Baseline Susceptibility and Resistance Allele Frequency in Ostrinia furnacalis in Relation to Cry1Ab Toxins in China
Abstract
:1. Introduction
2. Results
2.1. Baseline Susceptibility of ACB to Bt Cry1Ab Protein
2.2. ACB Larval Growth Inhibition to Bt Cry1Ab Protein
2.3. Resistance Allele Frequencies Related to Cry1Ab
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Field Collections
5.2. Susceptible Population
5.3. Cry1Ab Protein
5.4. Bt Corn
5.5. Baseline Bioassay
5.6. Resistance Allele Frequency
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.S.; Zhang, G.M.; Zhang, F. Factors influencing parasitism of Trichogramma dendrolimi on eggs of the Asian corn borer. Ostrinia furnacalis. BioControl 1998, 43, 273–287. [Google Scholar] [CrossRef]
- Afidchao, M.M.; Mustersa, C.J.M.; Snoo, G.R. Asian corn borer (ACB) and non-ACB pests in GM corn (Zea mays L.) in the Philippines. Pest Manag. Sci. 2013, 69, 792–801. [Google Scholar] [CrossRef] [PubMed]
- He, K.L.; Zhou, D.R.; Wang, Z.Y.; Wen, L.P.; Bai, S.X. Study on the damage and control tactics of Asian corn borer Ostrinia furnacalis (Guenée) in sweet corn field. Acta Phytophylacica Sin. 2002, 29, 199–204. [Google Scholar]
- Wang, Z.C.; Qian, H.T.; Dong, H.; Wang, J.H.; Wang, Z.Y.; Cong, B. Studies on the damage degree and yield loss by Asian corn borer. Plant Prot. 2008, 34, 112–115. [Google Scholar]
- Zhou, D.R.; Wang, Y.S.; Li, W.D. Studies on the identification of the dominant corn borer species in China. Acta Phytophylacica Sin. 1988, 15, 145–152. [Google Scholar]
- Wang, Z.Y.; Lu, X.; He, K.L.; Zhou, D.R. Review of history, present situation and prospect of the Asian maize borer research in China. J. Shenyang Agric. Univ. 2000, 31, 402–412. [Google Scholar]
- Gressel, J.; Hanafi, A.; Head, G.; Marasas, W.; Obilana, A.B.; Ochanda, J.; Souissi, T.; Tzotzos, G. Major heretofore intractable biotic constraints to African food security that may be amenable to novel biotechnological solutions. Crop Prot. 2004, 23, 661–689. [Google Scholar] [CrossRef] [Green Version]
- Suma, P.; Zappalà, L.; Mazzeo, G.; Siscaro, G. Lethal and sub-lethal effects of insecticides on natural enemies of citrus scale pests. BioControl 2009, 54, 651–661. [Google Scholar] [CrossRef]
- Andrade, G.S.; Pratissoli, D.; Dalvi, L.P.; Desneux, N.; Junior, H.J.G.S. Performance of four Trichogramma species (Hymenoptera: Trichogrammatidae) as biocontrol agents of Heliothis virescens (Lepidoptera: Noctuidae) under various temperature regimes. J. Pest Sci. 2011, 84, 313–320. [Google Scholar] [CrossRef]
- Baron, G.L.; Raine, N.E.; Brown, M.J.F. Impact of chronic exposure to a pyrethroid pesticide on bumblebees and interactions with a trypanosome parasite. J. Appl. Ecol. 2014, 51, 460–469. [Google Scholar] [CrossRef] [Green Version]
- He, K.L.; Wang, Z.Y.; Zhou, D.R.; Wen, L.P.; Song, Y.Y.; Yao, Z.Y. Evaluation of transgenic Bt corn for resistance to the Asian corn borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 2003, 96, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.L.; Parker, G.B.; Pershing, J.C.; Brown, S.M.; Sanders, P.R.; Duncan, D.R.; Stone, T.; Dean, D.A.; DeBoer, D.L.; Hart, J.; et al. Field evaluation of European corn borer control in progeny of 173 transgenic corn events expressing an insecticidal protein from Bacillus thuringiensis. Crop Sci. 1995, 35, 550–557. [Google Scholar] [CrossRef]
- Jansens, S.; Vliet, A.V.; Dickburt, C.; Buysse, L.; Piens, C.; Saey, B.; Wulf, A.D.; Gosselé, V.; Paez, A.; Göbel, E.; et al. Transgenic corn expressing a Cry9C insecticidal protein from Bacillus thuringiensis Protected from European corn borer damage. Crop Sci. 1997, 37, 1616–1624. [Google Scholar] [CrossRef]
- Shelton, A.M.; Zhao, J.Z.; Roush, R.T. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu. Rev. Entomol. 2002, 47, 845–881. [Google Scholar] [CrossRef]
- Tabashnik, B.E. Plant science. Communal benefits of transgenic corn. Science 2010, 330, 189–190. [Google Scholar] [CrossRef]
- Dively, G.P.; Venugopal, P.D.; Bean, D.; Whalen, J.; Holmstrom, K.; Kuhar, T.P.; Doughty, H.B.; Patton, T.; Cissel, W.; Hutchison, W.D. Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc. Natl. Acad. Sci. USA 2018, 115, 3320–3325. [Google Scholar] [CrossRef] [Green Version]
- International Service for the Acquisition of Agri-biotech Applications. Global Status of Commercialized Biotech/GM Crops in 2019: Biotech Crops Drive Social Economic Development and Sustainable Environment in the New Frontier; Brief No. 55; ISAAA: Ithaca, NY, USA, 2019. [Google Scholar]
- Tabashnik, B.E.; Gassmann, A.J.; Crowder, D.W.; Carriére, Y. Insect resistance to Bt crops: Evidence versus theory. Nat. Biotechnol. 2008, 26, 199–202. [Google Scholar] [CrossRef]
- Farias, J.R.; Andow, D.A.; Horikoshi, R.J.; Sorgatto, R.J.; Fresia, P.; Santos, A.C.; Omoto, C. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2014, 64, 150–158. [Google Scholar] [CrossRef]
- Storer, N.P.; Babcock, J.M.; Schlenz, M.; Meade, T.; Thompson, G.D.; Bing, J.W.; Huckaba, R.M. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J. Econ. Entomol. 2010, 103, 1031–1038. [Google Scholar] [CrossRef]
- Gassmann, A.J.; Petzold-Maxwell, J.L.; Keweshan, R.S.; Dunbar, M.W. Field-evolved resistance to Bt maize by western corn rootworm. PLoS ONE 2011, 6, e22629. [Google Scholar] [CrossRef] [Green Version]
- Meihls, L.N.; Higdon, M.L.; Siegfried, B.D.; Miller, N.J.; Sappington, T.W.; Ellersieck, M.R.; Spencer, T.A.; Hibbard, B.E. Increased survival of western corn rootworm on transgenic corn within three generations of on-plant greenhouse selection. Proc. Natl. Acad. Sci. USA 2008, 105, 19177–19182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, C.A.; Chiaravalle, W.; Dalla-Rizza, M.; Farias, J.R.; García-Degano, M.F.; Gastaminza, G.; Mota-Sánchez, D.; Murúa, M.G.; Omoto, C.; Pieralisi, B.K.; et al. Current situation of pests targeted by Bt crops in Latin America. Curr. Opin. Insect Sci. 2016, 15, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.J.G.; Lang, B.A.; Storer, N.P.; Siegfried, B.D. Selection for Cry1F resistance in the European corn borer and cross-resistance to other Cry toxins. Entomol. Exp. Appl. 2008, 126, 115–121. [Google Scholar] [CrossRef]
- Ostrem, J.S.; Pan, Z.; Flexner, J.L.; Owens, E.; Binning, R.; Higgins, L.S. Monitoring susceptibility of western bean cutworm (Lepidoptera: Noctuidae) field populations to Bacillus thuringiensis Cry1F protein. J. Econ. Entomol. 2016, 109, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Farhan, Y.; Schaafsma, A.W. Practical Resistance of Ostrinia nubilalis (Lepidoptera: Crambidae) to Cry1F Bacillus thuringiensis maize discovered in Nova Scotia, Canada. Sci. Rep. 2019, 9, 18247. [Google Scholar] [CrossRef] [Green Version]
- Van Rensburg, J.B.J. First report of field resistance by the stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. S. Afr. J. Plant Soil 2007, 24, 147–151. [Google Scholar] [CrossRef] [Green Version]
- He, K.L.; Wang, Z.Y.; Wen, L.P.; Bai, X.S.; Ma, X.; Yao, Z.Y. Determination of baseline susceptibility to Cry1Ab protein for Asian corn borer (Lep., Crambidae). J. Appl. Entomol. 2005, 129, 407–412. [Google Scholar] [CrossRef]
- Li, G.P.; Huang, J.R.; Ji, T.J.; Tian, C.H.; Zhao, X.C.; Feng, H.Q. Baseline susceptibility and resistance allele frequency in Ostrinia furnacalis related to Cry1 toxins in the Huanghuaihai summer corn region of China. Pest Manag. Sci. 2020, 76, 4311–4317. [Google Scholar] [CrossRef]
- Alcantara, E.; Estrada, A.; Alpuerto, V.; Head, G. Monitoring Cry1Ab susceptibility in Asian corn borer (Lepidoptera: Crambidae) on Bt corn in the Philippines. Crop Prot. 2011, 30, 554–559. [Google Scholar] [CrossRef]
- Stone, T.B.; Sims, S.R. Geographic Susceptibility of Heliothis virescens and Helicoverpa zea (Lepidoptera: Noctuidae) to Bacillus thuringiensis. J. Econ. Entomol. 1993, 86, 989–994. [Google Scholar] [CrossRef]
- Macron, P.C.R.G.; Young, L.J.; Steffey, K.L.; Siegfried, B. Baseline susceptibility of European corn borer (Lepidoptera: Crambidae) to Bacillus thuringiensis toxins. J. Econ. Entomol. 1999, 92, 279–285. [Google Scholar]
- Le, D.K.; Le, Q.K.; Tran, T.T.H.; Nguyen, D.V.; Dao, T.H.; Nguyen, T.T.; Truong, X.L.; Nguyen, Q.C.; Pham, H.P.; Phan, T.T.T.; et al. Baseline susceptibility of Asian corn borer (Ostrinia furnacalis (Guenée)) populations in Vietnam to Cry1Ab insecticidal protein. J. Asia-Pac. Entomol. 2019, 22, 493–498. [Google Scholar] [CrossRef]
- Liu, Z.X. Brief report of research on Asian corn borer control with Bt emulsion. Plant Prot. 1987, 13, 36–37. [Google Scholar]
- Robertson, J.L.; Preisler, H.K.; Ng, S.S.; Hickle, L.A.; Gelernter, W.D. Natural variation: A complicating factor in bioassays with chemical and microbial pesticides. J. Econ. Entomol. 1995, 88, 1–10. [Google Scholar] [CrossRef]
- Meade, T.; Hare, J.D. Effects of genetic and environmental host plant variation on the susceptibility of two noctuids to Bacillus thuringiensis. Entomol. Exp. Appl. 1994, 70, 165–178. [Google Scholar] [CrossRef]
- Lemes, A.R.; Davolos, C.C.; Legori, P.C.; Fernandes, O.A.; Ferré, J.; Lemos, M.V.; Desiderio, J.A. Synergism and antagonism between Bacillus thuringiensis Vip3A and Cry1 proteins in Heliothis virescens, Diatraea saccharalis and Spodoptera frugiperda. PLoS ONE 2014, 9, e107196. [Google Scholar] [CrossRef]
- Andow, D.A.; Alstad, D.N. F2 screen for rare resistance alleles. J. Econ. Entomol. 1998, 91, 572–578. [Google Scholar] [CrossRef]
- Andow, D.A.; Olson, D.M.; Hellmich, R.L.; Alstad, D.N.; Hutchison, W.D. Frequency of resistance to Bacillus thuringiensis toxin Cry1Ab in an Iowa population of European corn borer (Lepidoptera: Crambidae). J. Econ. Entomol. 2000, 93, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.Z.; Li, Y.X.; Collins, H.L.; Shelton, A.M. Examination of the F2 screen for rare resistance alleles to Bacillus thuringiensis toxins in the diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 2002, 95, 14–21. [Google Scholar] [CrossRef]
- Bentur, J.S.; Andow, D.A.; Cohen, M.B.; Romena, A.M.; Gould, F. Frequency of Alleles Conferring Resistance to a Bacillus thuringiensis Toxin in a Philippine Population of Scirpophaga incertulas (Lepidoptera: Pyralidae). J. Econ. Entomol. 2000, 93, 1515–1521. [Google Scholar] [CrossRef]
- Zhou, D.R.; Wang, Y.Y.; Liu, B.L.; Ju, Z.L. Studies on the mass rearing of corn borer: Development of a satisfactory artificial diet for larval growth. J. Plant Prot. 1980, 7, 113–122. [Google Scholar]
| Population | N | LC50 (95% FL) ng/cm2 a | LC95 (95% FL) ng/cm2 a | Slope ± SE | χ2 | df (χ2) |
|---|---|---|---|---|---|---|
| Luoyang | 1512 | 71.33 (45.61–102.64) a | 5752.34 (2532.54–21,631.84) a | 0.86 ± 0.11 | 10.30 | 19 |
| Xinxiang | 1512 | 66.31 (46.99–90.11) a | 3708.90 (1901.84–9857.31) a | 0.94 ± 0.10 | 8.78 | 19 |
| Xiaoxian | 1512 | 55.18 (35.31–80.01) a | 4596.70 (2166.61–14,375.89) a | 0.86 ± 0.10 | 11.51 | 19 |
| Dezhou | 1512 | 47.96 (35.01–61.51) a | 1052.27 (679.00–1981.37) b | 1.23 ± 0.13 | 7.73 | 19 |
| Jinan | 2160 | 28.35 (22.50–34.42) b | 305.75 (246.59–397.45) cd | 1.59 ± 0.11 | 8.56 | 28 |
| Qiqihar | 1512 | 27.86 (20.05–36.66) b | 922.94 (603.31–1625.22) b | 1.08 ± 0.09 | 7.77 | 19 |
| Gongzhuling | 1944 | 25.56 (20.23–30.95) b | 236.60 (190.22–311.10) cd | 1.70 ± 0.13 | 10.34 | 25 |
| Harbin | 1296 | 23.92 (7.76–31.17) bc | 1079.23 (684.77–1941.41) b | 0.99 ± 0.07 | 5.95 | 16 |
| Dongyang | 1944 | 13.67 (10.11–17.41) c | 183.25 (146.29–241.62) d | 1.46 ± 0.11 | 11.14 | 25 |
| Huanggang | 1728 | 13.60 (10.51–16.73) c | 138.11 (110.60–182.76) de | 1.63 ± 0.13 | 10.72 | 22 |
| Dalian | 1944 | 10.40 (7.54–13.60) cd | 229.70 (178.06–310.82) cd | 1.22 ± 0.08 | 6.57 | 25 |
| Heihe | 1512 | 8.92 (7.12–10.85) d | 109.66 (87.64–143.22) e | 1.51 ± 0.09 | 9.10 | 19 |
| Miyun | 1728 | 7.94 (5.83–10.26) de | 143.27 (111.27–194.29) de | 1.31 ± 0.09 | 10.46 | 22 |
| Kaili | 1728 | 7.11 (5.14–9.30) de | 145.37 (111.80–199.33) de | 1.26 ± 0.09 | 12.41 | 22 |
| Yingxian | 1512 | 6.98 (4.67–9.65) de | 1003.45 (567.19–2173.66) b | 0.76 ± 0.06 | 3.77 | 19 |
| Tongliao | 1512 | 6.69 (4.59–8.76) de | 53.76 (43.45–71.26) f | 1.82 ± 0.19 | 12.01 | 19 |
| Guangzhou | 1728 | 5.75 (4.59–6.98) e | 63.81 (51.90–81.29) f | 1.57 ± 0.09 | 6.76 | 22 |
| Chifeng | 1944 | 5.20 (3.54–7.18) ef | 250.65 (182.58–363.95) cd | 0.98 ± 0.06 | 13.13 | 25 |
| Dunhua | 1512 | 4.67 (3.55–5.87) ef | 84.38 (65.58–114.83) ef | 1.31 ± 0.09 | 11.29 | 19 |
| Deyang | 1944 | 3.90 (2.89–5.06) f | 109.87 (84.15–149.65) e | 1.14 ± 0.06 | 4.19 | 25 |
| Baicheng | 1728 | 2.97 (2.15–3.91) f | 99.81 (74.12–142.10) e | 1.08 ± 0.07 | 10.42 | 22 |
| Qinhuangdao | 1296 | 1.88 (1.39–2.40) g | 18.58 (14.74–24.64) h | 1.65 ± 0.13 | 12.72 | 16 |
| Shenyang | 2016 | 1.69 (1.13–2.31) g | 62.98 (48.62–86.72) f | 1.05 ± 0.08 | 10.41 | 26 |
| Chengde | 1512 | 1.36 (1.00–1.75) gh | 22.40 (17.39–30.43) gh | 1.35 ± 0.09 | 10.15 | 19 |
| Dandong | 1512 | 0.86 (0.44–1.38) h | 33.87 (25.47–49.09) g | 1.03 ± 0.10 | 7.09 | 19 |
| Laboratory population | 360 | 6.28 (4.46–8.50) de | 57.33 (38.35–98.88) fg | 1.71 ± 0.17 | 10.40 | 13 |
| Population | N | EC50 (95% FL) ng/cm2 a | EC95 (95% FL) ng/cm2 a | Slope ± SE | χ2 | df (χ2) |
|---|---|---|---|---|---|---|
| Xinxiang | 1512 | 10.40 (9.03–11.85) a | 172.86 (135.65–231.00) a | 1.35 ± 0.07 | 20.10 | 19 |
| Heihe | 1512 | 7.09 (6.14–8.09) b | 45.09 (36.42–58.99) cd | 2.05 ± 0.13 | 29.25 | 19 |
| Xiaoxian | 1512 | 4.56 (3.73–5.41) c | 77.17 (61.58–101.50) bc | 1.34 ± 0.08 | 23.03 | 19 |
| Dezhou | 1512 | 3.45 (2.65–4.30) c | 95.58 (73.09–133.61) b | 1.14 ± 0.08 | 7.05 | 19 |
| Qiqihar | 1512 | 3.30 (2.61–4.00) c | 55.24 (44.23–72.58) c | 1.34 ± 0.09 | 22.86 | 19 |
| Chifeng | 1944 | 1.68 (1.35–2.03) d | 45.73 (35.81–61.17) cd | 1.15 ± 0.06 | 19.61 | 25 |
| Dunhua | 1512 | 1.32 (0.86–1.80) de | 18.95 (15.41–24.65) e | 1.42 ± 0.13 | 15.44 | 19 |
| Harbin | 1296 | 1.25 (0.78–1.76) de | 34.71 (26.43–49.49) d | 1.14 ± 0.10 | 18.87 | 16 |
| Yingxian | 1512 | 1.23 (0.75–1.78) de | 49.26 (37.38–70.47) cd | 1.03 ± 0.09 | 6.80 | 19 |
| Luoyang | 1512 | 0.77 (0.38–1.27) e | 65.46 (47.00–102.33) bc | 0.85 ± 0.08 | 4.15 | 19 |
| Miyun | 1728 | 0.74 (0.56–0.93) e | 16.82 (13.26–22.53) ef | 1.21 ± 0.08 | 14.53 | 22 |
| Gongzhuling | 1944 | 0.68 (0.48–0.91) e | 26.28 (20.23–36.17) de | 1.04 ± 0.07 | 6.46 | 25 |
| Dalian | 1944 | 0.64 (0.46–0.84) e | 15.81 (12.46–21.18) ef | 1.18 ± 0.08 | 10.38 | 25 |
| Baicheng | 1728 | 0.64 (0.46–0.84) e | 15.10 (11.87–20.34) ef | 1.20 ± 0.09 | 4.07 | 22 |
| Tongliao | 1512 | 0.60 (0.43–0.77) e | 7.83 (6.33–10.26) g | 1.47 ± 0.12 | 4.58 | 19 |
| Qinhuangdao | 1296 | 0.60 (0.45–0.75) e | 3.75 (3.15–4.73) h | 2.08 ± 0.21 | 7.77 | 16 |
| Kaili | 1728 | 0.59 (0.40–0.79) ef | 18.12 (14.04–24.88) ef | 1.10 ± 0.08 | 3.61 | 22 |
| Dongyang | 1944 | 0.55 (0.38–0.74) ef | 12.62 (9.96–16.97) f | 1.21 ± 0.09 | 2.47 | 25 |
| Deyang | 1944 | 0.51 (0.35–0.68) ef | 9.84 (7.84–13.08) fg | 1.28 ± 0.10 | 11.24 | 25 |
| Huanggang | 1728 | 0.47 (0.30–0.67) ef | 20.19 (15.38–28.39) e | 1.01 ± 0.08 | 3.65 | 22 |
| Chengde | 1512 | 0.44 (0.29–0.59) ef | 4.17 (3.43–5.40) h | 1.68 ± 0.17 | 2.66 | 19 |
| Guangzhou | 1728 | 0.34 (0.20–0.49) f | 8.36 (6.57–11.34) g | 1.18 ± 0.11 | 3.78 | 22 |
| Jinan | 2160 | 0.20 (0.10–0.34) f | 34.56 (24.98–51.65) d | 0.74 ± 0.06 | 6.12 | 28 |
| Dandong | 1512 | 0.04 (0.00–0.20) g | 4.41 (2.13–6.92) gh | 0.81 ± 0.17 | 2.23 | 19 |
| Shenyang | 2016 | 0.03 (0.00–0.13) g | 3.87 (1.89–6.01) h | 0.76 ± 0.14 | 1.47 | 26 |
| Laboratory population | 360 | 0.46 (0.06–1.06) ef | 12.21 (7.35–33.76) fg | 1.16 ± 0.27 | 4.26 | 13 |
| Province | Location | Number of Isofemale Lines | Var (q) | E(q) (95% CI) | Number of Isofemale Lines with Resistance |
|---|---|---|---|---|---|
| Jilin | Dunhua | 61 | 6.18 × 10−5 | 0.0040 (0–1.95 × 10−2) | 0 |
| Liaoning | Shenyang | 56 | 7.27 × 10−5 | 0.0043 (0–2.10 × 10−2) | 0 |
| Heilongjiang | Qiqihar | 74 | 4.26 × 10−5 | 0.0033 (0–1.60 × 10−2) | 0 |
| Henan | Luoyang | 60 | 6.38 × 10−5 | 0.0040 (0–1.97 × 10−2) | 0 |
| Shanxi | Yingxian | 50 | 9.03 × 10−5 | 0.0048 (0–2.34 × 10−2) | 0 |
| Shandong | Dezhou | 55 | 7.53 × 10−5 | 0.0044 (0–2.15 × 10−2) | 0 |
| Jilin | Gongzhuling | 52 | 8.38 × 10−5 | 0.0046 (0–2.26 × 10−2) | 0 |
| Liaoning | Dalian | 75 | 4.15 × 10−5 | 0.0032 (0–1.57 × 10−2) | 0 |
| Inner Mongolia | Tongliao | 68 | 5.01 × 10−5 | 0.0036 (0–1.75 × 10−2) | 0 |
| Hebei | Qinhuangdao | 74 | 4.26 × 10−5 | 0.0033 (0–1.60 × 10−2) | 0 |
| Hubei | Huanggang | 58 | 6.80 × 10−5 | 0.0042 (0–2.03 × 10−2) | 0 |
| Zhejiang | Dongyang | 66 | 5.31 × 10−5 | 0.0037 (0–1.80 × 10−2) | 0 |
| Guizhou | Kaili | 53 | 8.08 × 10−5 | 0.0045 (0–1.95 × 10−2) | 0 |
| Total | - | 802 | 3.86 × 10−7 | 0.0003 (0–1.52 × 10−3) | 0 |
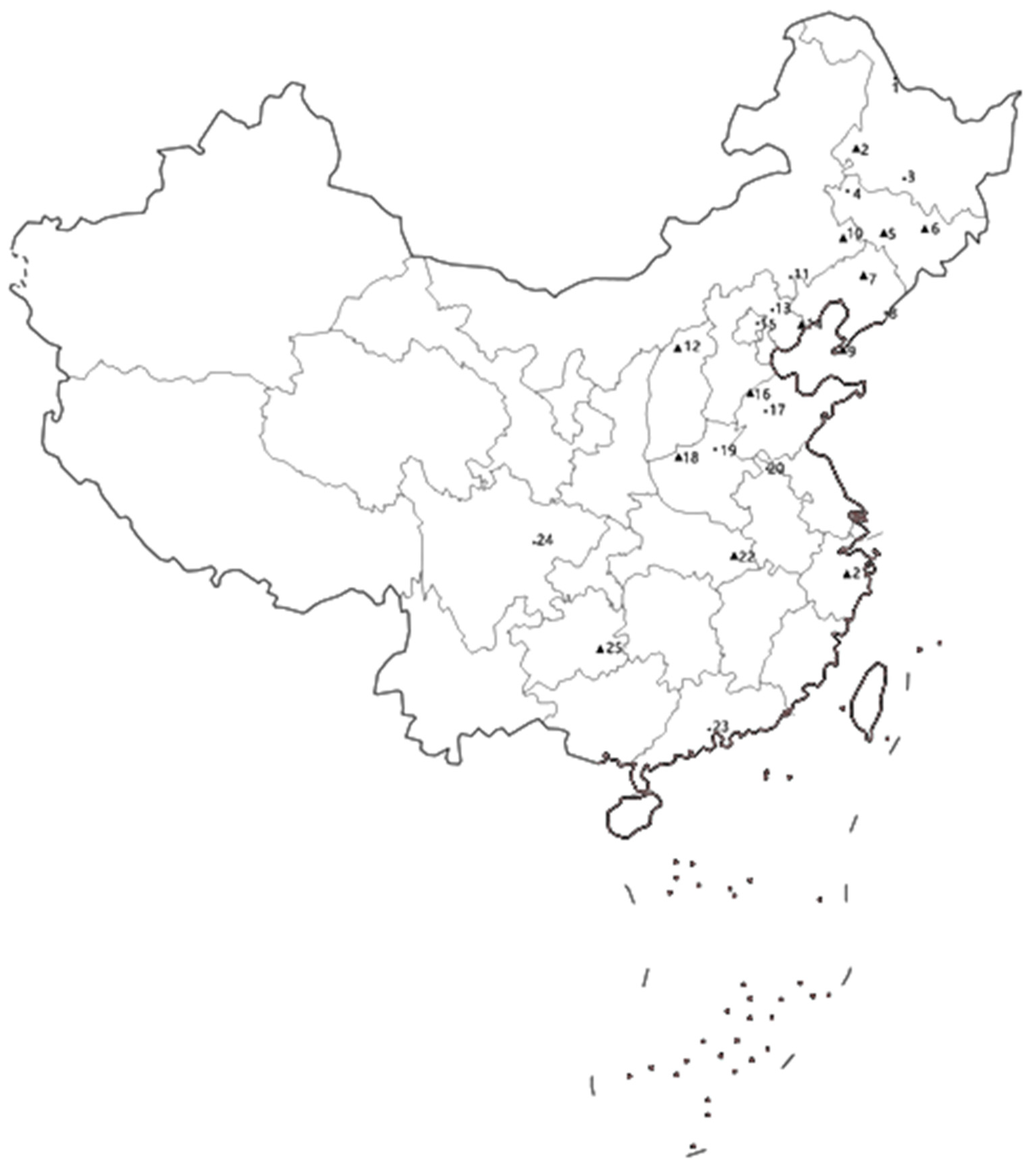
| Province | Location | Coordinates | Corn Ecological Region | Collected Insect Life Stage | Collection Month | Number of Collected Insects |
|---|---|---|---|---|---|---|
| Heilongjiang | Heihe (1) | 50°14′58.51″ N; 127°29′56.48″ E | NESpC | female moths | July, 2018 | 70 |
| Heilongjiang | Qiqihar (2) | 47°20′53.08″ N; 123°57′12.55″ E | NESpC | female moths | July, 2018 | 184 |
| Heilongjiang | Harbin (3) | 45°45′25.08″ N; 126°38′32.87″ E | NESpC | female moths | July, 2018 | 85 |
| Jilin | Baicheng (4) | 45°37′8.49″ N; 122°50′28.01″ E | NESpC | female moths | July, 2019 | 152 |
| Jilin | Gongzhuling (5) | 43°30′16.31″ N; 124°49′21.58″ E | NESpC | female moths | July, 2019 | 120 |
| Jilin | Dunhua (6) | 43°22′22.94″ N; 128°13′56.71″ E | NESpC | female moths | July, 2018 | 160 |
| Liaoning | Shenyang (7) | 41°47′48.36″ N; 123°25′44.75″ E | NESpC | female moths | June, 2018 | 195 |
| Liaoning | Dandong (8) | 40°7′27.47″ N; 124°22′58.96″ E | NESpC | female moths | July, 2018 | 203 |
| Liaoning | Dalian (9) | 38°54′52.52″ N; 121°37′7.04″ E | NESpC | female moths | June, 2019 | 180 |
| Inner Mongolia | Tongliao (10) | 43°37′2.74″ N; 122°15′47.23″ E | NESpC | female moths | June, 2019 | 169 |
| Inner Mongolia | Chifeng (11) | 42°16′31.14″ N; 118°57′24.50″ E | NESpC | female moths | June, 2019 | 220 |
| Shanxi | Yingxian (12) | 39°33′10.04″ N; 113°11′25.87″ E | NSpC | female moths | August, 2018 | 201 |
| Hebei | Chengde (13) | 40°58′34.33″ N; 117°56′20.95″ E | HHHSuC | female moths | June, 2019 | 117 |
| Hebei | Qinhuangdao (14) | 39°56′33.11″ N; 119°35′11.68″ E | HHHSuC | female moths | June, 2019 | 143 |
| Beijing | Miyun (15) | 40°22′34.25″ N; 116°50′34.62″ E | HHHSuC | female moths | August, 2019 | 132 |
| Shandong | Dezhou (16) | 37°27′14.28″ N; 116°18′26.74″ E | HHHSuC | female moths | August, 2018 | 174 |
| Shandong | Jinan (17) | 36°40′32.91″ N; 117°0′3.32″ E | HHHSuC | female moths | August, 2019 | 75 |
| Henan | Luoyang (18) | 34°39′46.95″ N; 112°26′4.08″ E | HHHSuC | female moths | August, 2018 | 186 |
| Henan | Xinxiang (19) | 35°18′9.42″ N; 113°53′2.37″ E | HHHSuC | female moths | August, 2018 | 130 |
| Anhui | Xiaoxian (20) | 34°11′16.44″ N; 116°56′43.66″ E | HHHSuC | female moths | September, 2018 | 182 |
| Zhejiang | Dongyang (21) | 29°17′21.91″ N; 120°14′30.66″ E | SEHiC | pupae | July, 2019 | 210 |
| Hubei | Huanggang (22) | 30°26′51.76″ N; 114°52′45.71″ E | SEHiC | female moths | August, 2019 | 266 |
| Guangdong | Guangzhou (23) | 23°7′30.64″ N; 113°16′50.29″ E | SEHiC | larvae | August, 2019 | 143 |
| Sichuan | Deyang (24) | 31°7′40.77″ N; 104°23′55.14″ E | SWHiC | larvae | September, 2019 | 172 |
| Guizhou | Kaili (25) | 26°34′0.80″ N; 107°58′52.75″ E | SWHiC | larvae | August, 2019 | 225 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, S.; Long, Y.; Wang, Y.; Zhao, W.; Shwe, S.M.; Wang, Z.; He, K.; Bai, S. Baseline Susceptibility and Resistance Allele Frequency in Ostrinia furnacalis in Relation to Cry1Ab Toxins in China. Toxins 2022, 14, 255. https://doi.org/10.3390/toxins14040255
Liu X, Liu S, Long Y, Wang Y, Zhao W, Shwe SM, Wang Z, He K, Bai S. Baseline Susceptibility and Resistance Allele Frequency in Ostrinia furnacalis in Relation to Cry1Ab Toxins in China. Toxins. 2022; 14(4):255. https://doi.org/10.3390/toxins14040255
Chicago/Turabian StyleLiu, Xiaobei, Shen Liu, Ying Long, Yueqin Wang, Wenlu Zhao, Su Mon Shwe, Zhenying Wang, Kanglai He, and Shuxiong Bai. 2022. "Baseline Susceptibility and Resistance Allele Frequency in Ostrinia furnacalis in Relation to Cry1Ab Toxins in China" Toxins 14, no. 4: 255. https://doi.org/10.3390/toxins14040255
APA StyleLiu, X., Liu, S., Long, Y., Wang, Y., Zhao, W., Shwe, S. M., Wang, Z., He, K., & Bai, S. (2022). Baseline Susceptibility and Resistance Allele Frequency in Ostrinia furnacalis in Relation to Cry1Ab Toxins in China. Toxins, 14(4), 255. https://doi.org/10.3390/toxins14040255






