Genomic Confirmation of the P-IIIe Subclass of Snake Venom Metalloproteinases and Characterisation of Its First Member, a Disintegrin-Like/Cysteine-Rich Protein
Abstract
:1. Introduction
2. Results
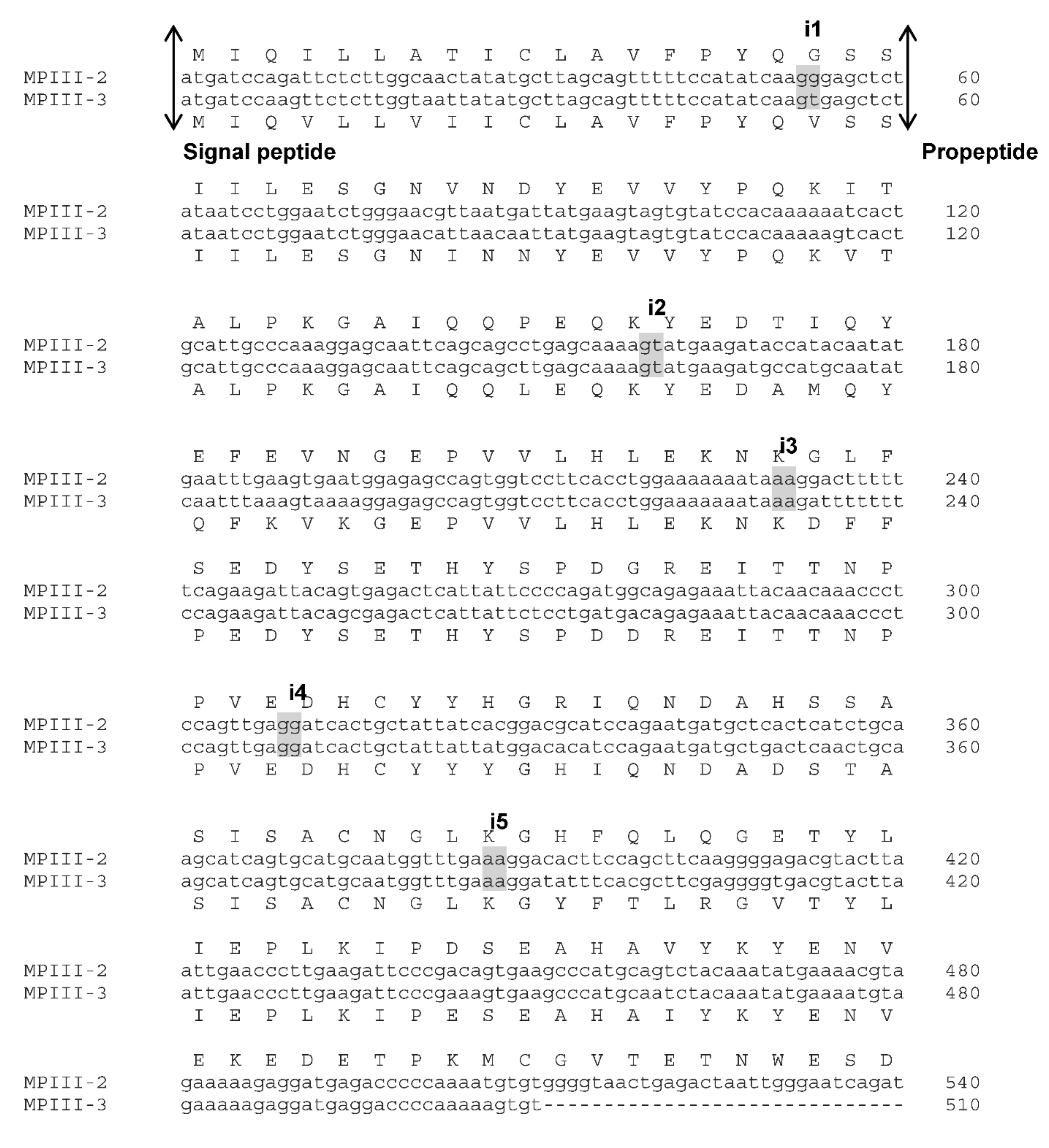
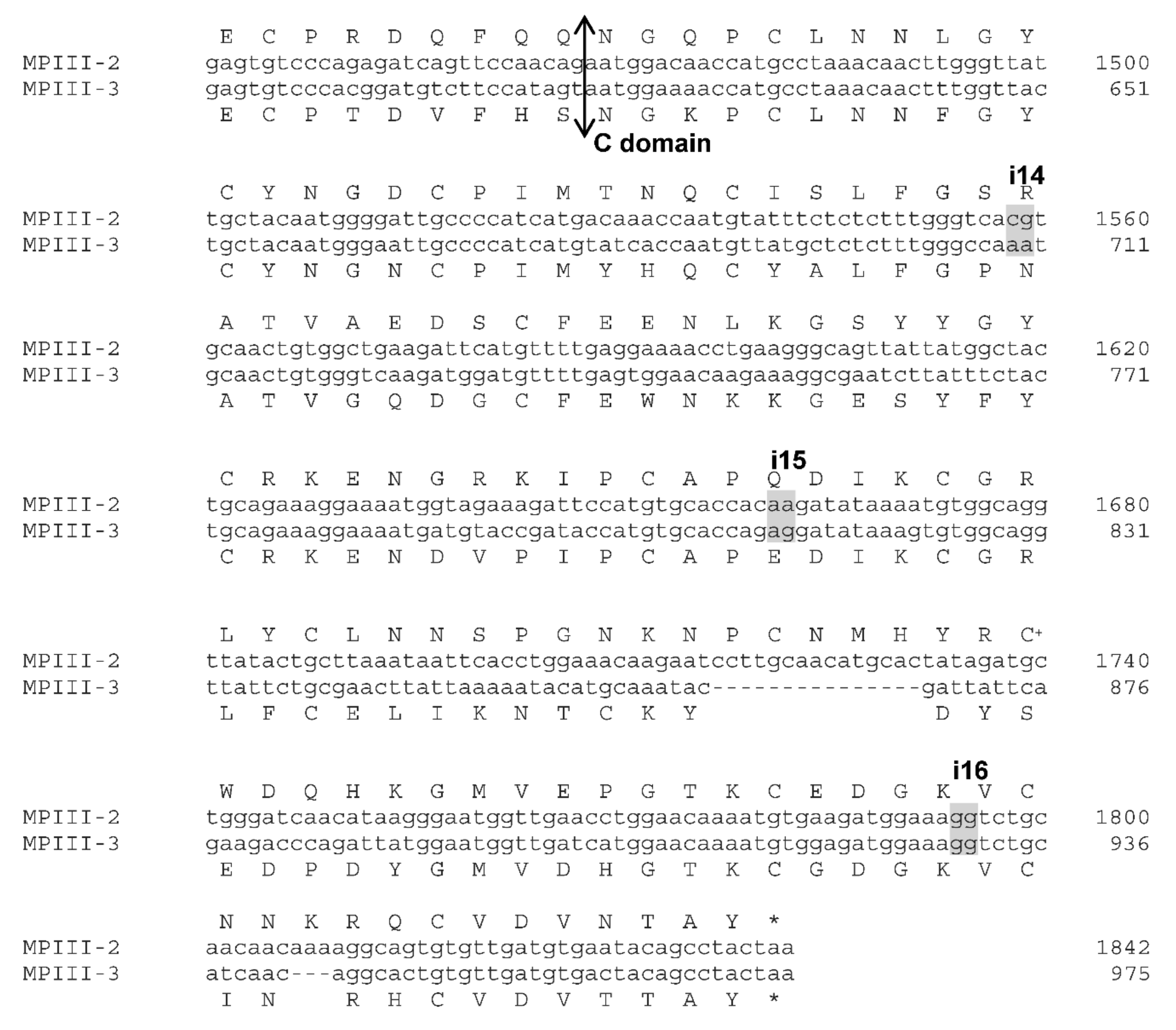
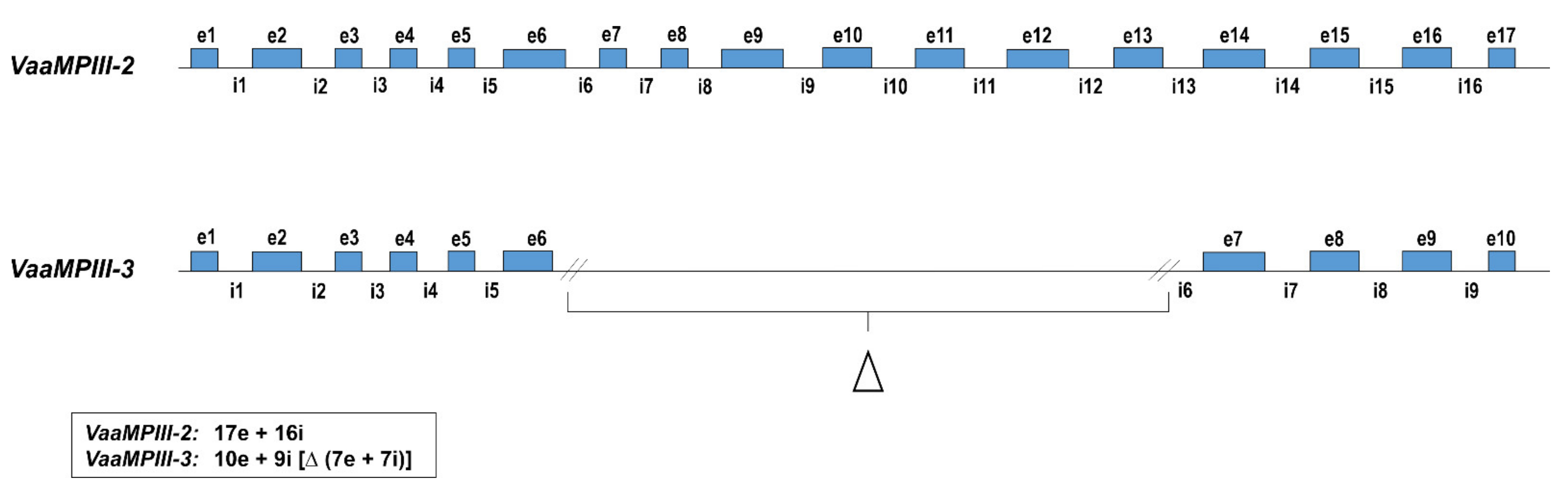
2.1. VaaMPIII-2 and VaaMPIII-3 Gene Structures
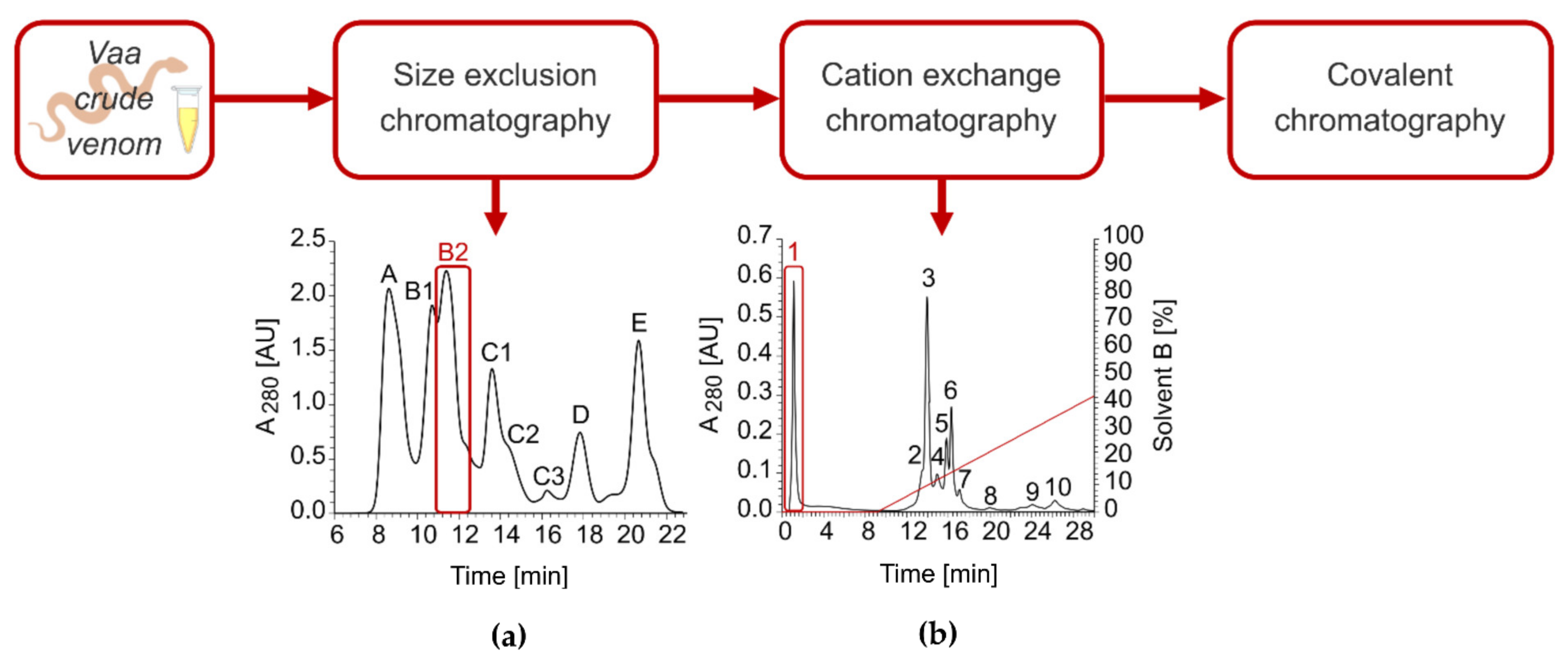
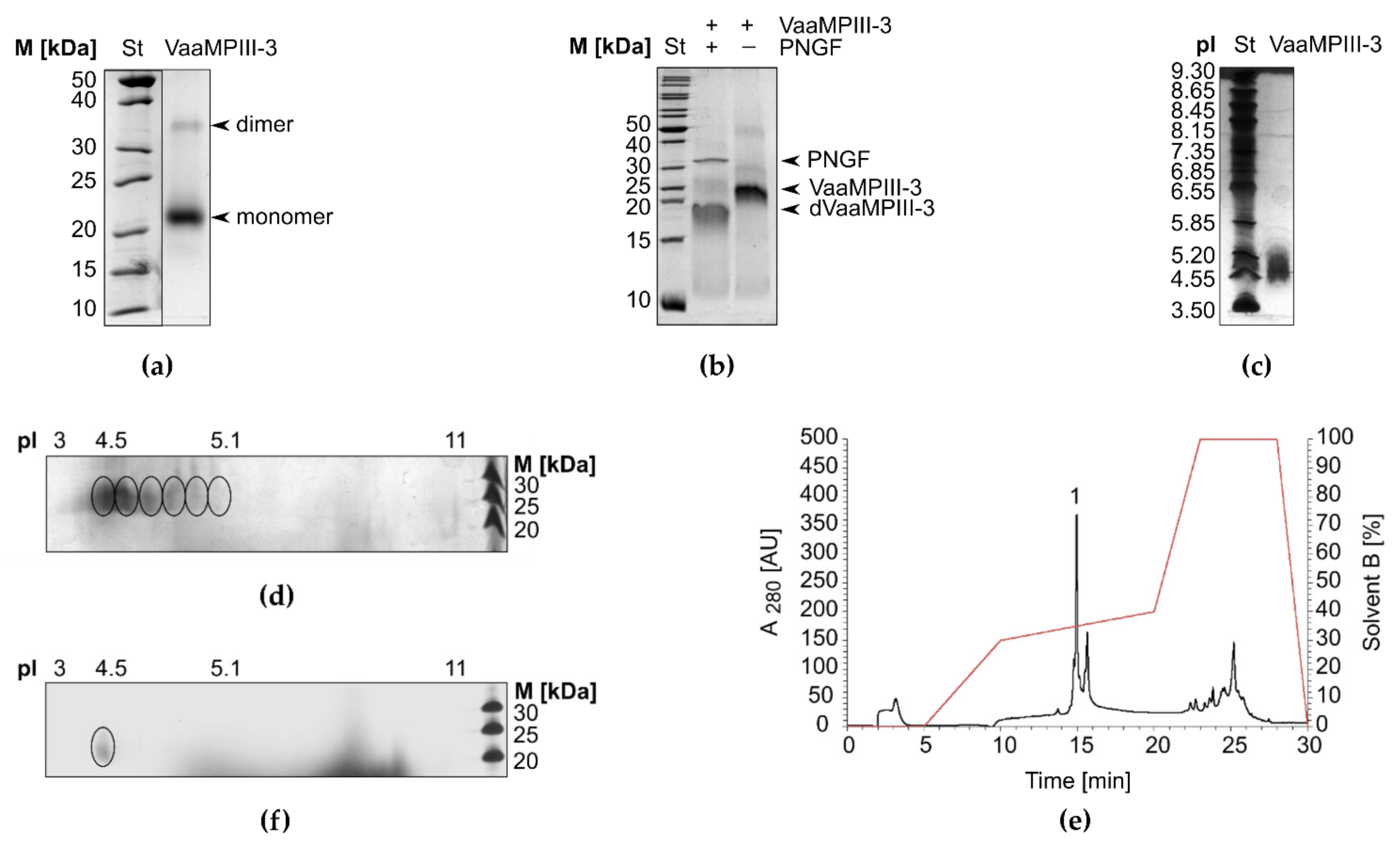
2.2. Isolation of VaaMPIII-3 from Crude Venom
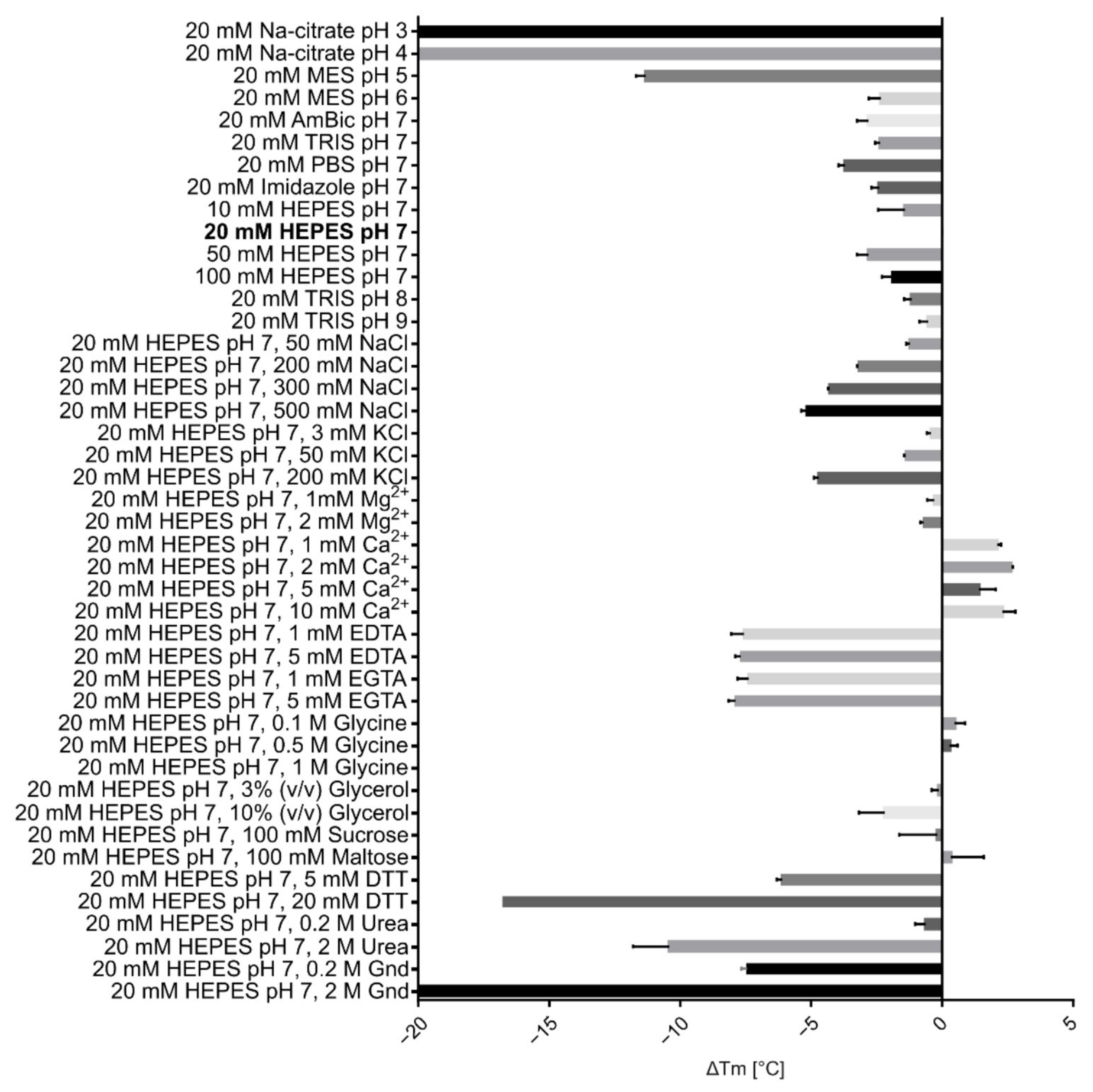
2.3. Physicochemical Characterisation of VaaMPIII-3
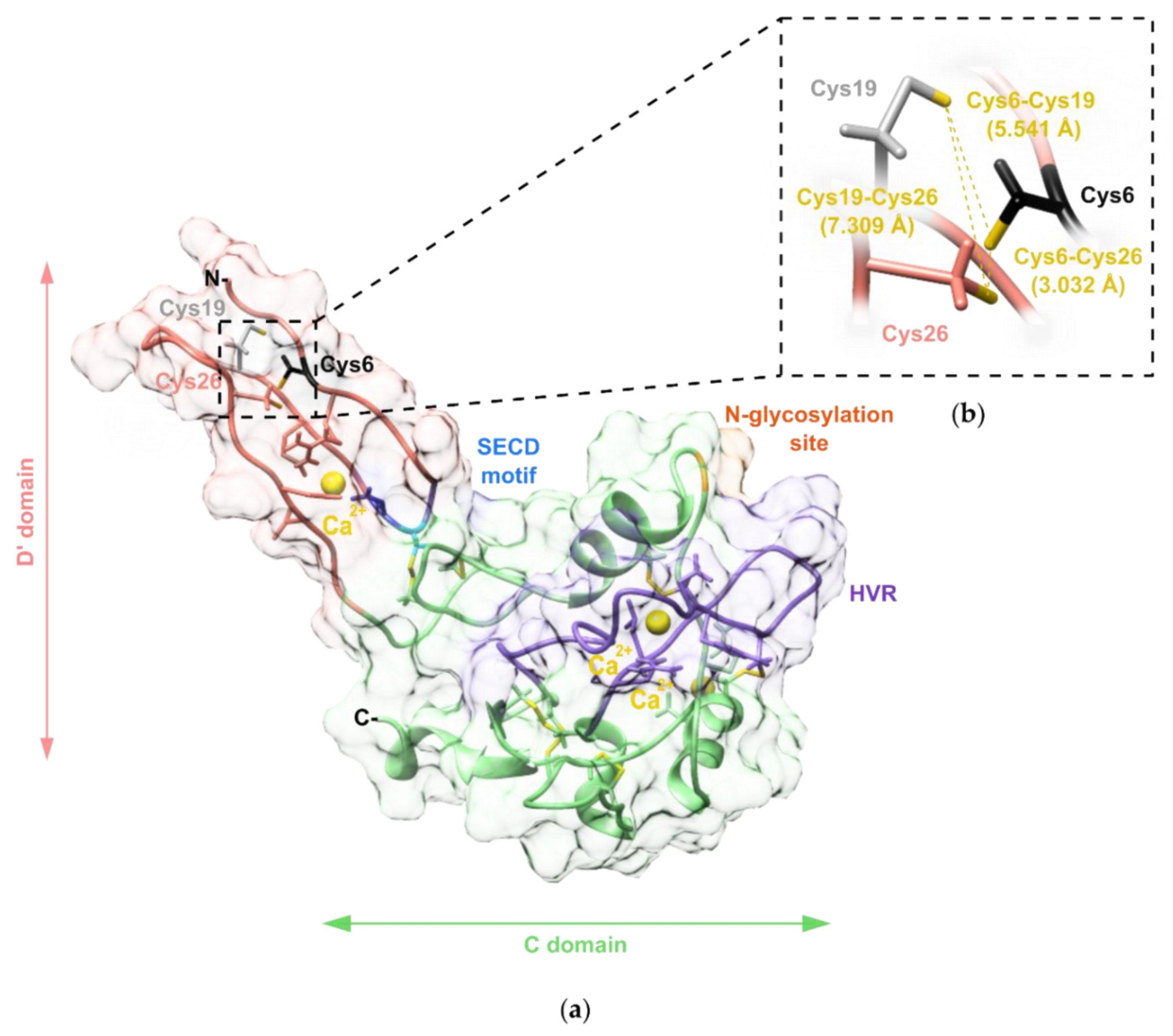
2.4. Modelling of the Three-Dimensional Structure of VaaMPIII-3
2.5. Determination of the Position of the Unpaired Cys Residue in VaaMPIII-3
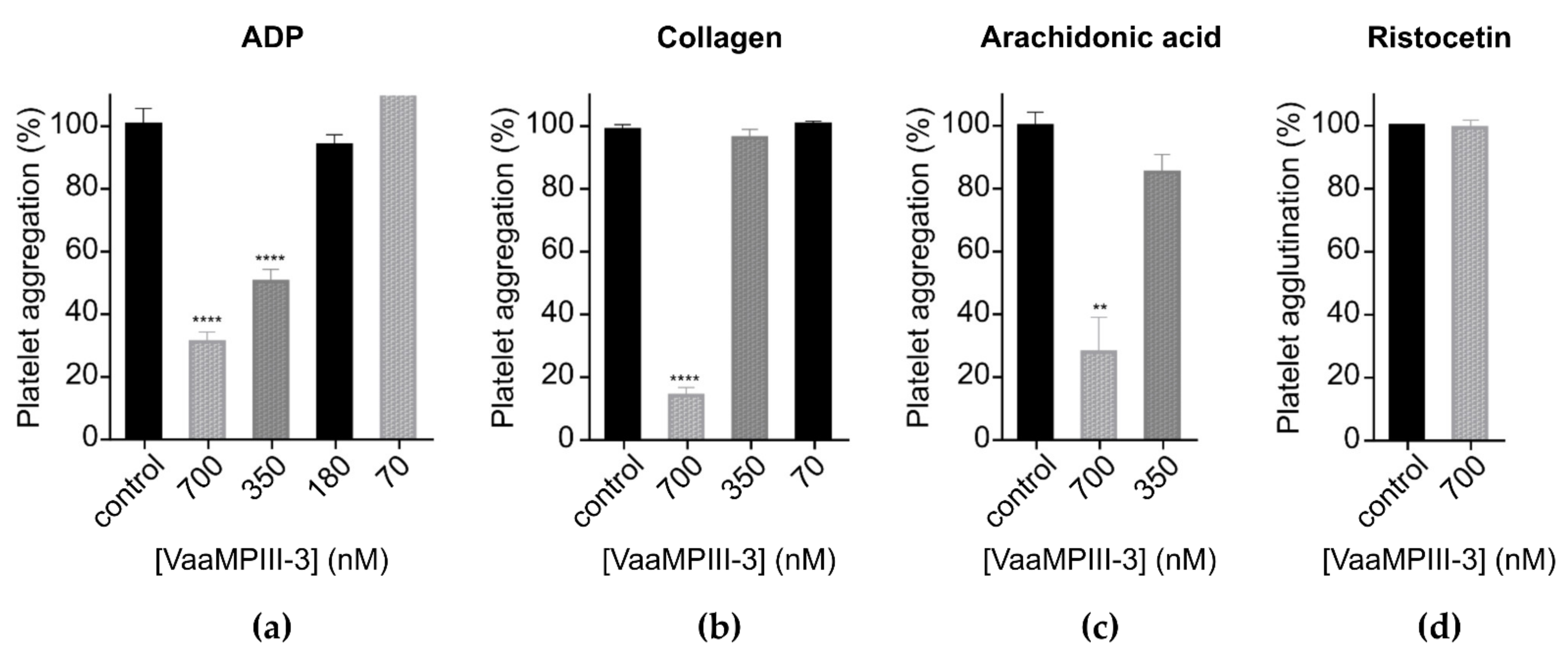
2.6. Functional Characterisation of VaaMPIII-3
3. Discussion
| DC Protein | Snake | Inhibition | Reference | |
|---|---|---|---|---|
| Induced by | IC50 (nM) | |||
| Alternagin-C | Bothrops alternatus | Collagen | [44] | |
| Balt-DC | Bothrops alternatus | Epinephrine, ristocetin | [18] | |
| BmooPAi | Bothrops moojeni | Ristocetin | [14] | |
| Catrocollastatin-C | Crotalus atrox | Collagen | 66 | [17] |
| Halysetin | Agkistrodon halys | Collagen | 420 | [13] |
| Jararhagin-C | Bothrops atrox | Collagen | 200 | [12,45] |
| Bothrops jararaca | ADP | |||
| Jaracetin | Bothrops jararaca | Collagen | [46] | |
| Leberagin-C | Macrovipera lebetina | Arachidonic acid | 50 | [11] |
| transmediterranea | Thrombin | 40 | ||
| VaaMPIII-3 | Vipera ammodytes | Collagen, ADP, arachidonic acid | This study | |
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Isolation of VaaMPIII-3 from Crude Venom of the Nose-Horned Viper
5.3. SDS-Polyacrylamide Gel Electrophoresis
5.4. Isoelectric Focusing
5.5. Two-Dimensional Gel Electrophoresis
5.6. Reversed Phase High-Performance Liquid Chromatography
5.7. N-Terminal Amino Acid Sequencing
5.8. Mass Spectrometry
5.9. Protein N-deglycosylation
5.10. Determination of the Position of the Free Cys Residue
5.11. Determination of the Thermal Stability of the Protein
5.12. Blood Coagulation Assays
5.13. Platelet Aggregation and Agglutination Assays
5.14. Platelet Receptor Binding Assays
5.15. Homology Modelling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mebs, D. Venomous and Poisonous Animals: A Handbook for Biologists, Toxicologists and Toxinologists, Physicians and Pharmacists; Medpharm Scientific Publishers: Stuttgart, Germany, 2002; ISBN 3-88763-093-9. [Google Scholar]
- Chippaux, J.-P. Epidemiology of Snakebites in Europe: A Systematic Review of the Literature. Toxicon 2012, 59, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Latinović, Z.; Leonardi, A.; Šribar, J.; Sajevic, T.; Žužek, M.C.; Frangež, R.; Halassy, B.; Trampuš-Bakija, A.; Pungerčar, J.; Križaj, I. Venomics of Vipera berus berus to Explain Differences in Pathology Elicited by Vipera ammodytes ammodytes Envenomation: Therapeutic Implications. J. Proteom. 2016, 146, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Karabuva, S.; Vrkić, I.; Brizić, I.; Ivić, I.; Lukšić, B. Venomous Snakebites in Children in Southern Croatia. Toxicon 2016, 112, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Karabuva, S.; Lukšić, B.; Brizić, I.; Latinović, Z.; Leonardi, A.; Križaj, I. Ammodytin L Is the Main Cardiotoxic Component of the Vipera ammodytes ammodytes Venom. Toxicon 2017, 139, 94–100. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wagstaff, S.C.; Harrison, R.A.; Renjifo, C.; Wüster, W. Domain Loss Facilitates Accelerated Evolution and Neofunctionalization of Duplicate Snake Venom Metalloproteinase Toxin Genes. Mol. Biol. Evol. 2011, 28, 2637–2649. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.W.; Serrano, S.M.T. Insights into and Speculations about Snake Venom Metalloproteinase (SVMP) Synthesis, Folding and Disulphide Bond Formation and Their Contribution to Venom Complexity. FEBS J. 2008, 275, 3016–3030. [Google Scholar] [CrossRef]
- Moura-da-Silva, A.M.; Theakston, R.D.; Crampton, J.M. Evolution of Disintegrin Cysteine-Rich and Mammalian Matrix-Degrading Metalloproteinases: Gene Duplication and Divergence of a Common Ancestor Rather than Convergent Evolution. J. Mol. Evol. 1996, 43, 263–269. [Google Scholar] [CrossRef]
- Fry, B.G. From Genome to “Venome”: Molecular Origin and Evolution of the Snake Venom Proteome Inferred from Phylogenetic Analysis of Toxin Sequences and Related Body Proteins. Genome Res. 2005, 15, 403–420. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, A.; Sajevic, T.; Pungerčar, J.; Križaj, I. Comprehensive Study of the Proteome and Transcriptome of the Venom of the Most Venomous European Viper: Discovery of a New Subclass of Ancestral Snake Venom Metalloproteinase Precursor-Derived Proteins. J. Proteome Res. 2019, 18, 2287–2309. [Google Scholar] [CrossRef]
- Limam, I.; Bazaa, A.; Srairi, N.; Taboubi, S.; Jebali, J.; Zouari-Kessentini, R.; Kallech-Ziri, O.; Mejdoub, H.; Hammami, A.; Mohamed, E.; et al. Leberagin-C, a Disintegrin-like/Cysteine-Rich Protein from Macrovipera lebetina transmediterranea Venom, Inhibits Alphavbeta3 Integrin-Mediated Cell Adhesion. Matrix Biol. 2009, 29, 117–126. [Google Scholar] [CrossRef]
- Usami, Y.; Fujimura, Y.; Miura, S.; Shima, H.; Yoshida, E.; Yoshioka, A.; Hirano, K.; Suzuki, M.; Titani, K. A 28-KDa Protein with Disintegrin-like Structure (Jararhagin-C) Purified from Bothrops jararaca Venom Inhibits Collagen- and ADP-Induced Platelet Aggregation. Biochem. Biophys. Res. Commun. 1994, 201, 331–339. [Google Scholar] [CrossRef]
- Liu, J.W.; Du, X.Y.; Liu, P.; Chen, X.; Xu, J.M.; Wu, X.F.; Zhou, Y.C. Purification, Characterization, and CDNA Sequence of Halysetin, a Disintegrin-like/Cysteine-Rich Protein from the Venom of Agkistrodon halys pallas. Biochem. Biophys. Res. Commun. 2000, 278, 112–118. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, B.B.; Mamede, C.C.N.; Matias, M.S.; Pereira, D.F.D.C.; de Queiroz, M.R.; Dias, E.H.V.; Silva, A.C.A.; Dantas, N.; Costa, J.D.O.; de Oliveira, F. A New Platelet-Aggregation-Inhibiting Factor Isolated from Bothrops moojeni Snake Venom. BioMed Res. Int. 2017, 2017, 4315832. [Google Scholar] [CrossRef] [Green Version]
- Terada, S.; Hirakawa, Y.; Fujimura, S. Primary Structure of a 29 KDa-Protein with Platelet Inhibitory Activity Isolated from the Venom of Agkistrodon halls brecicaudus. Fukuoka Univ. Sci. Rep. 1998, 28, 99–109. [Google Scholar]
- Souza, D.; Iemma, M.; Ferreira, L.L.; Faria, J.P.; Oliva, M.L.; Zingali, R.; Niewiarowski, S.; Selistre-de-Araujo, H. The Disintegrin-like Domain of the Snake Venom Metalloprotease Alternagin Inhibits A2β1 Integrin-Mediated Cell Adhesion. Arch. Biochem. Biophys. 2001, 384, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, K.; Shannon, J.D.; Jia, L.G.; Fox, J.W. Sequence and Biological Activity of Catrocollastatin-C: A Disintegrin-like/Cysteine-Rich Two-Domain Protein from Crotalus atrox Venom. Arch. Biochem. Biophys. 1997, 343, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.S.; de Sousa, B.B.; da Cunha Pereira, D.F.; Dias, E.H.V.; Mamede, C.C.N.; de Queiroz, M.R.; Silva, A.C.A.; Dantas, N.O.; Soares, A.M.; de Oliveira Costa, J.; et al. BaltDC: Purification, Characterization and Infrared Spectroscopy of an Antiplatelet DC Protein Isolated from Bothrops alternatus Snake Venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 36. [Google Scholar] [CrossRef] [Green Version]
- Moura-da-Silva, A.M.; Della-Casa, M.S.; David, A.S.; Assakura, M.T.; Butera, D.; Lebrun, I.; Shannon, J.D.; Serrano, S.M.T.; Fox, J.W. Evidence for Heterogeneous Forms of the Snake Venom Metalloproteinase Jararhagin: A Factor Contributing to Snake Venom Variability. Arch. Biochem. Biophys. 2003, 409, 395–401. [Google Scholar] [CrossRef]
- Komori, Y.; Sakai, K.; Masuda, K.; Nikai, A.T. Isolation and Biochemical Characterization of Rubelase, a Non-Hemorrhagic Elastase from Crotalus ruber ruber (Red Rattlesnake) Venom. Toxins 2011, 3, 900–910. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, S.; Oshikawa, K.; Terada, S.; Kimoto, E. Primary Structure and Autoproteolysis of Brevilysin H6 from the Venom of Gloydius halys brevicaudus. J. Biochem. 2000, 128, 167–173. [Google Scholar] [CrossRef]
- Takeya, H.; Nishida, S.; Nishino, N.; Makinose, Y.; Omori-Satoh, T.; Nikai, T.; Sugihara, H.; Iwanaga, S. Primary Structures of Platelet Aggregation Inhibitors (Disintegrins) Autoproteolytically Released from Snake Venom Hemorrhagic Metalloproteinases and New Fluorogenic Peptide Substrates for These Enzymes. J. Biochem. 1993, 113, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Huang, T.-F. Purification and Characterization of a Novel Metalloproteinase, Acurhagin, from Agkistrodon Acutus Venom. Thromb. Haemost. 2002, 87, 641–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petretski, J.H.; Kanashiro, M.M.; Rodrigues, F.R.; Alves, E.W.; Machado, O.L.; Kipnis, T.L. Edema Induction by the Disintegrin-like/Cysteine-Rich Domains from a Bothrops atrox Hemorrhagin. Biochem. Biophys. Res. Commun. 2000, 276, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, A.C.; Gay, C.C.; Moritz, M.N.D.O.; dos Santos, P.K.; Bustillo, S.; Rodríguez, J.P.; Acosta, O.C.; Biscoglio, M.J.; Selistre-De-Araujo, H.S.; Leiva, L.C. Purification of a Fragment Obtained by Autolysis of a PIIIb-SVMP from Bothrops alternatus Venom. Int. J. Biol. Macromol. 2018, 113, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assakura, M.T.; Silva, C.A.; Mentele, R.; Camargo, A.C.M.; Serrano, S.M.T. Molecular Cloning and Expression of Structural Domains of Bothropasin, a P-III Metalloproteinase from the Venom of Bothrops jararaca. Toxicon 2003, 41, 217–227. [Google Scholar] [CrossRef]
- Zang, J.; Zhu, Z.; Yu, Y.; Teng, M.; Niu, L.; Huang, Q.; Liu, Q.; Hao, Q. Purification, Partial Characterization and Crystallization of Acucetin, a Protein Containing both Disintegrin-like and Cysteine-Rich Domains Released by Auto-Proteolysis of a P-III-Type Metalloproteinase AaH-IV from Agkistrodon acutus Venom. Acta Crystallogr. D Biol. Crystallogr. 2003, 59, 2310–2312. [Google Scholar] [CrossRef] [Green Version]
- Kamiguti, A.S.; Moura-da-Silva, A.M.; Laing, G.D.; Knapp, T.; Zuzel, M.; Crampton, J.M.; Theakston, R.D. Collagen-Induced Secretion-Dependent Phase of Platelet Aggregation Is Inhibited by the Snake Venom Metalloproteinase Jararhagin. Biochim. Biophys. Acta 1997, 1335, 209–217. [Google Scholar] [CrossRef]
- Takeda, S. ADAM and ADAMTS Family Proteins and Snake Venom Metalloproteinases: A Structural Overview. Toxins 2016, 8, 155. [Google Scholar] [CrossRef] [Green Version]
- Menezes, M.C.; Paes Leme, A.F.; Melo, R.L.; Silva, C.A.; Della Casa, M.; Bruni, F.M.; Lima, C.; Lopes-Ferreira, M.; Camargo, A.C.M.; Fox, J.W.; et al. Activation of Leukocyte Rolling by the Cysteine-Rich Domain and the Hyper-Variable Region of HF3, a Snake Venom Hemorrhagic Metalloproteinase. FEBS Lett. 2008, 582, 3915–3921. [Google Scholar] [CrossRef] [Green Version]
- Kini, R.M.; Koh, C.Y. Metalloproteases Affecting Blood Coagulation, Fibrinolysis and Platelet Aggregation from Snake Venoms: Definition and Nomenclature of Interaction Sites. Toxins 2016, 8, 284. [Google Scholar] [CrossRef] [Green Version]
- Drew, E.D.; Janes, R.W. PDBMD2CD: Providing Predicted Protein Circular Dichroism Spectra from Multiple Molecular Dynamics-Generated Protein Structures. Nucleic Acids Res. 2020, 48, W17–W24. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Bulyáki, É.; Kun, J.; Moussong, É.; Lee, Y.-H.; Goto, Y.; Réfrégiers, M.; Kardos, J. BeStSel: A Web Server for Accurate Protein Secondary Structure Prediction and Fold Recognition from the Circular Dichroism Spectra. Nucleic Acids Res. 2018, 46, W315–W322. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wang, Y.; Zhang, Q.; Xia, Y.; Ge, W.; Guo, D. Prediction of Reversible Disulphide Based on Features from Local Structural Signatures. BMC Genom. 2017, 18, 279. [Google Scholar] [CrossRef] [PubMed]
- Giorgianni, M.W.; Dowell, N.L.; Griffin, S.; Kassner, V.A.; Selegue, J.E.; Carroll, S.B. The Origin and Diversification of a Novel Protein Family in Venomous Snakes. Proc. Natl. Acad. Sci. USA 2020, 117, 10911–10920. [Google Scholar] [CrossRef]
- Calvete, J.J.; Lomonte, B.; Saviola, A.J.; Bonilla, F.; Sasa, M.; Williams, D.J.; Undheim, E.A.B.; Sunagar, K.; Jackson, T.N.W. Mutual Enlightenment: A Toolbox of Concepts and Methods for Integrating Evolutionary and Clinical Toxinology via Snake Venomics and the Contextual Stance. Toxicon X 2021, 9–10, 100070. [Google Scholar] [CrossRef]
- Shental-Bechor, D.; Levy, Y. Effect of Glycosylation on Protein Folding: A Close Look at Thermodynamic Stabilization. Proc. Natl. Acad. Sci. USA 2008, 105, 8256–8261. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Gao, Y.; Zhu, Z.; Yu, Y.; Zhang, X.; Zang, J.; Teng, M.; Niu, L. Structural Basis of the Autolysis of AaHIV Suggests a Novel Target Recognizing Model for ADAM/Reprolysin Family Proteins. Biochem. Biophys. Res. Commun. 2009, 386, 159–164. [Google Scholar] [CrossRef]
- Calvete, J.J.; Moreno-Murciano, M.P.; Sanz, L.; Jürgens, M.; Schrader, M.; Raida, M.; Benjamin, D.C.; Fox, J.W. The Disulphide Bond Pattern of Catrocollastatin C, a Disintegrin-like/Cysteine-Rich Protein Isolated from Crotalus atrox Venom. Protein Sci. 2000, 9, 1365–1373. [Google Scholar] [CrossRef] [Green Version]
- Seidel, T.; Scholl, S.; Krebs, M.; Rienmüller, F.; Marten, I.; Hedrich, R.; Hanitzsch, M.; Janetzki, P.; Dietz, K.-J.; Schumacher, K. Regulation of the V-Type ATPase by Redox Modulation. Biochem. J. 2012, 448, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Passam, F.J.; Chiu, J. Allosteric disulphide bonds as reversible mechano-sensitive switches that control protein functions in the vasculature. Biophys. Rev. 2019, 11, 419–430. [Google Scholar] [CrossRef]
- Pinto, A.F.M.; Terra, R.M.S.; Guimaraes, J.A.; Fox, J.W. Mapping von Willebrand Factor A Domain Binding Sites on a Snake Venom Metalloproteinase Cysteine-Rich Domain. Arch. Biochem. Biophys. 2007, 457, 41–46. [Google Scholar] [CrossRef]
- Serrano, S.M.T.; Kim, J.; Wang, D.; Dragulev, B.; Shannon, J.D.; Mann, H.H.; Veit, G.; Wagener, R.; Koch, M.; Fox, J.W. The Cysteine-Rich Domain of Snake Venom Metalloproteinases Is a Ligand for von Willebrand Factor A Domains: Role in Substrate Targeting. J. Biol. Chem. 2006, 281, 39746–39756. [Google Scholar] [CrossRef] [Green Version]
- Cominetti, M.R.; Terruggi, C.H.B.; Ramos, O.H.P.; Fox, J.W.; Mariano-Oliveira, A.; De Freitas, M.S.; Figueiredo, C.C.; Morandi, V.; Selistre-de-Araujo, H.S. Alternagin-C, a Disintegrin-like Protein, Induces Vascular Endothelial Cell Growth Factor (VEGF) Expression and Endothelial Cell Proliferation in vitro. J. Biol. Chem. 2004, 279, 18247–18255. [Google Scholar] [CrossRef] [Green Version]
- Clissa, P.B.; Lopes-Ferreira, M.; Della-Casa, M.S.; Farsky, S.H.P.; Moura-da-Silva, A.M. Importance of Jararhagin Disintegrin-like and Cysteine-Rich Domains in the Early Events of Local Inflammatory Response. Toxicon 2006, 47, 591–596. [Google Scholar] [CrossRef]
- De Luca, M.; Ward, C.M.; Ohmori, K.; Andrews, R.K.; Berndt, M.C. Jararhagin and Jaracetin: Novel Snake Venom Inhibitors of the Integrin Collagen Receptor, Alpha 2 Beta 1. Biochem. Biophys. Res. Commun. 1995, 206, 570–576. [Google Scholar] [CrossRef]
- Jia, L.G.; Wang, X.M.; Shannon, J.D.; Bjarnason, J.B.; Fox, J.W. Function of Disintegrin-like/Cysteine-Rich Domains of Atrolysin A. Inhibition of Platelet Aggregation by Recombinant Protein and Peptide Antagonists. J. Biol. Chem. 1997, 272, 13094–13102. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.G.; Wang, X.M.; Shannon, J.D.; Bjarnason, J.B.; Fox, J.W. Inhibition of Platelet Aggregation by the Recombinant Cysteine-Rich Domain of the Hemorrhagic Snake Venom Metalloproteinase, Atrolysin A. Arch. Biochem. Biophys. 2000, 373, 281–286. [Google Scholar] [CrossRef]
- Swieringa, F.; Kuijpers, M.J.E.; Heemskerk, J.W.M.; van der Meijden, P.E.J. Targeting Platelet Receptor Function in Thrombus Formation: The Risk of Bleeding. Blood Rev. 2014, 28, 9–21. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet Integrin AIIbβ3: Signal Transduction, Regulation, and Its Therapeutic Targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Moura-da-Silva, A.M.; Línica, A.; Della-Casa, M.S.; Kamiguti, A.S.; Ho, P.L.; Crampton, J.M.; Theakston, R.D. Jararhagin ECD-Containing Disintegrin Domain: Expression in Escherichia coli and Inhibition of the Platelet-Collagen Interaction. Arch. Biochem. Biophys. 1999, 369, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Menezes, M.C.; Imbert, L.; Kitano, E.S.; Vernet, T.; Serrano, S.M.T. Recombinant Expression of the Precursor of the Hemorrhagic Metalloproteinase HF3 and Its Non-Catalytic Domains Using a Cell-Free Synthesis System. Amino Acids 2016, 48, 2205–2214. [Google Scholar] [CrossRef]
- Ivaska, J.; Käpylä, J.; Pentikäinen, O.; Hoffrén, A.M.; Hermonen, J.; Huttunen, P.; Johnson, M.S.; Heino, J. A Peptide Inhibiting the Collagen Binding Function of Integrin Alpha2I Domain. J. Biol. Chem. 1999, 274, 3513–3521. [Google Scholar] [CrossRef] [Green Version]
- Kamiguti, A.S.; Gallagher, P.; Marcinkiewicz, C.; Theakston, R.D.G.; Zuzel, M.; Fox, J.W. Identification of Sites in the Cysteine-Rich Domain of the Class P-III Snake Venom Metalloproteinases Responsible for Inhibition of Platelet Function. FEBS Lett. 2003, 549, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Sajevic, T.; Leonardi, A.; Kovačič, L.; Lang-Balija, M.; Kurtović, T.; Pungerčar, J.; Halassy, B.; Trampuš-Bakija, A.; Križaj, I. VaH3, One of the Principal Hemorrhagins in Vipera ammodytes ammodytes Venom, Is a Homodimeric P-IIIc Metalloproteinase. Biochimie 2013, 95, 1158–1170. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Premrov Bajuk, B.; Zrimšek, P.; Kotnik, T.; Leonardi, A.; Križaj, I.; Jakovac Strajn, B. Insect Protein-Based Diet as Potential Risk of Allergy in Dogs. Animals 2021, 11, 1942. [Google Scholar] [CrossRef]
- Wu, T.; Yu, J.; Gale-Day, Z.; Woo, A.; Suresh, A.; Hornsby, M.; Gestwicki, J.E. Three Essential Resources to Improve Differential Scanning Fluorimetry (DSF) Experiments. bioRxiv 2020. [Google Scholar] [CrossRef]
- Šali, A.; Blundell, T.L. Comparative Protein Modelling by Satisfaction of Spatial Restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI Search and Sequence Analysis Tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A Program to Check the Stereochemical Quality of Protein Structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]

| Secondary Structure Element | Secondary Structure Contents (%) | |
|---|---|---|
| 3D Model | Experimental Data | |
| α-Helix | 14.8 | 10.9 |
| Antiparallel β-sheet | 27.8 | 27.4 |
| Turn | 13.1 | 15.8 |
| Other | 44.2 | 45.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Požek, K.; Leonardi, A.; Pungerčar, J.; Rao, W.; Gao, Z.; Liu, S.; Laustsen, A.H.; Trampuš Bakija, A.; Reberšek, K.; Podgornik, H.; et al. Genomic Confirmation of the P-IIIe Subclass of Snake Venom Metalloproteinases and Characterisation of Its First Member, a Disintegrin-Like/Cysteine-Rich Protein. Toxins 2022, 14, 232. https://doi.org/10.3390/toxins14040232
Požek K, Leonardi A, Pungerčar J, Rao W, Gao Z, Liu S, Laustsen AH, Trampuš Bakija A, Reberšek K, Podgornik H, et al. Genomic Confirmation of the P-IIIe Subclass of Snake Venom Metalloproteinases and Characterisation of Its First Member, a Disintegrin-Like/Cysteine-Rich Protein. Toxins. 2022; 14(4):232. https://doi.org/10.3390/toxins14040232
Chicago/Turabian StylePožek, Kity, Adrijana Leonardi, Jože Pungerčar, Weiqiao Rao, Zijian Gao, Siqi Liu, Andreas Hougaard Laustsen, Alenka Trampuš Bakija, Katarina Reberšek, Helena Podgornik, and et al. 2022. "Genomic Confirmation of the P-IIIe Subclass of Snake Venom Metalloproteinases and Characterisation of Its First Member, a Disintegrin-Like/Cysteine-Rich Protein" Toxins 14, no. 4: 232. https://doi.org/10.3390/toxins14040232
APA StylePožek, K., Leonardi, A., Pungerčar, J., Rao, W., Gao, Z., Liu, S., Laustsen, A. H., Trampuš Bakija, A., Reberšek, K., Podgornik, H., & Križaj, I. (2022). Genomic Confirmation of the P-IIIe Subclass of Snake Venom Metalloproteinases and Characterisation of Its First Member, a Disintegrin-Like/Cysteine-Rich Protein. Toxins, 14(4), 232. https://doi.org/10.3390/toxins14040232







