The Isolated Mouse Jejunal Afferent Nerve Assay as a Tool to Assess the Effect of Botulinum Neurotoxins in Visceral Nociception
Abstract
1. Introduction
2. Results
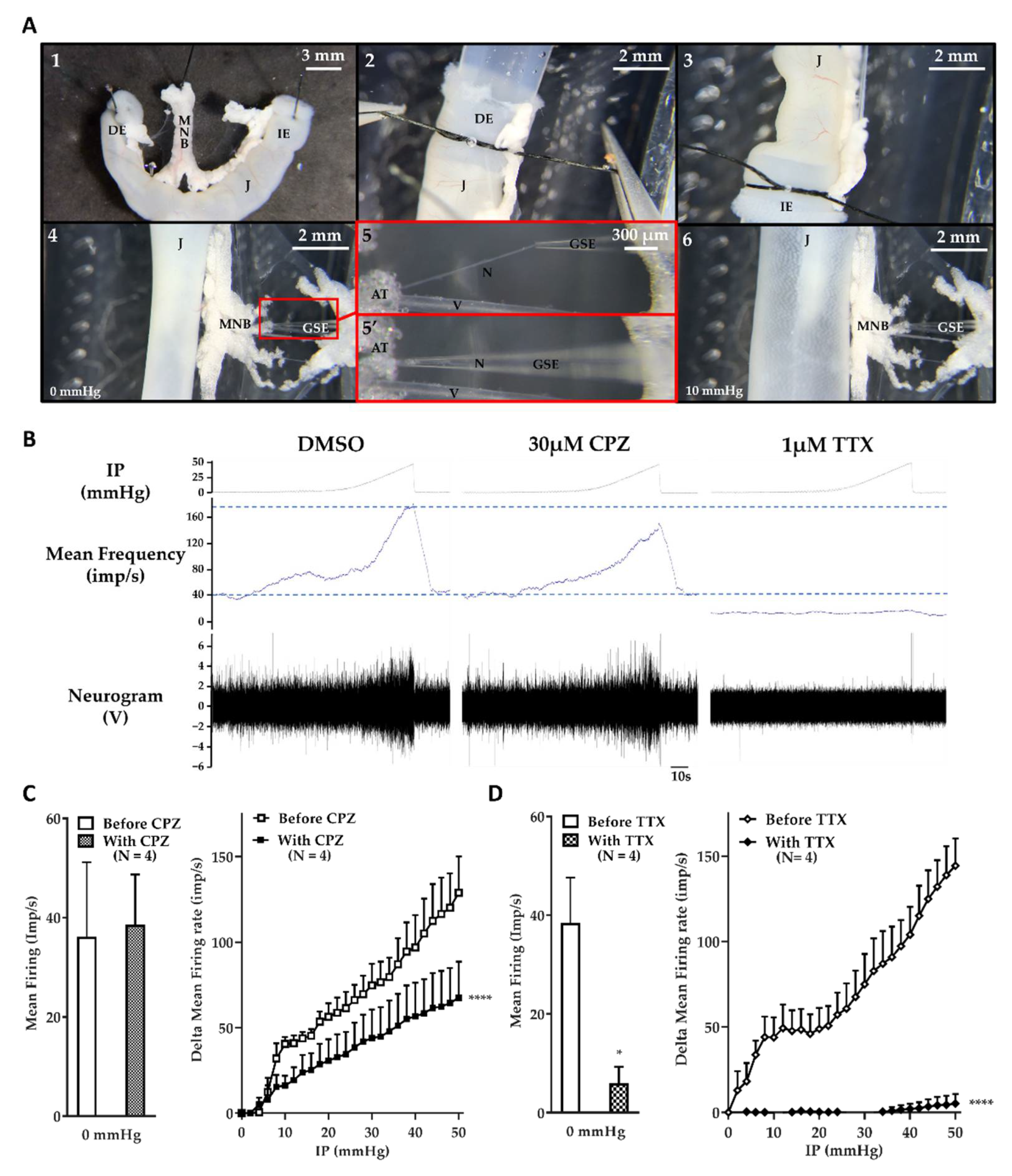
2.1. The Jejunum Mesenteric Nerve Assay: A Sensitive Assay
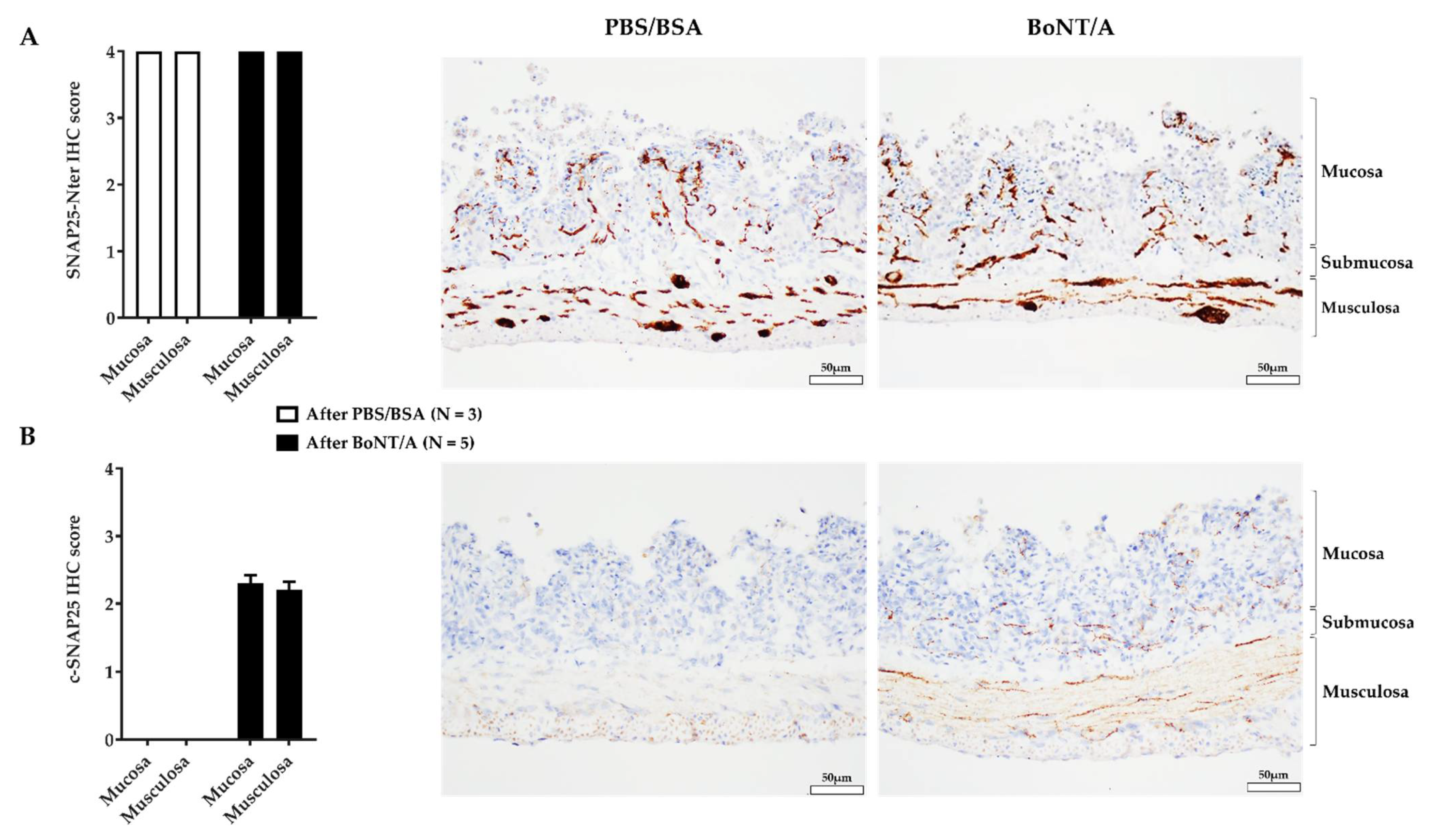
2.2. An Effective BoNT/A Intoxication
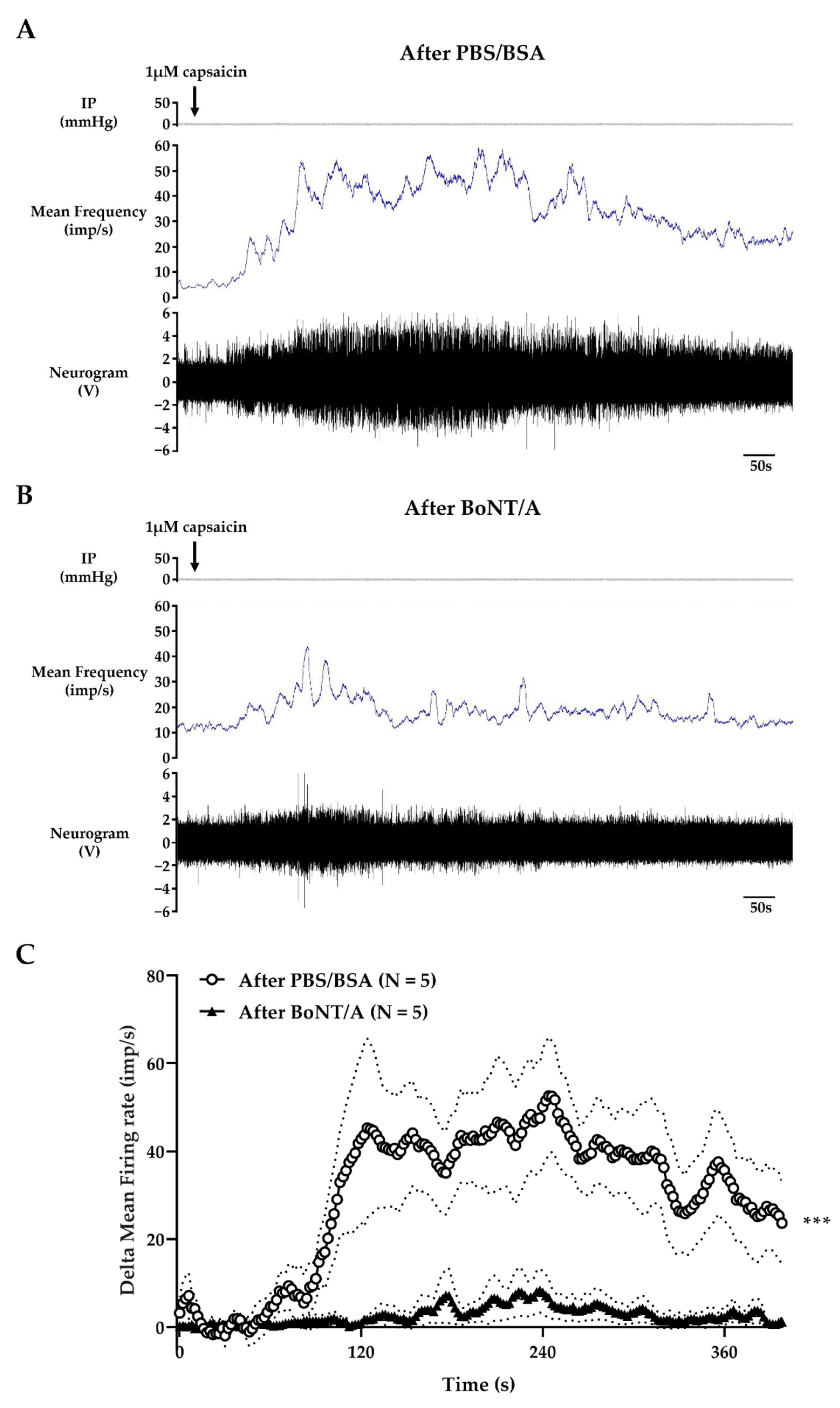
2.3. BoNT/A Inhibits Jejunal Multiunit Afferent Nerve Discharge
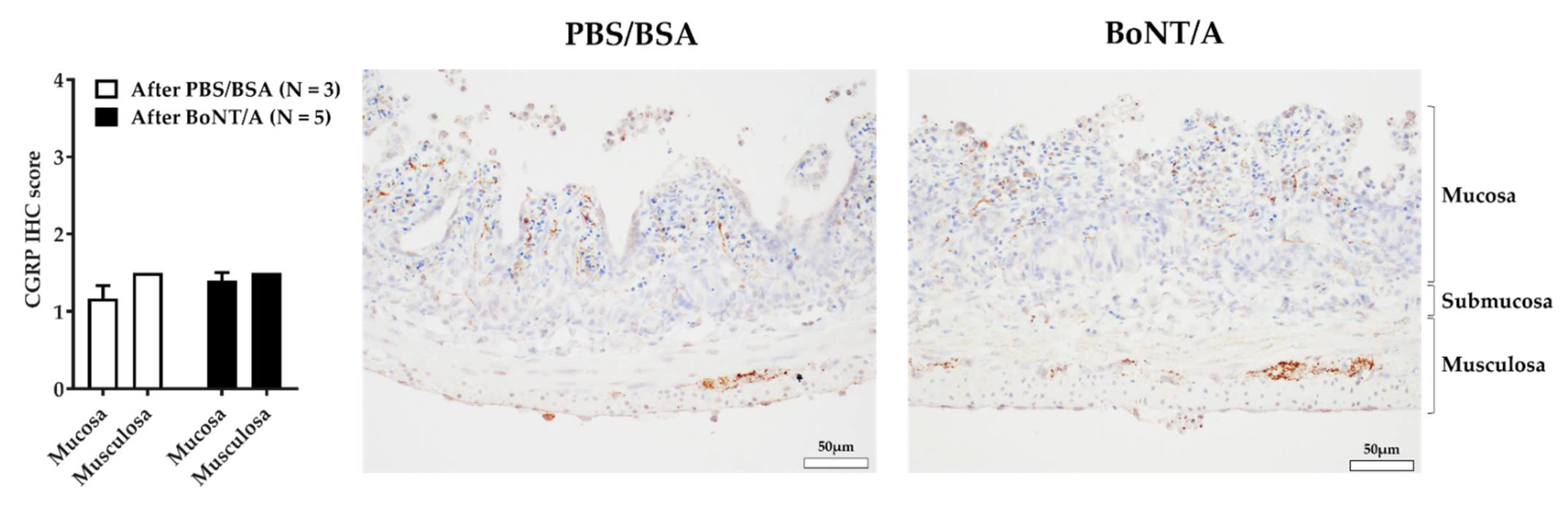
2.4. BoNT/A Does Not Change the Quantity of Calcitonin Gene-Related Peptide in the Nerve Endings
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Drugs
5.2. Jejunum Segments Preparation
5.3. Multiunit Discharge Recording on Mesenteric Nerve
5.4. Mechanical Stimulation
5.5. Pharmacological Stimulation
5.6. Treatments
5.7. Immunohistochemistry
5.8. Statistical Analysis
5.9. List of Abbreviations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng Chen, Z.; Morris, J.G.; Rodriguez, R.L.; Wagle Shukla, A.; Tapia-Núñez, J.; Okun, M.S. Emerging opportunities for serotypes of botulinum neurotoxins. Toxins 2012, 4, 1196–1222. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Rizo, J.; Südhof, T.C. Snares and Munc18 in synaptic vesicle fusion. Nat. Rev. Neurosci. 2002, 3, 641–653. [Google Scholar] [CrossRef]
- Südhof, T.C. The molecular machinery of neurotransmitter release (Nobel lecture). Angew. Chem. Int. Ed. Engl. 2014, 53, 12696–12717. [Google Scholar] [CrossRef] [PubMed]
- Whitcup, S.M. The History of Botulinum Toxins in Medicine: A Thousand Year Journey. In Botulinum Toxin Therapy; Handbook of Experimental Pharmacology; Whitcup, S.M., Hallett, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–10. ISBN 978-3-030-66306-3. [Google Scholar]
- Sarzyńska-Długosz, I.; Szczepańska-Szerej, A.; Drużdż, A.; Łukomski, T.; Ochudło, S.; Fabian, A.; Sobolewski, P.; Mariańska, K.; Maciejewska, J.; Mulek, E.; et al. Real-world effectiveness of abobotulinumtoxinA (Dysport®) in adults with upper limb spasticity in routine clinical practice: An observational study. Neurol. Neurochir. Pol. 2020, 54, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R.L.; Balcaitiene, J.; Berard, H.; Maisonobe, P.; Goh, K.J.; Kumthornthip, W.; Mazlan, M.; Latif, L.A.; Delos Santos, M.M.D.; Chotiyarnwong, C.; et al. Early AbobotulinumtoxinA (Dysport®) in Post-Stroke Adult Upper Limb Spasticity: ONTIME Pilot Study. Toxins 2018, 10, 253. [Google Scholar] [CrossRef]
- Simpson, D.M. Treatment of spasticity with botulinum toxin. Muscle Nerve 2000, 23, 447–449. [Google Scholar] [CrossRef]
- O’Brien, C.F. Treatment of spasticity with botulinum toxin. Clin. J. Pain 2002, 18, S182–S190. [Google Scholar] [CrossRef]
- Swartling, C.; Naver, H.; Pihl-Lundin, I.; Hagforsen, E.; Vahlquist, A. Sweat gland morphology and periglandular innervation in essential palmar hyperhidrosis before and after treatment with intradermal botulinum toxin. J. Am. Acad. Dermatol. 2004, 51, 739–745. [Google Scholar] [CrossRef]
- Doft, M.A.; Hardy, K.L.; Ascherman, J.A. Treatment of Hyperhidrosis with Botulinum Toxin. Aesthetic Surg. J. 2012, 32, 238–244. [Google Scholar] [CrossRef]
- Fuster Torres, M.A.; Berini Aytés, L.; Gay Escoda, C. Salivary gland application of botulinum toxin for the treatment of sialorrhea. Med. Oral Patol. Oral Cir. Bucal. 2007, 12, E511–E517. [Google Scholar] [PubMed]
- Pavone, F.; Luvisetto, S. Botulinum neurotoxin for pain management: Insights from animal models. Toxins 2010, 2, 2890–2913. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-M.; Chung, M. Botulinum Toxin for Neuropathic Pain: A Review of the Literature. Toxins 2015, 7, 3127–3154. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.A.W.; Adamson, G.D.; Al-Jefout, M.; Becker, C.M.; D’Hooghe, T.M.; Dunselman, G.A.J.; Fazleabas, A.; Giudice, L.C.; Horne, A.W.; Hull, M.L.; et al. Research Priorities for Endometriosis: Recommendations From a Global Consortium of Investigators in Endometriosis. Reprod. Sci. 2017, 24, 202–226. [Google Scholar] [CrossRef]
- Han, E.; Nguyen, L.; Sirls, L.; Peters, K. Current best practice management of interstitial cystitis/bladder pain syndrome. Ther. Adv. Urol. 2018, 10, 197–211. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.; Popoff, M.R. Engineering Botulinum Neurotoxins for Enhanced Therapeutic Applications and Vaccine Development. Toxins 2021, 13, 1. [Google Scholar] [CrossRef]
- Tracey, I.; Mantyh, P.W. The Cerebral Signature for Pain Perception and Its Modulation. Neuron 2007, 55, 377–391. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Pain, Disability, and Chronic Illness Behavior; Osterweis, M.; Kleinman, A.; Mechanic, D. The Anatomy and Physiology of Pain; National Academies Press (US): Cambridge, MA, USA, 1987. [Google Scholar]
- Melzack, R. From the gate to the neuromatrix. Pain 1999, 82, S121–S126. [Google Scholar] [CrossRef]
- Graham-Engeland, J.E.; Zawadzki, M.J.; Slavish, D.C.; Smyth, J.M. Depressive Symptoms and Momentary Mood Predict Momentary Pain among Rheumatoid Arthritis Patients. Ann. Behav. Med. 2016, 50, 12–23. [Google Scholar] [CrossRef]
- Turner, P.V.; Pang, D.S.; Lofgren, J.L. A Review of Pain Assessment Methods in Laboratory Rodents. Comp. Med. 2019, 69, 451–467. [Google Scholar] [CrossRef]
- Gebhart, G.F.; Bielefeldt, K. Physiology of Visceral Pain. Compr. Physiol. 2016, 6, 1609–1633. [Google Scholar] [CrossRef] [PubMed]
- Sanvictores, T.; Tadi, P. Neuroanatomy, Autonomic Nervous System Visceral Afferent Fibers and Pain. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Zagorodnyuk, V.P.; Gibbins, I.L.; Costa, M.; Brookes, S.J.H.; Gregory, S.J. Properties of the major classes of mechanoreceptors in the guinea pig bladder. J. Physiol. 2007, 585, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Zagorodnyuk, V.P.; Keightley, L.J.; Brookes, S.J.H.; Spencer, N.J.; Costa, M.; Nicholas, S.J. Functional changes in low- and high-threshold afferents in obstruction-induced bladder overactivity. Am. J. Physiol.-Ren. Physiol. 2019, 316, F1103–F1113. [Google Scholar] [CrossRef] [PubMed]
- Collins, V.M.; Daly, D.M.; Liaskos, M.; McKay, N.G.; Sellers, D.; Chapple, C.; Grundy, D. OnabotulinumtoxinA significantly attenuates bladder afferent nerve firing and inhibits ATP release from the urothelium. BJU Int. 2013, 112, 1018–1026. [Google Scholar] [CrossRef]
- Nullens, S.; Deiteren, A.; Jiang, W.; Keating, C.; Ceuleers, H.; Francque, S.; Grundy, D.; De Man, J.G.; De Winter, B.Y. In Vitro Recording of Mesenteric Afferent Nerve Activity in Mouse Jejunal and Colonic Segments. JoVE 2016, 54576. [Google Scholar] [CrossRef]
- Rong, W.; Hillsley, K.; Davis, J.B.; Hicks, G.; Winchester, W.J.; Grundy, D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice: TRPV1 receptor and gut sensitivity. J. Physiol. 2004, 560, 867–881. [Google Scholar] [CrossRef]
- Messeguer, A.; Planells-Cases, R.; Ferrer-Montiel, A. Physiology and pharmacology of the vanilloid receptor. Curr. Neuropharmacol. 2006, 4, 1–15. [Google Scholar] [CrossRef]
- Miranda, A.; Nordstrom, E.; Mannem, A.; Smith, C.; Banerjee, B.; Sengupta, J.N. The Role of TRPV1 in Mechanical and Chemical Visceral Hyperalgesia Following Experimental Colitis. Neuroscience 2007, 148, 1021–1032. [Google Scholar] [CrossRef]
- Jones, R.C.W. The Mechanosensitivity of Mouse Colon Afferent Fibers and Their Sensitization by Inflammatory Mediators Require Transient Receptor Potential Vanilloid 1 and Acid-Sensing Ion Channel 3. J. Neurosci. 2005, 25, 10981–10989. [Google Scholar] [CrossRef]
- Huang, C.-J.; Schild, L.; Moczydlowski, E.G. Use-dependent block of the voltage-gated Na(+) channel by tetrodotoxin and saxitoxin: Effect of pore mutations that change ionic selectivity. J. Gen. Physiol. 2012, 140, 435–454. [Google Scholar] [CrossRef]
- de Lera Ruiz, M.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Peigneur, S.; Tytgat, J. Neurotoxins and Their Binding Areas on Voltage-Gated Sodium Channels. Front. Pharmacol. 2011, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Hockley, J.R.F.; González-Cano, R.; McMurray, S.; Tejada-Giraldez, M.A.; McGuire, C.; Torres, A.; Wilbrey, A.L.; Cibert-Goton, V.; Nieto, F.R.; Pitcher, T.; et al. Visceral and somatic pain modalities reveal Na V 1.7-independent visceral nociceptive pathways: Role of Na V 1.7 in visceral nociception. J. Physiol. 2017, 595, 2661–2679. [Google Scholar] [CrossRef]
- Marcil, J.; Walczak, J.-S.; Guindon, J.; Ngoc, A.H.; Lu, S.; Beaulieu, P. Antinociceptive effects of tetrodotoxin (TTX) in rodents. Br. J. Anaesth 2006, 96, 761–768. [Google Scholar] [CrossRef]
- González-Cano, R.; Tejada, M.Á.; Artacho-Cordón, A.; Nieto, F.R.; Entrena, J.M.; Wood, J.N.; Cendán, C.M. Effects of Tetrodotoxin in Mouse Models of Visceral Pain. Mar. Drugs 2017, 15, 188. [Google Scholar] [CrossRef]
- Hagen, N.A.; Lapointe, B.; Ong–Lam, M.; Dubuc, B.; Walde, D.; Gagnon, B.; Love, R.; Goel, R.; Hawley, P.; Ngoc, A.H.; et al. A multicentre open-label safety and efficacy study of tetrodotoxin for cancer pain. Curr. Oncol. 2011, 18, e109–e116. [Google Scholar] [CrossRef]
- Donald, S.; Elliott, M.; Gray, B.; Hornby, F.; Lewandowska, A.; Marlin, S.; Favre-Guilmard, C.; Périer, C.; Cornet, S.; Kalinichev, M.; et al. A comparison of biological activity of commercially available purified native botulinum neurotoxin serotypes A1 to F1 in vitro, ex vivo, and in vivo. Pharmacol. Res. Perspect. 2018, 6, e00446. [Google Scholar] [CrossRef]
- Ruthel, G.; Burnett, J.C.; Nuss, J.E.; Wanner, L.M.; Tressler, L.E.; Torres-Melendez, E.; Sandwick, S.J.; Retterer, C.J.; Bavari, S. Post-Intoxication Inhibition of Botulinum Neurotoxin Serotype A within Neurons by Small-Molecule, Non-Peptidic Inhibitors. Toxins 2011, 3, 207–217. [Google Scholar] [CrossRef]
- Grando, S.a.; Zachary, C.b. The non-neuronal and nonmuscular effects of botulinum toxin: An opportunity for a deadly molecule to treat disease in the skin and beyond. Br. J. Dermatol. 2018, 178, 1011–1019. [Google Scholar] [CrossRef]
- Lacković, Z. Botulinum Toxin and Pain. Handb. Exp. Pharmacol. 2021, 263, 251–264. [Google Scholar] [CrossRef]
- Marinelli, S.; Vacca, V.; Ricordy, R.; Uggenti, C.; Tata, A.M.; Luvisetto, S.; Pavone, F. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS ONE 2012, 7, e47977. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, J.; Steinhoff, M.; Dolly, J.O. TNFα induces co-trafficking of TRPV1/TRPA1 in VAMP1-containing vesicles to the plasmalemma via Munc18–1/syntaxin1/SNAP-25 mediated fusion. Sci. Rep. 2016, 6, 21226. [Google Scholar] [CrossRef] [PubMed]
- Nugent, M.; Yusef, Y.R.; Meng, J.; Wang, J.; Dolly, J.O. A SNAP-25 cleaving chimera of botulinum neurotoxin /A and /E prevents TNFα−induced elevation of the activities of native TRP channels on early postnatal rat dorsal root ganglion neurons. Neuropharmacology 2018, 138, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Morenilla-Palao, C.; Planells-Cases, R.; García-Sanz, N.; Ferrer-Montiel, A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J. Biol. Chem. 2004, 279, 25665–25672. [Google Scholar] [CrossRef]
- Zhang, X.; Strassman, A.M.; Novack, V.; Brin, M.F.; Burstein, R. Extracranial injections of botulinum neurotoxin type A inhibit intracranial meningeal nociceptors’ responses to stimulation of TRPV1 and TRPA1 channels: Are we getting closer to solving this puzzle? Cephalalgia 2016, 36, 875–886. [Google Scholar] [CrossRef]
- Burstein, R.; Blumenfeld, A.M.; Silberstein, S.D.; Manack Adams, A.; Brin, M.F. Mechanism of Action of OnabotulinumtoxinA in Chronic Migraine: A Narrative Review. Headache 2020, 60, 1259–1272. [Google Scholar] [CrossRef]
- Li, X.; Coffield, J.A. Structural and Functional Interactions between Transient Receptor Potential Vanilloid Subfamily 1 and Botulinum Neurotoxin Serotype A. PLoS ONE 2016, 11, e0143024. [Google Scholar] [CrossRef]
- Vilceanu, D.; Stucky, C.L. TRPA1 Mediates Mechanical Currents in the Plasma Membrane of Mouse Sensory Neurons. PLoS ONE 2010, 5, e12177. [Google Scholar] [CrossRef]
- Rapp, D.E.; Turk, K.W.; Bales, G.T.; Cook, S.P. Botulinum toxin type a inhibits calcitonin gene-related peptide release from isolated rat bladder. J. Urol. 2006, 175, 1138–1142. [Google Scholar] [CrossRef]
- Durham, P.L.; Cady, R.; Cady, R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: Implications for migraine therapy. Headache 2004, 44, 35–42; discussion 42–43. [Google Scholar] [CrossRef]
- Meunier, F.A.; Colasante, C.; Faille, L.; Gastard, M.; Molgó, J. Upregulation of calcitonin gene-related peptide at mouse motor nerve terminals poisoned with botulinum type-A toxin. Pflugers Arch. 1996, 431, R297–R298. [Google Scholar] [CrossRef] [PubMed]
- Daly, D.M.; Park, S.J.; Valinsky, W.C.; Beyak, M.J. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse: Impaired afferent satiety signalling in obesity. J. Physiol. 2011, 589, 2857–2870. [Google Scholar] [CrossRef] [PubMed]
- Stakenborg, N.; Gomez-Pinilla, P.J.; Verlinden, T.J.M.; Wolthuis, A.M.; D’Hoore, A.; Farré, R.; Herijgers, P.; Matteoli, G.; Boeckxstaens, G.E. Comparison between the cervical and abdominal vagus nerves in mice, pigs, and humans. Neurogastroenterol. Motil. 2020, 32, e13889. [Google Scholar] [CrossRef] [PubMed]
- Rostock, C.; Schrenk-Siemens, K.; Pohle, J.; Siemens, J. Human vs. Mouse Nociceptors—Similarities and Differences. Neuroscience 2018, 387, 13–27. [Google Scholar] [CrossRef]
- Greenwood-Van Meerveld, B.; Prusator, D.K.; Johnson, A.C. Animal models of gastrointestinal and liver diseases. Animal models of visceral pain: Pathophysiology, translational relevance, and challenges. Am. J. Physiol. Gastrointest Liver Physiol. 2015, 308, G885–G903. [Google Scholar] [CrossRef]
- Regmi, B.; Shah, M.K. Possible implications of animal models for the assessment of visceral pain. Anim. Model. Exp. Med. 2020, 3, 215–228. [Google Scholar] [CrossRef]
- Peng, L.; Berntsson, R.P.-A.; Tepp, W.H.; Pitkin, R.M.; Johnson, E.A.; Stenmark, P.; Dong, M. Botulinum neurotoxin D-C uses synaptotagmin I and II as receptors, and human synaptotagmin II is not an effective receptor for type B, D-C and G toxins. J. Cell Sci. 2012, 125, 3233–3242. [Google Scholar] [CrossRef]
- Elliott, M.; Favre-Guilmard, C.; Liu, S.M.; Maignel, J.; Masuyer, G.; Beard, M.; Boone, C.; Carré, D.; Kalinichev, M.; Lezmi, S.; et al. Engineered botulinum neurotoxin B with improved binding to human receptors has enhanced efficacy in preclinical models. Sci. Adv. 2019, 5, eaau7196. [Google Scholar] [CrossRef]
- Smith, C.P.; Boone, T.B.; de Groat, W.C.; Chancellor, M.B.; Somogyi, G.T. Effect of stimulation intensity and botulinum toxin isoform on rat bladder strip contractions. Brain Res. Bull. 2003, 61, 165–171. [Google Scholar] [CrossRef]
- van Uhm, J.I.M.; Beckers, G.M.A.; van der Laarse, W.J.; Meuleman, E.J.H.; Geldof, A.A.; Nieuwenhuijzen, J.A. Development of an in vitro model to measure bioactivity of botulinum neurotoxin A in rat bladder muscle strips. BMC Urol. 2014, 14, 37. [Google Scholar] [CrossRef]
- Maignel-Ludop, J.; Huchet, M.; Krupp, J. Botulinum Neurotoxins Serotypes A and B induce paralysis of mouse striated and smooth muscles with different potencies. Pharmacol. Res. Perspect. 2017, 5, e00289. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Zabbarova, I.V.; Birder, L.A.; de Groat, W.C.; McCarthy, C.J.; Hanna-Mitchell, A.T.; Kanai, A.J. Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder. Eur. Urol. 2012, 62, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Périer, C.; Martin, V.; Cornet, S.; Favre-Guilmard, C.; Rocher, M.-N.; Bindler, J.; Wagner, S.; Andriambeloson, E.; Rudkin, B.; Marty, R.; et al. Recombinant botulinum neurotoxin serotype A1 in vivo characterization. Pharmacol. Res. Perspect. 2021, 9, e00857. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Retailleau, K.; Martin, V.; Lezmi, S.; Nicoleau, C.; Maignel, J. The Isolated Mouse Jejunal Afferent Nerve Assay as a Tool to Assess the Effect of Botulinum Neurotoxins in Visceral Nociception. Toxins 2022, 14, 205. https://doi.org/10.3390/toxins14030205
Retailleau K, Martin V, Lezmi S, Nicoleau C, Maignel J. The Isolated Mouse Jejunal Afferent Nerve Assay as a Tool to Assess the Effect of Botulinum Neurotoxins in Visceral Nociception. Toxins. 2022; 14(3):205. https://doi.org/10.3390/toxins14030205
Chicago/Turabian StyleRetailleau, Kevin, Vincent Martin, Stephane Lezmi, Camille Nicoleau, and Jacquie Maignel. 2022. "The Isolated Mouse Jejunal Afferent Nerve Assay as a Tool to Assess the Effect of Botulinum Neurotoxins in Visceral Nociception" Toxins 14, no. 3: 205. https://doi.org/10.3390/toxins14030205
APA StyleRetailleau, K., Martin, V., Lezmi, S., Nicoleau, C., & Maignel, J. (2022). The Isolated Mouse Jejunal Afferent Nerve Assay as a Tool to Assess the Effect of Botulinum Neurotoxins in Visceral Nociception. Toxins, 14(3), 205. https://doi.org/10.3390/toxins14030205




