic review. Phys. Ther. 2013, 93, 1646–1660. [Google Scholar] [CrossRef]
- Bajpai, S.; Yelavarthi, R. Effect of Intervertebral Level on Interlaminar Epidural Steroid Injection in Lumbar Spinal Canal Stenosis: A Randomized Controlled Trial. Anesth Essays Res. 2020, 14, 100–103. [Google Scholar] [CrossRef]
- Carassiti, M.; Pascarella, G.; Strumia, A.; Russo, F.; Papalia, G.F.; Cataldo, R.; Gargano, F.; Costa, F.; Pierri, M.; De Tommasi, F.; et al. Epidural Steroid Injections for Low Back Pain: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 19, 231. [Google Scholar] [CrossRef]
- Kim, M.; Cho, S.; Noh, Y.; Goh, D.; Son, H.J.; Huh, J.; Kang, S.S.; Hwang, B. Changes in pain scores and walking distance after epidural steroid injection in patients with lumbar central spinal stenosis. Medicine 2022, 101, e29302. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Wu, J.; Gao, X.; Wei, Q.; Lai, X.; An, J. Long-Term Outcomes of Epidurals with Lidocaine with or without Steroids for Lumbar Disc Herniation and Spinal Stenosis: A Meta-Analysis. Pain Physician 2020, 23, 365–374. [Google Scholar]
- Lee, J.H.; Shin, K.H.; Park, S.J.; Lee, G.J.; Lee, C.H.; Kim, D.H.; Kim, D.H.; Yang, H.S. Comparison of Clinical Efficacy between Transforaminal and Interlaminar Epidural Injections in Lumbosacral Disc Herniation: A Systematic Review and Meta-Analysis. Pain Physician 2018, 21, 433–448. [Google Scholar] [CrossRef]
- Lee, J.H.; An, J.H.; Lee, S.H. Comparison of the effectiveness of interlaminar and bilateral transforaminal epidural steroid injections in treatment of patients with lumbosacral disc herniation and spinal stenosis. Clin. J. Pain 2009, 25, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Lutz, G.E.; Vad, V.B.; Wisneski, R.J. Fluoroscopic transforaminal lumbar epidural steroids: An outcome study. Arch. Phys. Med. Rehabil. 1998, 79, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Gharibo, C.G.; Varlotta, G.P.; Rhame, E.E.; Liu, E.C.; Bendo, J.A.; Perloff, M.D. Interlaminar versus transforaminal epidural steroids for the treatment of subacute lumbar radicular pain: A randomized, blinded, prospective outcome study. Pain Physician 2011, 14, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Kamble, P.C.; Sharma, A.; Singh, V.; Natraj, B.; Devani, D.; Khapane, V. Outcome of single level disc prolapse treated with transforaminal steroid versus epidural steroid versus caudal steroids. Eur. Spine J. 2016, 25, 217–221. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Lee, J.W.; Lee, G.Y.; Kang, H.S. Lumbar facet joint injection: Feasibility as an alternative method in high-risk patients. Eur. Radiol. 2013, 23, 3153–3160. [Google Scholar] [CrossRef]
- Shim, E.; Lee, J.W.; Lee, E.; Im, T.; Kang, Y.; Ahn, J.M.; Kang, H.S. Facet joint injection versus epidural steroid injection for lumbar spinal stenosis: Intra-individual study. Clin. Radiol. 2017, 72, 96.e7–96.e14. [Google Scholar] [CrossRef]
- Aoki, K.R. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache 2003, 43 (Suppl. S1), S9–S15. [Google Scholar] [CrossRef]
- Kumar, R. Therapeutic use of botulinum toxin in pain treatment. Neuronal. Signal. 2018, 2, Ns20180058. [Google Scholar] [CrossRef]
- Kwak, D.G.; Kwak, S.G.; Lee, A.Y.; Chang, M.C. Outcome of intra-articular lumbar facet joint corticosteroid injection according to the severity of facet joint arthritis. Exp. Ther. Med. 2019, 18, 4132–4136. [Google Scholar] [CrossRef]
- Yamada, T.; Horikawa, M.; Sato, T.; Kahyo, T.; Takanashi, Y.; Ushirozako, H.; Kurosu, K.; Al Mamun, M.; Mihara, Y.; Oe, S.; et al. Hypertrophy of the ligamentum flavum in lumbar spinal canal stenosis is associated with abnormal accumulation of specific lipids. Sci. Rep. 2021, 11, 23515. [Google Scholar] [CrossRef]
- Netzer, C.; Urech, K.; Hügle, T.; Benz, R.M.; Geurts, J.; Schären, S. Characterization of subchondral bone histopathology of facet joint osteoarthritis in lumbar spinal stenosis. J. Orthop. Res. 2016, 34, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Boswell, M.V.; Colson, J.D.; Sehgal, N.; Dunbar, E.E.; Epter, R. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician 2007, 10, 229–253. [Google Scholar] [CrossRef] [PubMed]
- Somayaji, H.S.; Saifuddin, A.; Casey, A.T.; Briggs, T.W. Spinal cord infarction following therapeutic computed tomography-guided left L2 nerve root injection. Spine 2005, 30, E106–E108. [Google Scholar] [CrossRef] [PubMed]
- Stoll, A.; Sanchez, M. Epidural hematoma after epidural block: Implications for its use in pain management. Surg. Neurol. 2002, 57, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Wybier, M.; Gaudart, S.; Petrover, D.; Houdart, E.; Laredo, J.D. Paraplegia complicating selective steroid injections of the lumbar spine. Report of five cases and review of the literature. Eur. Radiol. 2010, 20, 181–189. [Google Scholar] [CrossRef]
- Ambrosio, L.; Vadalà, G.; Russo, F.; Pascarella, G.; De Salvatore, S.; Papalia, G.F.; Ruggiero, A.; Di Folco, M.; Carassiti, M.; Papalia, R.; et al. Interventional Minimally Invasive Treatments for Chronic Low Back Pain Caused by Lumbar Facet Joint Syndrome: A Systematic Review. Glob. Spine J. 2022, 21925682221142264, (Ahead of print). [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, S.K.; Ahnn, J. Botulinum Toxin as a Pain Killer: Players and Actions in Antinociception. Toxins 2015, 7, 2435–2453. [Google Scholar] [CrossRef]
- Schizas, C.; Theumann, N.; Burn, A.; Tansey, R.; Wardlaw, D.; Smith, F.W.; Kulik, G. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine 2010, 35, 1919–1924. [Google Scholar] [CrossRef]
| FI Group | TFESI Group | p Value | |
|---|---|---|---|
| Age, years | 69.4 ± 7.5 | 69.2 ± 7.1 | 0.870 |
| Sex (M:F), n | 18:32 | 21:29 | 0.539 |
| Level (L2–3:L3–4:L4–5), n | 1:4:45 | 0:1:49 | 0.204 |
| Pain duration, months | 28.9 ± 17.3 | 28.4 ± 16.9 | 0.889 |
| Stenosis grade (severe:extreme) | 12:38 | 20:30 | 0.086 |
| Initial NRS | 6.5 ± 0.7 | 6.6 ± 0.7 | 0.564 |
| Initial MODI | 62.0 ± 6.1 | 61.6 ± 5.6 | 0.758 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Choi, H.H.; Chang, M.C. The Effectiveness of Facet Joint Injection with Steroid and Botulinum Toxin in Severe Lumbar Central Spinal Stenosis: A Randomized Controlled Trial. Toxins 2023, 15, 11. https://doi.org/10.3390/toxins15010011
Lee SH, Choi HH, Chang MC. The Effectiveness of Facet Joint Injection with Steroid and Botulinum Toxin in Severe Lumbar Central Spinal Stenosis: A Randomized Controlled Trial. Toxins. 2023; 15(1):11. https://doi.org/10.3390/toxins15010011
Chicago/Turabian StyleLee, Sang Hoon, Hyun Hee Choi, and Min Cheol Chang. 2023. "The Effectiveness of Facet Joint Injection with Steroid and Botulinum Toxin in Severe Lumbar Central Spinal Stenosis: A Randomized Controlled Trial" Toxins 15, no. 1: 11. https://doi.org/10.3390/toxins15010011
APA StyleLee, S. H., Choi, H. H., & Chang, M. C. (2023). The Effectiveness of Facet Joint Injection with Steroid and Botulinum Toxin in Severe Lumbar Central Spinal Stenosis: A Randomized Controlled Trial. Toxins, 15(1), 11. https://doi.org/10.3390/toxins15010011





