Intramuscular Neural Distribution of the Serratus Anterior Muscle: Regarding Botulinum Neurotoxin Injection for Treating Myofascial Pain Syndrome
Abstract
:1. Introduction
2. Results
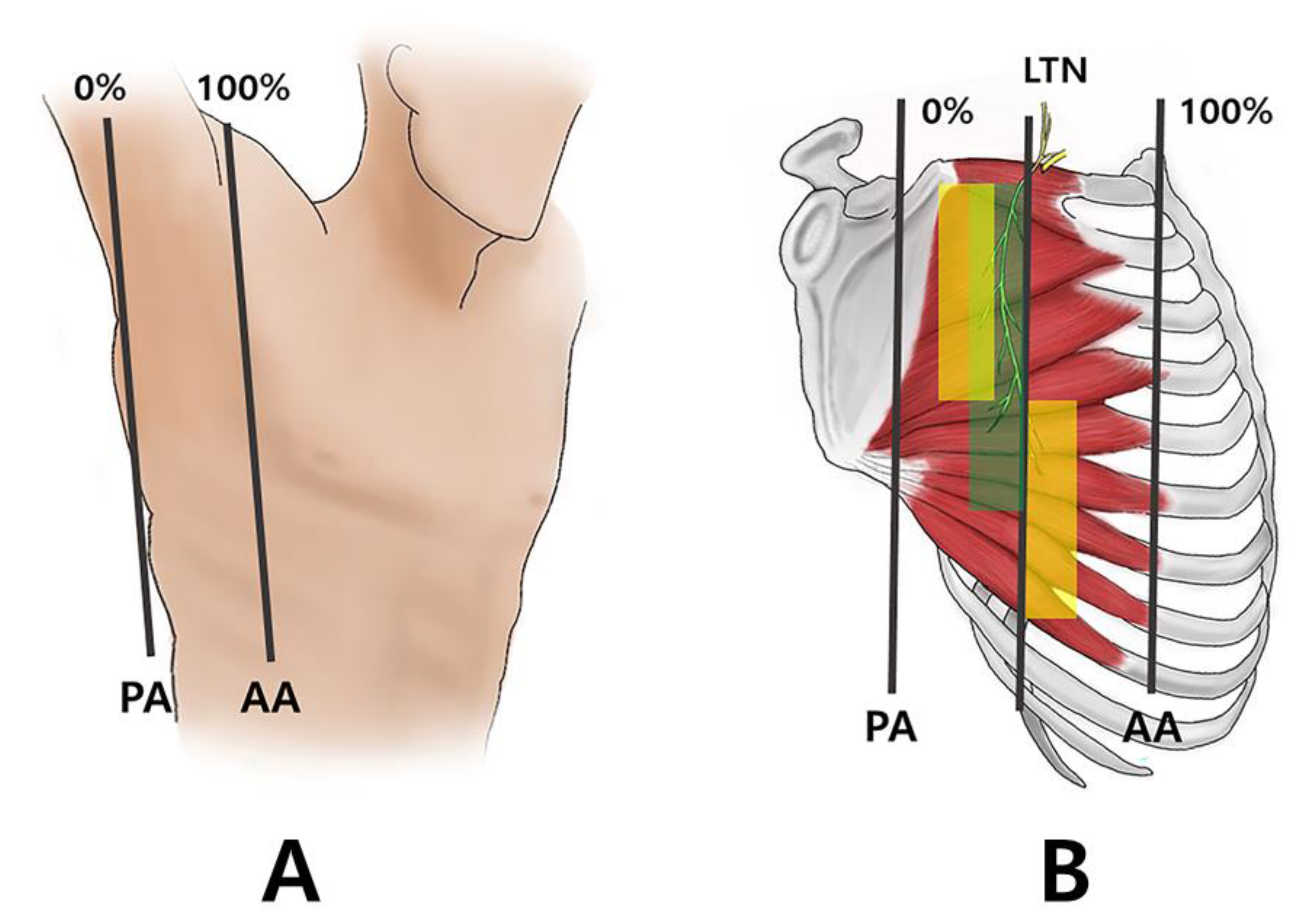
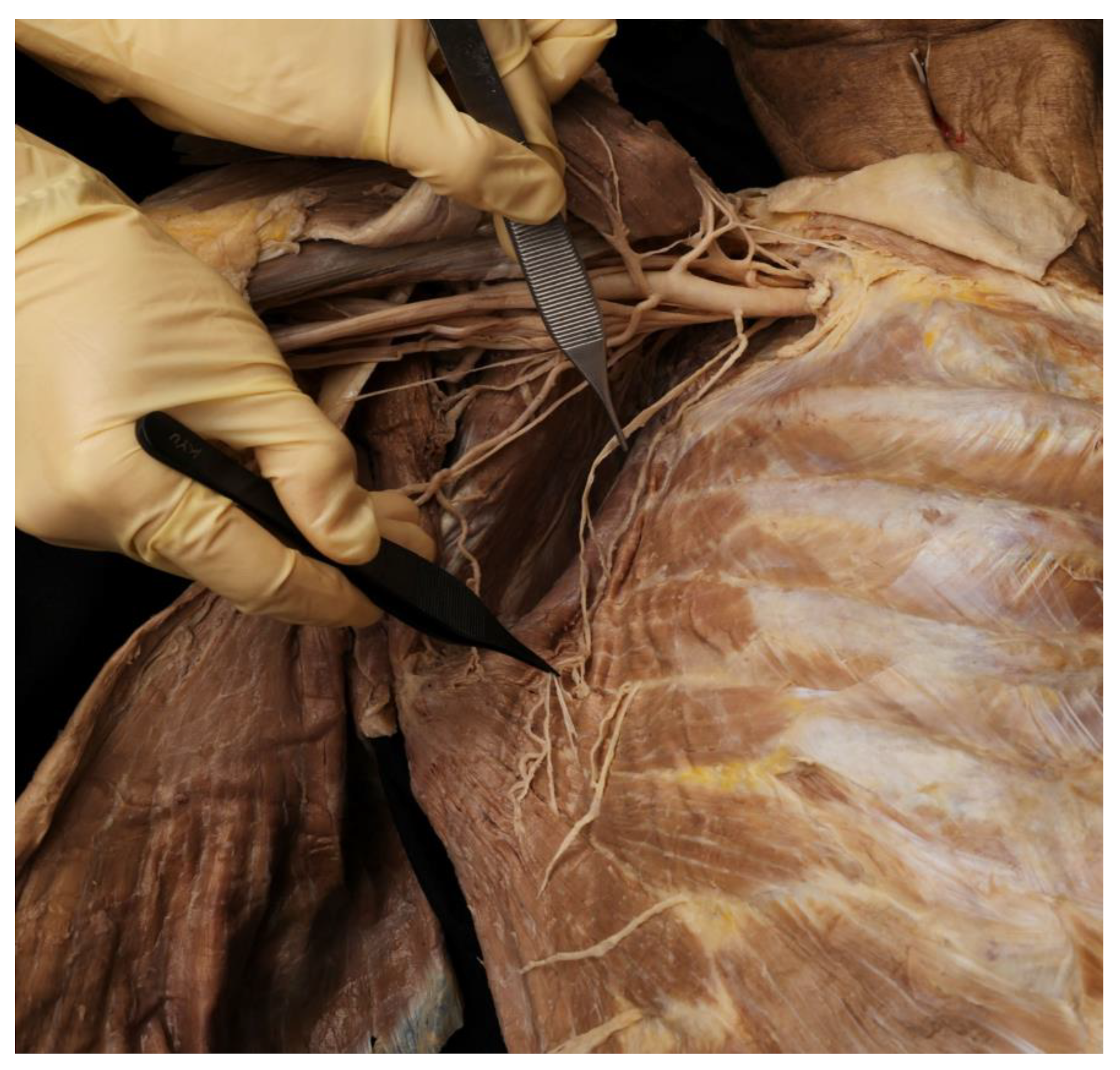
2.1. Running of the Thoracic Nerve Trunk
2.2. Intramuscular Arborization Patterns of the SA Muscle
3. Discussion
3.1. Anatomy of the SA Muscle
3.2. MPS in SA Muscle
4. Materials and Methods
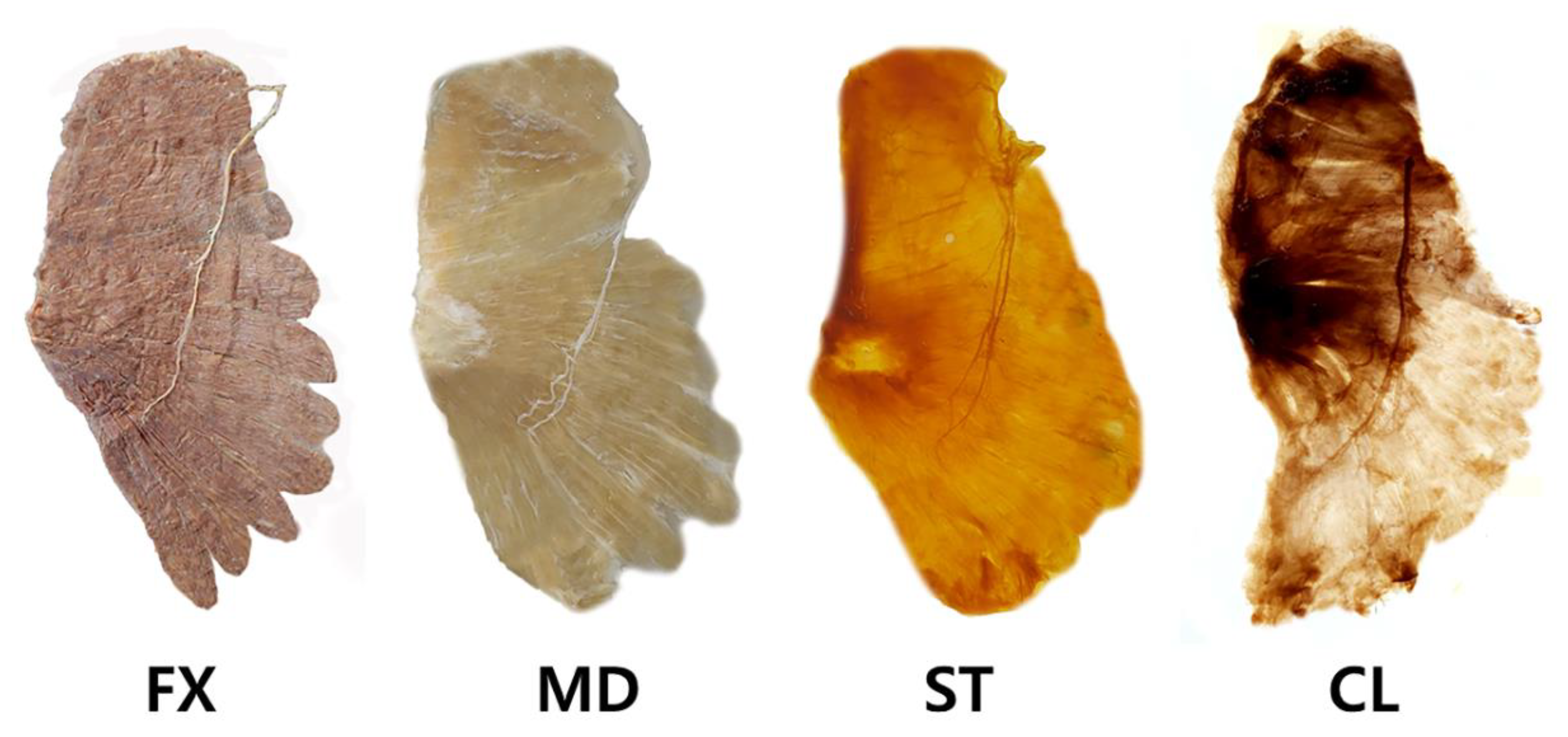
Modified Sihler Staining
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarez, D.J.; Rockwell, P.G. Trigger points: Diagnosis and management. Am. Fam. Physician 2002, 65, 653–660. [Google Scholar] [PubMed]
- Bautista, A.; Webb, C.; Rosenquist, R. Serratus Anterior Muscle Pain Syndrome: A Diagnostic Conundrum. Pain Med. 2017, 18, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.M.; Simons, D.G. Travell, Simons & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2019. [Google Scholar]
- Lee, S.T.; Moon, J.; Lee, S.H.; Cho, K.H.; Im, S.H.; Kim, M.; Min, K. Changes in Activation of Serratus Anterior, Trapezius and Latissimus Dorsi With Slouched Posture. Ann. Rehabil. Med. 2016, 40, 318–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfredini, D.; Cocilovo, F.; Stellini, E.; Favero, L.; Guarda-Nardini, L. Surface electromyography findings in unilateral myofascial pain patients: Comparison of painful vs. non painful sides. Pain Med. 2013, 14, 1848–1853. [Google Scholar] [CrossRef] [Green Version]
- Szyszka-Sommerfeld, L.; Machoy, M.; Lipski, M.; Wozniak, K. The Diagnostic Value of Electromyography in Identifying Patients with Pain-Related Temporomandibular Disorders. Front. Neurol. 2019, 10, 180. [Google Scholar] [CrossRef]
- Saxena, A.; Chansoria, M.; Tomar, G.; Kumar, A. Myofascial pain syndrome: An overview. J. Pain Palliat. Care Pharmacother. 2015, 29, 16–21. [Google Scholar] [CrossRef]
- Lluch, E.; Arguisuelas, M.D.; Coloma, P.S.; Palma, F.; Rey, A.; Falla, D. Effects of deep cervical flexor training on pressure pain thresholds over myofascial trigger points in patients with chronic neck pain. J. Manipulative Physiol. Ther. 2013, 36, 604–611. [Google Scholar] [CrossRef]
- Javanshir, K.; Ortega-Santiago, R.; Mohseni-Bandpei, M.A.; Miangolarra-Page, J.C.; Fernandez-de-Las-Penas, C. Exploration of somatosensory impairments in subjects with mechanical idiopathic neck pain: A preliminary study. J. Manipulative Physiol. Ther. 2010, 33, 493–499. [Google Scholar] [CrossRef]
- Iglesias-Gonzalez, J.J.; Munoz-Garcia, M.T.; Rodrigues-de-Souza, D.P.; Alburquerque-Sendin, F.; Fernandez-de-Las-Penas, C. Myofascial trigger points, pain, disability, and sleep quality in patients with chronic nonspecific low back pain. Pain Med. 2013, 14, 1964–1970. [Google Scholar] [CrossRef]
- Duyur Cakit, B.; Genc, H.; Altuntas, V.; Erdem, H.R. Disability and related factors in patients with chronic cervical myofascial pain. Clin. Rheumatol. 2009, 28, 647–654. [Google Scholar] [CrossRef]
- Childers, M.K. Targeting the neuromuscular junction in skeletal muscles. Am. J. Phys. Med. Rehabil. 2004, 83, S38–S44. [Google Scholar] [CrossRef]
- Comella, C.L. The treatment of cervical dystonia with botulinum toxins. J. Neural Transm. 2008, 115, 579–583. [Google Scholar] [CrossRef]
- Yi, K.H.; Choi, Y.J.; Cong, L.; Lee, K.L.; Hu, K.S.; Kim, H.J. Effective botulinum toxin injection guide for treatment of cervical dystonia. Clin. Anat. 2020, 33, 192–198. [Google Scholar] [CrossRef]
- Vasileiadis, G.I.; Sakellariou, V.I.; Papagelopoulos, P.J.; Zoubos, A.B. Posttraumatic focal dystonia of the shoulder. Orthopedics 2012, 35, e977–e980. [Google Scholar] [CrossRef] [Green Version]
- Climent, J.M.; Kuan, T.S.; Fenollosa, P.; Martin-Del-Rosario, F. Botulinum toxin for the treatment of myofascial pain syndromes involving the neck and back: A review from a clinical perspective. Evid. Based Complement. Alternat. Med. 2013, 2013, 381459. [Google Scholar] [CrossRef] [Green Version]
- Kwanchuay, P.; Petchnumsin, T.; Yiemsiri, P.; Pasuk, N.; Srikanok, W.; Hathaiareerug, C. Efficacy and Safety of Single Botulinum Toxin Type A (Botox(R)) Injection for Relief of Upper Trapezius Myofascial Trigger Point: A Randomized, Double-Blind, Placebo-Controlled Study. J. Med. Assoc. Thai 2015, 98, 1231–1236. [Google Scholar]
- Xie, P.; Qin, B.; Yang, F.; Yu, T.; Yu, J.; Wang, J.; Zheng, H. Lidocaine Injection in the Intramuscular Innervation Zone Can Effectively Treat Chronic Neck Pain Caused by MTrPs in the Trapezius Muscle. Pain Physician 2015, 18, E815–E826. [Google Scholar]
- Mor, N.; Tang, C.; Blitzer, A. Temporomandibular Myofacial Pain Treated with Botulinum Toxin Injection. Toxins 2015, 7, 2791–2800. [Google Scholar] [CrossRef] [Green Version]
- Chaurand, J.; Pacheco-Ruiz, L.; Orozco-Saldivar, H.; Lopez-Valdes, J. Efficacy of botulinum toxin therapy in treatment of myofascial pain. J. Oral. Sci. 2017, 59, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Layeeque, R.; Hochberg, J.; Siegel, E.; Kunkel, K.; Kepple, J.; Henry-Tillman, R.S.; Dunlap, M.; Seibert, J.; Klimberg, V.S. Botulinum toxin infiltration for pain control after mastectomy and expander reconstruction. Ann. Surg. 2004, 240, 608–613; discussion 613–604. [Google Scholar] [CrossRef]
- Rosales, R.L.; Kong, K.H.; Goh, K.J.; Kumthornthip, W.; Mok, V.C.; Delgado-De Los Santos, M.M.; Chua, K.S.; Abdullah, S.J.; Zakine, B.; Maisonobe, P.; et al. Botulinum toxin injection for hypertonicity of the upper extremity within 12 weeks after stroke: A randomized controlled trial. Neurorehabil. Neural Repair 2012, 26, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, B.B.; Cozens, J.A.; Bamford, J.M.; Chamberlain, M.A. Use of botulinum toxin in stroke patients with severe upper limb spasticity. J. Neurol. Neurosurg. Psychiatry 1996, 61, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brashear, A.; Gordon, M.F.; Elovic, E.; Kassicieh, V.D.; Marciniak, C.; Do, M.; Lee, C.H.; Jenkins, S.; Turkel, C.; Botox Post-Stroke Spasticity Study, G. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N. Engl. J. Med. 2002, 347, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S.; Jahnke, M.T.; Luecke, D.; Mauritz, K.H. Short-term electrical stimulation enhances the effectiveness of Botulinum toxin in the treatment of lower limb spasticity in hemiparetic patients. Neurosci. Lett. 1995, 201, 37–40. [Google Scholar] [CrossRef]
- Van Campenhout, A.; Verhaegen, A.; Pans, S.; Molenaers, G. Botulinum toxin type A injections in the psoas muscle of children with cerebral palsy: Muscle atrophy after motor end plate-targeted injections. Res. Dev. Disabil. 2013, 34, 1052–1058. [Google Scholar] [CrossRef]
- Gracies, J.M.; Lugassy, M.; Weisz, D.J.; Vecchio, M.; Flanagan, S.; Simpson, D.M. Botulinum toxin dilution and endplate targeting in spasticity: A double-blind controlled study. Arch. Phys. Med. Rehabil. 2009, 90, 9–16.e12. [Google Scholar] [CrossRef]
- Kinnett, D. Botulinum toxin A injections in children: Technique and dosing issues. Am. J. Phys. Med. Rehabil. 2004, 83, S59–S64. [Google Scholar] [CrossRef]
- Hsu, T.S.; Dover, J.S.; Arndt, K.A. Effect of volume and concentration on the diffusion of botulinum exotoxin A. Arch. Dermatol. 2004, 140, 1351–1354. [Google Scholar] [CrossRef]
- Peng, H.P.; Peng, J.H. Complications of botulinum toxin injection for masseter hypertrophy: Incidence rate from 2036 treatments and summary of causes and preventions. J. Cosmet. Dermatol. 2018, 17, 33–38. [Google Scholar] [CrossRef]
- Song, J.H.; Cho, E.S.; Kim, S.T.; Ahn, H.J. Change of distribution and timing of bite force after botulinum toxin type A injection evaluated by a computerized occlusion analysis system. Yonsei Med. J. 2014, 55, 1123–1129. [Google Scholar] [CrossRef] [Green Version]
- Rafferty, K.L.; Liu, Z.J.; Ye, W.; Navarrete, A.L.; Nguyen, T.T.; Salamati, A.; Herring, S.W. Botulinum toxin in masticatory muscles: Short- and long-term effects on muscle, bone, and craniofacial function in adult rabbits. Bone 2012, 50, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Yi, K.H.; Cong, L.; Bae, J.H.; Park, E.S.; Rha, D.W.; Kim, H.J. Neuromuscular structure of the tibialis anterior muscle for functional electrical stimulation. Surg. Radiol. Anat. 2017, 39, 77–83. [Google Scholar] [CrossRef]
- Yi, K.H.; Rha, D.W.; Lee, S.C.; Cong, L.; Lee, H.J.; Lee, Y.W.; Kim, H.J.; Hu, K.S. Intramuscular nerve distribution pattern of ankle invertor muscles in human cadaver using sihler stain. Muscle Nerve 2016, 53, 742–747. [Google Scholar] [CrossRef]
- Rha, D.W.; Yi, K.H.; Park, E.S.; Park, C.; Kim, H.J. Intramuscular nerve distribution of the hamstring muscles: Application to treating spasticity. Clin. Anat. 2016, 29, 746–751. [Google Scholar] [CrossRef]
- Yi, K.-H.; Lee, H.-J.; Seo, K.K.; Kim, H.-J. Intramuscular Neural Arborization of the Latissimus Dorsi Muscle: Application of Botulinum Neurotoxin Injection in Flap Reconstruction. Toxins 2022, 14, 107. [Google Scholar] [CrossRef]
- Yi, K.H.; Lee, H.J.; Seo, K.K.; Kim, H.J. Botulinum neurotoxin injection guidelines regarding flap surgeries in breast reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2021, 75, 503–505. [Google Scholar] [CrossRef]
- Yi, K.H.; Lee, J.H.; Kim, H.M.; Kim, H.J. The botulinum neurotoxin for pain control after breast reconstruction: Neural distribution of the pectoralis major muscle. Reg. Anesth. Pain Med. 2022. [Google Scholar] [CrossRef]
- Yi, K.H.; Lee, H.J.; Lee, J.H.; Lee, K.L.; Kim, H.J. Effective botulinum neurotoxin injection in treating iliopsoas spasticity. Clin. Anat. 2021, 34, 431–436. [Google Scholar] [CrossRef]
- Yi, K.H.; Lee, H.J.; Choi, Y.J.; Lee, K.; Lee, J.H.; Kim, H.J. Anatomical guide for botulinum neurotoxin injection: Application to cosmetic shoulder contouring, pain syndromes, and cervical dystonia. Clin. Anat. 2021, 34, 822–828. [Google Scholar] [CrossRef]
- Yi, K.H.; Lee, K.L.; Lee, J.H.; Hu, H.W.; Lee, K.; Seo, K.K.; Kim, H.J. Guidelines for botulinum neurotoxin injections in piriformis syndrome. Clin. Anat. 2021, 34, 1028–1034. [Google Scholar] [CrossRef]
- Yi, K.H.; Lee, H.J.; Lee, J.H.; Seo, K.K.; Kim, H.J. Application of Botulinum Neurotoxin Injections in TRAM Flap for Breast Reconstruction: Intramuscular Neural Arborization of the Rectus Abdominis Muscle. Toxins 2021, 13, 269. [Google Scholar] [CrossRef]
- Yi, K.H.; Lee, J.H.; Lee, D.K.; Hu, H.W.; Seo, K.K.; Kim, H.J. Anatomical locations of the motor endplates of sartorius muscle for botulinum toxin injections in treatment of muscle spasticity. Surg. Radiol. Anat. 2021, 43, 2025–2030. [Google Scholar] [CrossRef] [PubMed]
- Hamada, J.; Igarashi, E.; Akita, K.; Mochizuki, T. A cadaveric study of the serratus anterior muscle and the long thoracic nerve. J. Shoulder Elbow Surg. 2008, 17, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Agur, A.M.R.; Dalley, A.F. Clinically Oriented Anatomy; Wolters Kluwer India Pvt, Ltd.: Gurugram, India, 2019. [Google Scholar]
- Webb, A.L.; O’Sullivan, E.; Stokes, M.; Mottram, S. A novel cadaveric study of the morphometry of the serratus anterior muscle: One part, two parts, three parts, four? Anat. Sci. Int. 2018, 93, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasu, H.; Yamaguchi, K.; Nimura, A.; Akita, K. An anatomic study of structure and innervation of the serratus anterior muscle. Surg. Radiol. Anat. 2012, 34, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Fishbain, D.A.; Goldberg, M.; Meagher, B.R.; Steele, R.; Rosomoff, H. Male and female chronic pain patients categorized by DSM-III psychiatric diagnostic criteria. Pain 1986, 26, 181–197. [Google Scholar] [CrossRef]
- Tough, E.A.; White, A.R.; Richards, S.; Campbell, J. Variability of criteria used to diagnose myofascial trigger point pain syndrome—Evidence from a review of the literature. Clin. J. Pain. 2007, 23, 278–286. [Google Scholar] [CrossRef]
- Torres Lacomba, M.; Mayoral del Moral, O.; Coperias Zazo, J.L.; Gerwin, R.D.; Goni, A.Z. Incidence of myofascial pain syndrome in breast cancer surgery: A prospective study. Clin. J. Pain 2010, 26, 320–325. [Google Scholar] [CrossRef] [Green Version]
- McPartland, J.M. Travell trigger points—Molecular and osteopathic perspectives. J. Am. Osteopath Assoc. 2004, 104, 244–249. [Google Scholar]
- Shah, J.P.; Gilliams, E.A. Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: An application of muscle pain concepts to myofascial pain syndrome. J. Bodyw. Mov. Ther. 2008, 12, 371–384. [Google Scholar] [CrossRef]
- Hsieh, R.L.; Lee, W.C. Are the effects of botulinum toxin injection on myofascial trigger points placebo effects or needling effects? Arch. Phys. Med. Rehabil. 2008, 89, 792–793; author reply 793. [Google Scholar] [CrossRef]
- Tepper, S.J. Treatment of headache pain with botulinum neurotoxins. Pain Pract. 2004, 4 (Suppl. 1), S38–S46. [Google Scholar] [CrossRef]
- Ondo, W.G.; Vuong, K.D.; Derman, H.S. Botulinum toxin A for chronic daily headache: A randomized, placebo-controlled, parallel design study. Cephalalgia 2004, 24, 60–65. [Google Scholar] [CrossRef]
- Troost, B.T. Botulinum toxin type A (Botox) in the treatment of migraine and other headaches. Expert Rev. Neurother. 2004, 4, 27–31. [Google Scholar] [CrossRef]
- Giamberardino, M.A.; Affaitati, G.; Fabrizio, A.; Costantini, R. Myofascial pain syndromes and their evaluation. Best Pract. Res. Clin. Rheumatol. 2011, 25, 185–198. [Google Scholar] [CrossRef]
- Robertson, C.E.; Garza, I. Critical analysis of the use of onabotulinumtoxinA (botulinum toxin type A) in migraine. Neuropsychiatr. Dis. Treat 2012, 8, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, R.; Yaksh, T.L. Therapeutic use of botulinum toxin in migraine: Mechanisms of action. Br. J. Pharmacol. 2014, 171, 4177–4192. [Google Scholar] [CrossRef]
- Kamanli, A.; Kaya, A.; Ardicoglu, O.; Ozgocmen, S.; Zengin, F.O.; Bayik, Y. Comparison of lidocaine injection, botulinum toxin injection, and dry needli.ing to trigger points in myofascial pain syndrome. Rheumatol. Int. 2005, 25, 604–611. [Google Scholar] [CrossRef]
- Gobel, H.; Heinze, A.; Heinze-Kuhn, K.; Jost, W.H. Evidence-based medicine: Botulinum toxin A in migraine and tension-type headache. J. Neurol. 2001, 248 (Suppl. 1), 34–38. [Google Scholar] [CrossRef]
- Dodick, D.W. Botulinum neurotoxin for the treatment of migraine and other primary headache disorders: From bench to bedside. Headache 2003, 43 (Suppl. 1), S25–S33. [Google Scholar] [CrossRef]
- Chan, V.W.; McCabe, E.J.; MacGregor, D.L. Botox treatment for migraine and chronic daily headache in adolescents. J. Neurosci. Nurs. 2009, 41, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.H.; Lee, H.J.; Choi, Y.J.; Lee, J.H.; Hu, K.S.; Kim, H.J. Intramuscular Neural Distribution of Rhomboid Muscles: Evaluation for Botulinum Toxin Injection Using Modified Sihler’s Method. Toxins 2020, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Wiater, J.M.; Flatow, E.L. Long thoracic nerve injury. Clin. Orthop. Relat. Res. 1999, 368, 17–27. [Google Scholar] [CrossRef]
- Helgadottir, H.; Kristjansson, E.; Einarsson, E.; Karduna, A.; Jonsson, H., Jr. Altered activity of the serratus anterior during unilateral arm elevation in patients with cervical disorders. J. Electromyogr. Kinesiol. 2011, 21, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Liem, R.S.; Douwe van Willigen, J. In toto staining and preservation of peripheral nervous tissue. Stain Technol. 1988, 63, 113–120. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, K.-H.; Lee, J.-H.; Kim, H.-J. Intramuscular Neural Distribution of the Serratus Anterior Muscle: Regarding Botulinum Neurotoxin Injection for Treating Myofascial Pain Syndrome. Toxins 2022, 14, 271. https://doi.org/10.3390/toxins14040271
Yi K-H, Lee J-H, Kim H-J. Intramuscular Neural Distribution of the Serratus Anterior Muscle: Regarding Botulinum Neurotoxin Injection for Treating Myofascial Pain Syndrome. Toxins. 2022; 14(4):271. https://doi.org/10.3390/toxins14040271
Chicago/Turabian StyleYi, Kyu-Ho, Ji-Hyun Lee, and Hee-Jin Kim. 2022. "Intramuscular Neural Distribution of the Serratus Anterior Muscle: Regarding Botulinum Neurotoxin Injection for Treating Myofascial Pain Syndrome" Toxins 14, no. 4: 271. https://doi.org/10.3390/toxins14040271
APA StyleYi, K.-H., Lee, J.-H., & Kim, H.-J. (2022). Intramuscular Neural Distribution of the Serratus Anterior Muscle: Regarding Botulinum Neurotoxin Injection for Treating Myofascial Pain Syndrome. Toxins, 14(4), 271. https://doi.org/10.3390/toxins14040271





