Warming and Salt Intrusion Affect Microcystin Production in Tropical Bloom-Forming Microcystis
Abstract
:1. Introduction
2. Results
2.1. Effect of Temperature and Salinity on Biomass Indicators
2.2. Microcystin Concentrations
2.3. Microcystin Quota
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sampling Locations and Microcystis Strains
5.2. Growth Experiment
5.3. Microcystin (MC) Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Strain | Temp (°C) | DW = DW0 + a × Sal | r2 |
|---|---|---|---|
| MBC | 27 | 287.9 + 11.91 × Sal | 0.9375 |
| 31 | 281.6 + 18.57 × Sal | 0.9844 | |
| 35 | 291.3 + 12.77 × Sal | 0.6967 | |
| 37 | 325.2 + 11.09 × Sal | 0.9807 | |
| MTV | 27 | 228.4 + 26.05 × Sal | 0.8876 |
| 31 | 251.3 + 24.25 × Sal | 0.8612 | |
| 35 | 290.7 + 16.13 × Sal | 0.9141 | |
| 37 | 255.4 + 17.81 × Sal | 0.9487 |
| Indicator | M. aeruginosa MBC | M. aeruginosa MTV | |
|---|---|---|---|
| Particles | slope | F3,8 = 0.3903; p = 0.7634 | F3,8 = 1.7188; p = 0.2400 |
| intercept | F3,11 = 1.0647; p = 0.4034 | F3,11 = 2.0534; p = 0.1648 | |
| Chlorophyll-a | slope | F3,8 = 2.6897; p = 0.1170 | F3,8 = 0.3372; p = 0.7992 |
| intercept | F3,11 = 0.7597; p = 0.5398 | F3,11 = 1.6620; p = 0.2322 | |
| Dry-weight | slope | F3,8 = 1.0432; p = 0.4245 | F3,8 = 0.8387; p = 0.5097 |
| intercept | F3,11 = 1.4221; p = 0.2887 | F3,11 = 0.4524; p = 0.7208 |
Appendix B
| Microcystis aeruginosa MBC | ||||||
|---|---|---|---|---|---|---|
| Temp (°C) | Sal. ‰ | dmMC-RR | MC-RR | MC-YR | dmMC-LR | MC-LR |
| 27 | 0.5 | 4.2 | 31.4 | 12.9 | 2.8 | 48.8 |
| 27 | 4 | 2.6 | 24.6 | 12.1 | 2.7 | 58.0 |
| 27 | 8 | 1.2 | 21.9 | 11.6 | 1.3 | 64.0 |
| 27 | 12 | 1.8 | 23.4 | 11.5 | 1.0 | 62.3 |
| 27 | 16 | wash out * | ---* | ---* | ---* | ---* |
| 31 | 0.5 | 4.2 | 32.3 | 14.0 | 2.4 | 47.1 |
| 31 | 4 | 1.9 | 27.7 | 13.8 | 1.3 | 55.2 |
| 31 | 8 | 1.5 | 27.4 | 13.6 | 1.0 | 56.6 |
| 31 | 12 | 1.9 | 28.6 | 10.9 | 0.7 | 57.9 |
| 31 | 16 | 2.4 | 29.0 | 13.1 | 1.4 | 54.2 |
| 35 | 0.5 | 15.5 | 43.2 | 8.6 | <LOQ | 32.7 |
| 35 | 4 | 7.8 | 30.9 | 11.2 | <LOQ | 50.1 |
| 35 | 8 | 5.7 | 28.3 | 11.8 | <LOQ | 54.2 |
| 35 | 12 | 6.2 | 31.9 | 10.4 | <LOQ | 51.5 |
| 35 | 16 | 8.8 | 33.6 | 10.5 | <LOQ | 47.1 |
| 37 | 0.5 | <LOQ | <LOQ | 11.6 | <LOQ | 88.4 |
| 37 | 4 | <LOQ | 68.3 | 6.2 | <LOQ | 25.5 |
| 37 | 8 | <LOQ | 67.1 | 6.1 | <LOQ | 26.8 |
| 37 | 12 | <LOQ | 61.6 | 5.5 | <LOQ | 32.9 |
| 37 | 16 | <LOQ | 49.3 | 7.3 | <LOQ | 43.4 |
Appendix C
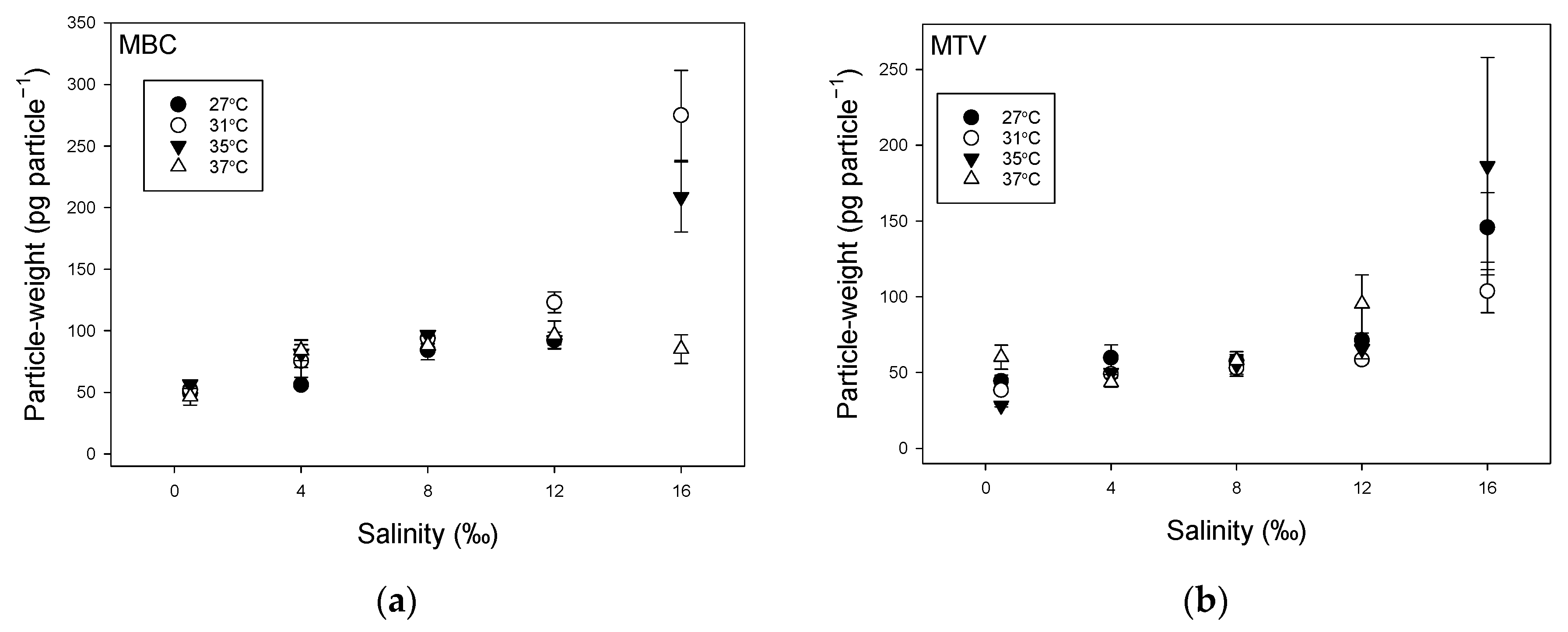
Appendix D
| WC Medium Supplied (L per Day) | |||||||
|---|---|---|---|---|---|---|---|
| MBC | MTV | ||||||
| 27 °C | 31 °C | 35 °C | 37 °C | 27 °C | 31 °C | 35 °C | 37 °C |
| 0.340 | 0.366 | 0.349 | 0.391 | 0.362 | 0.347 | 0.332 | 0.387 |
| 0.354 | 0.368 | 0.351 | 0.389 | 0.353 | 0.366 | 0.338 | 0.388 |
| 0.369 | 0.366 | 0.351 | 0.386 | 0.362 | 0.345 | 0.347 | 0.387 |
| 0.367 | 0.363 | 0.348 | 0.385 | 0.347 | 0.354 | 0.352 | 0.390 |
| 0.370 | 0.361 | 0.344 | 0.403 | 0.364 | 0.356 | 0.347 | 0.413 |
| 0.366 | 0.363 | 0.356 | 0.401 | 0.356 | 0.358 | 0.348 | 0.374 |
| 0.365 | 0.364 | 0.355 | 0.398 | 0.339 | 0.357 | 0.353 | 0.370 |
| 0.369 | 0.363 | 0.353 | 0.397 | 0.358 | 0.352 | 0.349 | 0.366 |
| 0.360 | 0.362 | 0.360 | 0.425 | 0.364 | 0.352 | 0.358 | 0.366 |
| 0.358 | 0.357 | 0.352 | 0.328 | 0.350 | 0.351 | 0.356 | 0.365 |
| 0.362 | 0.361 | 0.355 | 0.395 | 0.323 | 0.353 | 0.348 | 0.370 |
| 0.363 | 0.358 | 0.352 | 0.361 | 0.364 | 0.348 | 0.345 | 0.364 |
| 0.358 | 0.346 | 0.338 | 0.359 | 0.360 | 0.334 | 0.351 | 0.352 |
| 0.364 | 0.384 | 0.381 | 0.376 | 0.378 | 0.340 | 0.394 | |
| 0.366 | 0.354 | 0.350 | 0.347 | 0.354 | 0.366 | ||
| 0.357 | 0.351 | 0.350 | 0.346 | 0.362 | |||
| Averages added (L)/dilution rate (d−1) | Averages added (L)/dilution rate (d−1) | ||||||
| 0.362 L | 0.362 L | 0.353 L | 0.386 L | 0.356 L | 0.353 L | 0.348 L | 0.377 L |
| 0.289 d−1 | 0.289 d−1 | 0.282 d−1 | 0.309 d−1 | 0.284 d−1 | 0.282 d−1 | 0.278 d−1 | 0.301 d−1 |
Appendix E
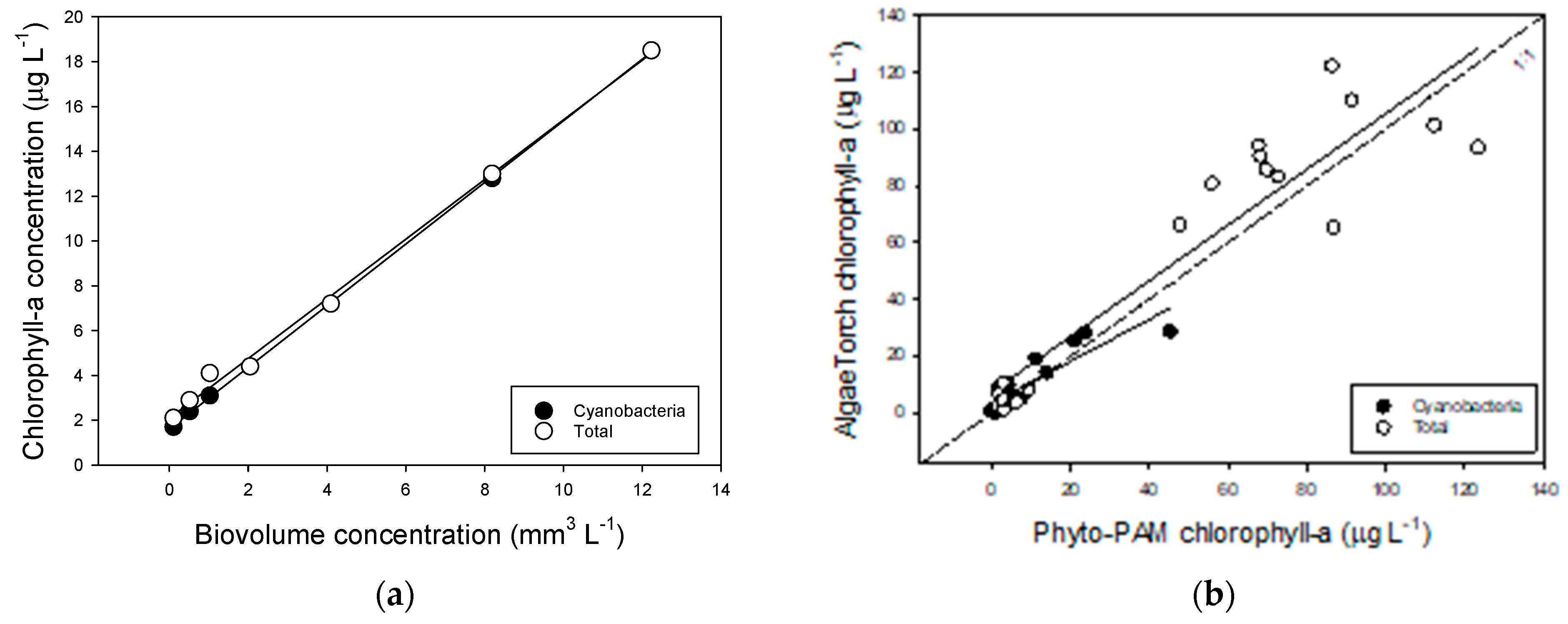
References
- Paerl, H.W.; Barnard, M.A. Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human- and climatically-altered world. Harmful Algae 2020, 96, 101845. [Google Scholar] [CrossRef] [PubMed]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Bui, T.; Dao, T.S.; Faassen, E.; Lürling, M. Cyanobacterial Blooms and Microcystins in Southern Vietnam. Toxins 2018, 10, 471. [Google Scholar]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, S.; Laplante, B.; Meisner, C.; Wheeler, D.; Yan, J. The Impact of Sea Level Rise on Developing Countries: A Comparative Analysis. Clim. Chang. 2007, 93, 379–388. [Google Scholar] [CrossRef]
- MRC. Adaptation to Climate Change in the Countries of the Lower Mekong Basin: Regional Synthesis Report; MRC Technical Paper No. 24; Mekong River Commission: Vientiane, Laos, 2009; p. 89. [Google Scholar]
- MONRE. Climate Change, Sea Level Rise Scenarios for Vietnam; MONRE: Hanoi, Vietnam, 2012. [Google Scholar]
- Asian Development Bank. Viet Nam: Environment and Climate Change Assessment; Asian Development Bank: Mandaluyong City, Philippines, 2013. [Google Scholar]
- MONRE. Climate Change, Sea Level Rise Scenarios for Vietnam; MONRE: Hanoi, Vietnam, 2009. [Google Scholar]
- Paerl, H.W.; Huisman, J. Climate. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [Green Version]
- Giani, A.; Taranu, Z.E.; von Rückert, G.; Gregory-Eaves, I. Comparing key drivers of cyanobacteria biomass in temperate and tropical systems. Harmful Algae 2020, 97, 101859. [Google Scholar] [CrossRef]
- Urrutia-Cordero, P.; Zhang, H.; Chaguaceda, F.; Geng, H.; Hansson, L.A. Climate warming and heat waves alter harmful cyanobacterial blooms along the benthic-pelagic interface. Ecology 2020, 101, e03025. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.F.; Oishi, S. Effects of environmental factors on toxicity of a cyanobacterium (Microcystis aeruginosa) under culture conditions. Appl. Environ. Microbiol. 1985, 49, 1342–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Westhuizen, A.J.; Eloff, J.N. Effect of temperature and light on the toxicity and growth of the blue-green alga Microcystisaeruginosa (UV-006). Planta 1985, 163, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Mowe, M.A.D.; Porojan, C.; Abbas, F.; Mitrovic, S.M.; Lim, R.P.; Furey, A.; Yeo, D.C.J. Rising temperatures may increase growth rates and microcystin production in tropical Microcystis species. Harmful Algae 2015, 50, 88–98. [Google Scholar] [CrossRef]
- Lürling, M.; van Oosterhout, F.; Faassen, E. Eutrophication and Warming Boost Cyanobacterial Biomass and Microcystins. Toxins 2017, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Bui, T.; Dao, T.S.; Vo, T.G.; Lürling, M. Warming Affects Growth Rates and Microcystin Production in Tropical Bloom-Forming Microcystis Strains. Toxins 2018, 10, 123. [Google Scholar] [CrossRef] [Green Version]
- Kotera, A.; Sakamoto, T.; Khang, N.; Yokozawa, M. Regional consequences of seawater intrusion on rice productivity and land use in coastal area of the Mekong River Delta. Jpn. Agric. Res. Q. 2008, 42, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Trung, N.H.; Tri, V.P.D.; Esteban, N.D.T.T. 10—Possible Impacts of Seawater Intrusion and Strategies for Water Management in Coastal Areas in the Vietnamese Mekong Delta in the Context of Climate Change. In Coastal Disasters and Climate Change in Vietnam; Elsevier: Oxford, UK, 2014; pp. 219–232. [Google Scholar]
- Dat, T.Q.; Trung, N.H.; Likitdecharote, K. Modelling the influence of river discharge and sea level rise on salinity intrusion in the Mekong Delta. In The First Environment Asia International Conference on Environmental Supporting in Food and Energy Security: Crisis and Opportunity; Thai Society of Higher Education Institutes on Environment: Bangkok, Thailand, 2011. [Google Scholar]
- Lam, M.P.; Van, P.D.T.; Tran, Q.D. Application of a one-dimensional hydrodynamic model to assess the historical saline intrusion dynamics and to predict the future dynamics in the main river network in the TraVinh Province. J. Sci. CanThoUniv. Part A Nat. Sci. Technol. Environ. 2013, 25, 68–75. [Google Scholar]
- Moisander, P.H.; Paerl, H.W. Growth, primary productivity, and nitrogen fixation potential of Nodularia spp. (cyanophyceae) in water from a subtropical estuary in the United States. J. Phycol. 2000, 36, 645–658. [Google Scholar] [CrossRef]
- Tonk, L.; Bosch, K.; Visser, P.M.; Huisman, J. Salt tolerance of the harmful cyanobacterium Microcystis aeruginosa. Aquat. Microb. Ecol. 2007, 46, 117–123. [Google Scholar] [CrossRef]
- Black, K.; Yilmaz, M.; Phlips, E. Growth and Toxin Production by Microcystis aeruginosa PCC 7806 (Kutzing) Lemmerman at Elevated Salt Concentrations. J. Environ. Prot. 2011, 2, 669–674. [Google Scholar] [CrossRef] [Green Version]
- Lehman, P.W.; Boyer, G.; Hall, C.; Waller, S.; Gehrts, K. Distribution and toxicity of a new colonial Microcystis aeruginosa bloom in the San Francisco Bay Estuary, California. Hydrobiologia 2005, 541, 87–99. [Google Scholar] [CrossRef]
- Sinclair, J.L.; Hall, S.; Berkman, J.A.; Boyer, G.; Burkholder, J.; Burns, J.; Carmichael, W.; DuFour, A.; Frazier, W.; Morton, S.L.; et al. Occurrence of Cyanobacterial Harmful Algal Blooms Workgroup report. Adv. Exp. Med. Biol. 2008, 619, 45–103. [Google Scholar] [PubMed]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A review of microcystin detections in Estuarine and Marine waters: Environmental implications and human health risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Georges des Aulnois, M.; Roux, P.; Caruana, A.; Réveillon, D.; Briand, E.; Hervé, F.; Savar, V.; Bormans, M.; Amzil, Z. Physiological and Metabolic Responses of Freshwater and Brackish-Water Strains of Microcystisaeruginosa Acclimated to a Salinity Gradient: Insight into Salt Tolerance. Appl. Environ. Microbiol. 2019, 85, e01614-19. [Google Scholar] [CrossRef]
- Ohkubo, N.; Yagi, O.; Okada, M. Effects of Temperature and Illumination on the Growth of Blue-green Alga Microcystis viridis. Jpn. J. Limnol. 1991, 52, 255–261. [Google Scholar] [CrossRef]
- Geada, P.; Pereira, R.N.; Vasconcelos, V.; Vicente, A.A.; Fernandes, B.D. Assessment of synergistic interactions between environmental factors on Microcystis aeruginosa growth and microcystin production. Algal Res. 2017, 27, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Yagi, O.; Ohkubo, N.; Tomioka, N.; Okada, M. Effect of irradiance and temperature on photosynthetic activity of the cyanobacterium Microcystis spp. Environ. Technol. 1994, 15, 389–394. [Google Scholar] [CrossRef]
- NOAA National Centers for Environmental Information. Global Temperature and Precipitation Maps. Available online: https://www.ncdc.noaa.gov/temp-and-precip/global-maps/ (accessed on 24 March 2021).
- Eastham, J.; Mpelasoka, F.; Mainuddin, M.; Ticehurst, C.; Dyce, P.; Hodgson, G.; Ali, R.; Kirby, M. Mekong River Basin Water Resources Assessment: Impacts of Climate Change. In Water for a Healthy Country National Research Flagship Report; Commonwealth Scientific and Industrial Research Organisation (CSIRO): Canberra, Australia, 2008. [Google Scholar]
- Office of the UN Resident Coordinator. Viet Nam: Drought and Saltwater Intrusion; Flash Update No. 3; United Nations in Vietnam: Ha Noi, Vietnam, 2020. [Google Scholar]
- Robson, B.J.; Hamilton, D.P. Summer flow event induces a cyanobacterial bloom in a seasonal western Australia estuary. Mar. Freshw. Res. 2003, 54, 139–151. [Google Scholar] [CrossRef]
- Wangwibulkit, S.; Limsuwan, C.; Chuchird, N. Effects of salinity and pH on the growth of blue-green algae, Oscillatoria sp. and Microcystis sp., isolated from Pacific white shrimp (Litopenaeusvannamei) ponds. Kasetsart Univ. Fish. Res. Bull. 2008, 32, 1–9. [Google Scholar]
- Liu, L.; Huang, Q.; Qin, B. Characteristics and roles of Microcystis extracellular polymeric substances (EPS) in cyanobacterial blooms: A short review. J. Freshwater Ecol. 2018, 33, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Kruk, C.; Segura, A.M.; Nogueira, L.; Alcántara, I.; Calliari, D.; Martínez de la Escalera, G.; Carballo, C.; Cabrera, C.; Sarthou, F.; Scavone, P.; et al. A multilevel trait-based approach to the ecological performance of Microcystis aeruginosa complex from headwaters to the ocean. Harmful Algae 2017, 70, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Kudela, R.M.; Mekebri, A.; Crane, D.; Oates, S.C.; Tinker, M.T.; Staedler, M.; Miller, W.A.; Toy-Choutka, S.; Dominik, C.; et al. Evidence for a novel marine harmful algal bloom: Cyanotoxin (microcystin) transfer from land to sea otters. PLoS ONE 2010, 5, e12576. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, G.; Huisman, J.; Matthijs, H.C. Potassium sensitivity differs among strains of the harmful cyanobacterium Microcystis and correlates with the presence of salt tolerance genes. FEMS Microbiol. Lett. 2015, 362, fnv121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, Y.; Hodoki, Y.; Sano, T.; Tada, K.; Watanabe, M.M. Adaptation of the Freshwater Bloom-Forming Cyanobacterium Microcystisaeruginosa to Brackish Water Is Driven by Recent Horizontal Transfer of Sucrose Genes. Front. Microbiol. 2018, 9, 1150. [Google Scholar] [CrossRef] [Green Version]
- Melero-Jiménez, I.J.; Martín-Clemente, E.; García-Sánchez, M.J.; Bañares-España, E.; Flores-Moya, A. The limit of resistance to salinity in the freshwater cyanobacterium Microcystisaeruginosa is modulated by the rate of salinity increase. Ecol. Evol. 2020, 10, 5045–5055. [Google Scholar] [CrossRef] [Green Version]
- Melero-Jiménez, I.J.; Martín-Clemente, E.; García-Sánchez, M.J.; Flores-Moya, A.; Bañares-España, E. Adaptation of the toxic fresh-water cyanobacterium Microcystis aeruginosa to salinity is achieved by the selection of spontaneous mutants. Phycol. Res. 2019, 67, 192–201. [Google Scholar] [CrossRef]
- Available online: https://web.stanford.edu/group/Urchin/mineral.html (accessed on 24 March 2021).
- Warr, S.R.C.; Reed, R.H.; Stewart, W.D.P. Carbohydrate accumulation in osmotically stressed cyanobacteria (blue-green algae): Interactions of temperature and salinity. New Phytol. 1985, 100, 285–292. [Google Scholar] [CrossRef]
- Gianuzzi, L.; Krock, B.; CrettazMinaglia, M.C.; Rosso, L.; Houghton, C.; Sedan, D.; Malanga, G.; Espinosa, M.; Andrinolo, D.; Hernando, M. Growth, toxin production, active oxygen species and catalase activity of Microcystis aeruginosa (Cyanophyceae) exposed to temperature stress. Comp. Biochem. Physiol. Part C 2016, 189, 22–30. [Google Scholar] [CrossRef] [Green Version]
- CrettazMinaglia, M.C.; Rosso, L.; Aranda, J.O.; Goñi, S.; Sedan, D.; Andrinolo, D.; Giannuzzi, L. Mathematical modeling of Microcystis aeruginosa growth and [D-Leu1] microcystin-LR production in culture media at different temperatures. Harmful Algae 2017, 67, 13–25. [Google Scholar]
- Zilliges, Y.; Kehr, J.C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS ONE 2011, 6, e17615. [Google Scholar] [CrossRef] [Green Version]
- Kaebernick, M.; Neilan, B.A.; Börner, T.; Dittmann, E. Light and the Transcriptional Response of the Microcystin Biosynthesis Gene Cluster. Appl. Environ. Microbiol. 2000, 66, 3387–3392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; De Meester, L.; et al. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef] [Green Version]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA; American Water Works Foundation: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2005; p. 2671. [Google Scholar]
- Hoshaw, R.; Rosowski, J.R. Methods for microscopic algae. In Handbook of Phycological Methods: Culture Methods and Growth Measurements; Stein, R., Ed.; Cambridge University Press: London, UK, 1973; pp. 53–68. [Google Scholar]
- Lürling, M.; Beekman, W. Palmelloids formation in Chlamydomonas reinhardtii: Defence against rotifer predators? Ann. Limnol. 2006, 42, 65–72. [Google Scholar] [CrossRef]
- Lürling, M.; Faassen, E.J. Dog Poisonings Associated with a Microcystis aeruginosa Bloom in the Netherlands. Toxins 2013, 5, 556–567. [Google Scholar] [CrossRef] [PubMed]
- NNI. Water—Spectrophotometric Determination of Chlorophyll-A Content; NEN 6520+ C1; Netherlands Normalization Institute: Delft, The Netherlands, 2011. (In Dutch) [Google Scholar]





| Strain | Temp. (°C) | Salinity (‰) | pH | TN (mg L−1) | TP (mg L−1) | N-NH4 (mg L−1) | N-NO3 (mg L−1) | P-PO4 (mg L−1) | CHLa (µg L−1) |
|---|---|---|---|---|---|---|---|---|---|
| MBC | 37.4 | 7.5 | 9.77 | 9.00 | 0.33 | 0.67 | <0.01 | <0.02 | 1520 |
| MTV | 33.1 | 0.5 | 9.58 | 19.5 | 1.75 | 1.14 | 0.28 | 0.02 | 4352 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trung, B.; Vollebregt, M.E.; Lürling, M. Warming and Salt Intrusion Affect Microcystin Production in Tropical Bloom-Forming Microcystis. Toxins 2022, 14, 214. https://doi.org/10.3390/toxins14030214
Trung B, Vollebregt ME, Lürling M. Warming and Salt Intrusion Affect Microcystin Production in Tropical Bloom-Forming Microcystis. Toxins. 2022; 14(3):214. https://doi.org/10.3390/toxins14030214
Chicago/Turabian StyleTrung, Bui, Marlies E. Vollebregt, and Miquel Lürling. 2022. "Warming and Salt Intrusion Affect Microcystin Production in Tropical Bloom-Forming Microcystis" Toxins 14, no. 3: 214. https://doi.org/10.3390/toxins14030214
APA StyleTrung, B., Vollebregt, M. E., & Lürling, M. (2022). Warming and Salt Intrusion Affect Microcystin Production in Tropical Bloom-Forming Microcystis. Toxins, 14(3), 214. https://doi.org/10.3390/toxins14030214






