Synthesis of Rice Husk-Based MCM-41 for Removal of Aflatoxin B1 from Peanut Oil
Abstract
:1. Introduction
2. Results and Discussion
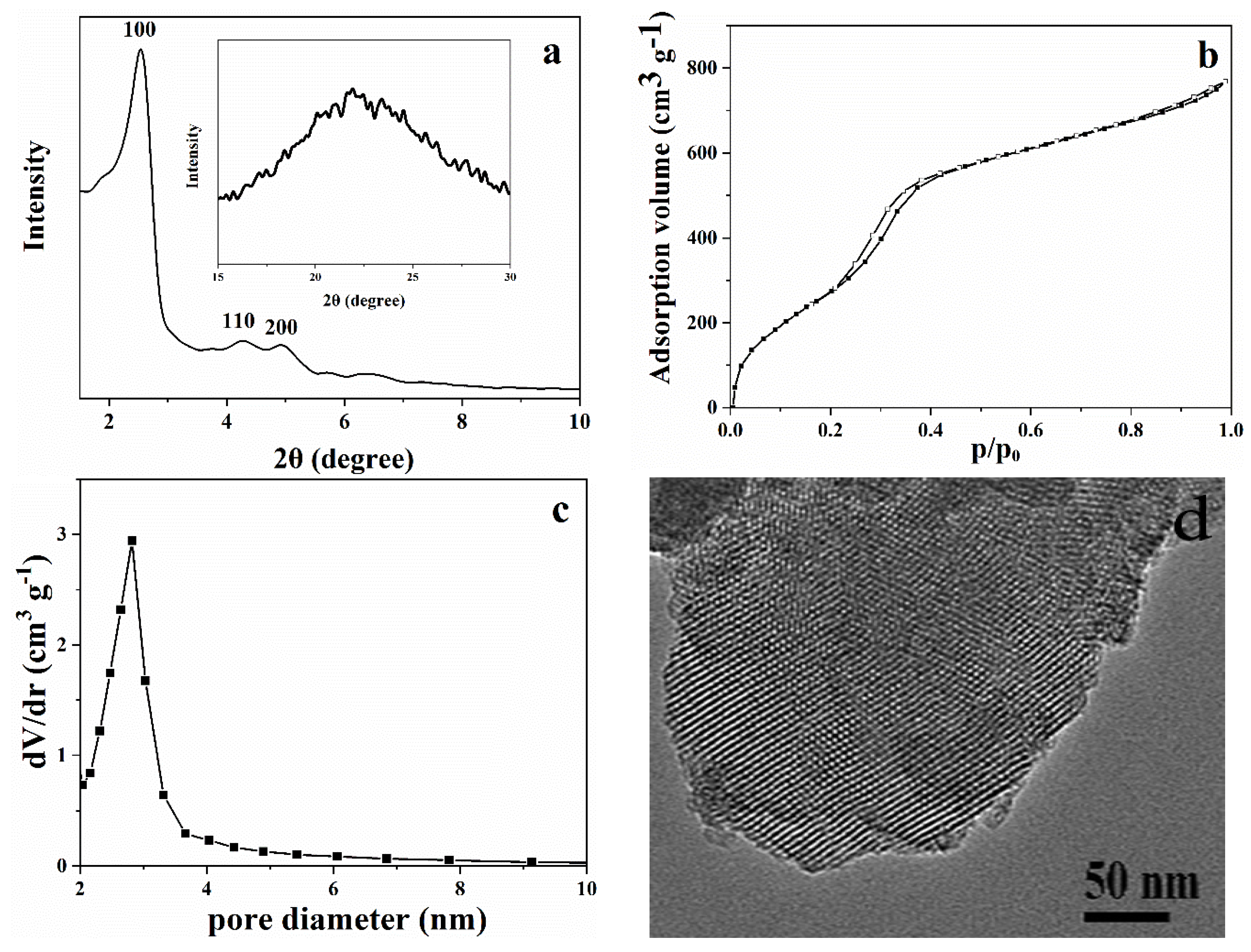
2.1. Characterization of MCM-41
2.2. Effect of Adsorbent Dose
2.3. Adsorption Kinetics
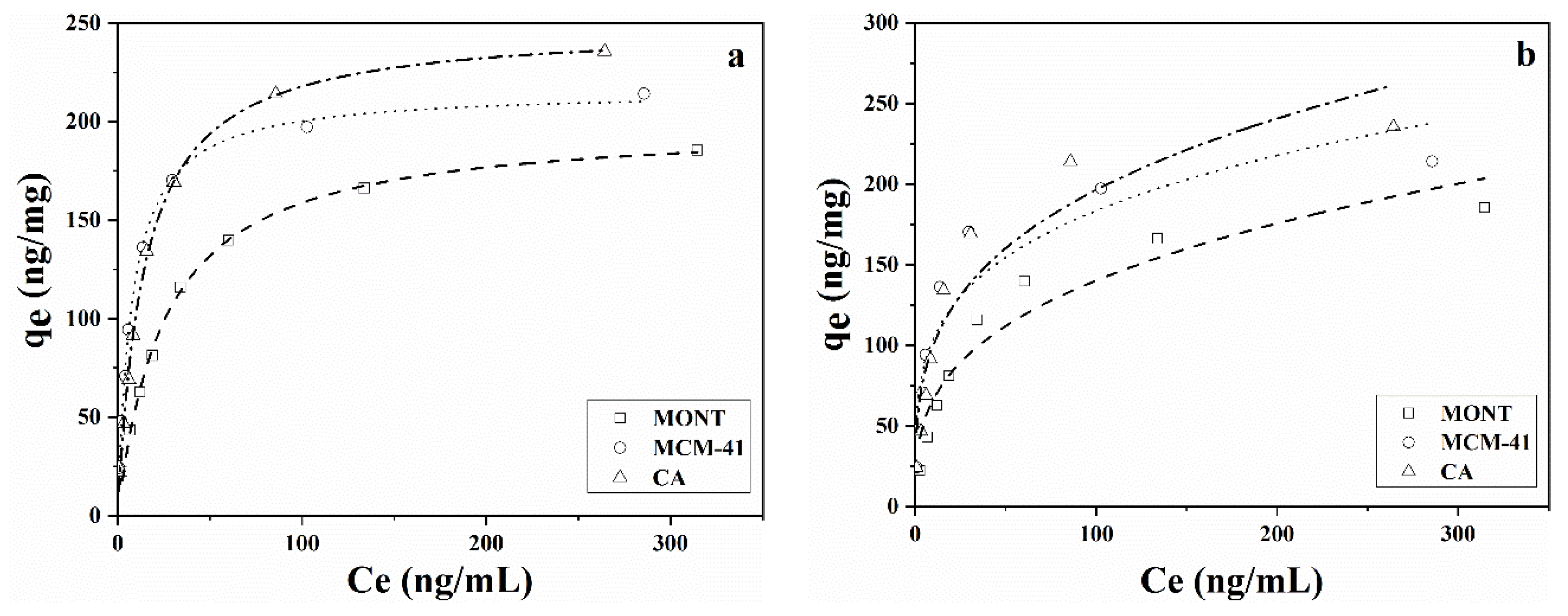
2.4. Adsorption Isotherm
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Synthesis of MCM-41 from Rice Husk
4.3. Characterization of Rice Husk-Based MCM-41
4.4. Adsorption Experiments
4.4.1. Batch Adsorption Experiments
4.4.2. Determination of AFB1 Concentration
4.5. Adsorption Kinetics
4.6. Adsorption Isotherms
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020, 137, 111161. [Google Scholar] [CrossRef]
- Ding, X.; Wu, L.; Li, P.; Zhang, Z.; Zhou, H.; Bai, Y.; Chen, X.; Jiang, J. Risk Assessment on Dietary Exposure to Aflatoxin B1 in Post-Harvest Peanuts in the Yangtze River Ecological Region. Toxins 2015, 7, 4157–4174. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liang, B.; Zhang, Y.; Zhong, X.; Luo, X.; Huang, J.; Wang, Y.; Cheng, W.; Chen, K. Probabilistic risk assessment of dietary exposure to aflatoxin B1 in Guangzhou, China. Sci. Rep. 2020, 10, 7973. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, Z.; Zheng, T.; Song, L.; Chen, N. Removal of aflatoxin in different quality crude peanut oils by alkali refining. China Oils Fats 2019, 44, 82–85. [Google Scholar] [CrossRef]
- Laureth, J.C.U.; Christ, D.; Ganascini, D.; Coelho, S.R.M. Effect of Ozone Application on the Fungal Count and Lipid Quality of Peanut Grains. J. Agric. Sci. 2019, 11, p271. [Google Scholar] [CrossRef]
- Moghaddam, A.F.; Sarlak, Z.; Hosseini, H. Application of Probiotics in Aflatoxins Risk Reduction in Foods: A Review. Int. J. Environ. Sci. Nat. Resour. 2019, 22, 88–96. [Google Scholar] [CrossRef]
- Mao, J.; He, B.; Zhang, L.; Li, P.; Zhang, Q.; Ding, X.; Zhang, W. A Structure Identification and Toxicity Assessment of the Degradation Products of Aflatoxin B1 in Peanut Oil under UV Irradiation. Toxins 2016, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Diao, E.; Shen, X.; Zhang, Z.; Ji, N.; Ma, W.; Dong, H. Safety evaluation of aflatoxin B1in peanut oil after ultraviolet irradiation detoxification in a photodegradation reactor. Int. J. Food Sci. Technol. 2015, 50, 41–47. [Google Scholar] [CrossRef]
- Magzoub, R.; Yassin, A.; Abdel-Rahim, A.; Gubartallah, E.; Miskam, M.; Saad, B.; Sabar, S. Photocatalytic detoxification of aflatoxins in Sudanese peanut oil using immobilized titanium dioxide. Food Control. 2019, 95, 206–214. [Google Scholar] [CrossRef]
- Ji, J.; Xie, W. Removal of aflatoxin B1 from contaminated peanut oils using magnetic attapulgite. Food Chem. 2021, 339, 128072. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, S.; Peng, X.; Geng, J.; Chen, C.; Li, M. Al–Fe PILC preparation, characterization and its potential adsorption capacity for aflatoxin B1. Appl. Clay Sci. 2013, 83-84, 231–237. [Google Scholar] [CrossRef]
- Fowler, J.; Li, W.; Bailey, C. Effects of a Calcium Bentonite Clay in Diets Containing Aflatoxin when Measuring Liver Residues of Aflatoxin B1 in Starter Broiler Chicks. Toxins 2015, 7, 3455–3464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jebali, A.; Ardakani, S.A.Y.; Sedighi, N.; Hekmatimoghaddam, S. Erratum to: Nanocellulose conjugated with retinoic acid: Its capability to adsorb aflatoxin B1. Cellulose 2015, 22, 2139. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Liu, N.; Yang, L.; Wang, J.; Song, S.; Nie, D.; Yang, X.; Hou, J.; Wu, A. Cross-linked chitosan polymers as generic adsorbents for simultaneous adsorption of multiple mycotoxins. Food Control 2015, 57, 362–369. [Google Scholar] [CrossRef]
- Afriyie-Gyawu, E.; Mackie, J.; Dash, B.; Wiles, M.; Taylor, J.; Huebner, H.; Tang, L.; Guan, H.; Wang, J.-S.; Phillips, T. Chronic toxicological evaluation of dietary NovaSil Clay in Sprague-Dawley rats. Food Addit. Contam. 2005, 22, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Tuo, Y.; Cai, M. Immobilization of copper(II) on mesoporous MCM-41: A highly efficient and recyclable catalyst for tandem oxidative annulation of amidines and methylarenes. J. Porous Mater. 2020, 27, 1039–1049. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Yang, X.; Gao, X.; Wang, H.; Chang, Y. A rapid and efficient extraction method based on industrial MCM-41-miniaturized matrix solid-phase dispersion extraction with response surface methodology for simultaneous quantification of six flavonoids in Pollen typhae by ultra-high-performance liquid chromatography. J. Sep. Sci. 2019, 42, 2426–2434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, H.; Chen, Y.; Hao, T.; Li, F.; Yuan, D.; Wang, X.; Zhao, L.; Gao, J. Study on the preparation of amine-modified silicate MCM-41 adsorbent and its H2S removal performance. Prog. React. Kinet. Mech. 2019, 45. [Google Scholar] [CrossRef] [Green Version]
- Bouchikhi, N.; Adjdir, M.; Bendeddouche, K.C.; Bouazza, D.; Mokhtar, A.; Bennabi, F.; Tabti, H.A.; Sehmi, A.; Miloudi, H. Enhancement of adsorption capacity of low cost mesoporous MCM-41 and their antibacterial and antifungal activities. Mater. Res. Express 2020, 6, 1250j7. [Google Scholar] [CrossRef]
- Patel, D.B.; Singh, S.; Bandyopadhyaya, R. Enrichment of benzene from benzene–water mixture by adsorption in silylated mesoporous silica. Microporous Mesoporous Mater. 2011, 137, 49–55. [Google Scholar] [CrossRef]
- Nien, K.C.; Chang, F.T.; Chang, M.B. Adsorption of mesitylene via mesoporous adsorbents. J. Air Waste Manag. Assoc. 2017, 67, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, T.; Sun, D.; Chen, W.; Li, G.; Li, Y. NH2-MCM-41 supported on nitrogen-doped graphene as bifunctional composites for removing phenol compounds: Synergistic effect between catalytic degradation and adsorption. Carbon 2019, 147, 312–322. [Google Scholar] [CrossRef]
- Liu, F.; Yan, X.; Fan, F.; Zhao, C.; Liu, R.; Gao, Y.; Wang, Y. Application of micro–meso hierarchical porous carbon for toluene adsorption treatment. Micro Nano Lett. 2016, 11, 372–377. [Google Scholar] [CrossRef]
- Lin, R.; Liang, Z.; Yang, C.; Shi, W.; Cui, F.; Zhao, Z. Selective and enhanced adsorption of the monosubstituted benzenes on the Fe-modified MCM-41: Contribution of the substituent groups. Chemosphere 2019, 237, 124546. [Google Scholar] [CrossRef] [PubMed]
- Anbia, M.; Lashgari, M. Synthesis of amino-modified ordered mesoporous silica as a new nano sorbent for the removal of chlorophenols from aqueous media. Chem. Eng. J. 2009, 150, 555–560. [Google Scholar] [CrossRef]
- Jia, W.; Li, Q.; Zhang, L.; Hou, L.; Liu, T.; Bhavana, G.; Yang, J. Highly efficient photocatalytic reduction of CO2 on amine-functionalized Ti-MCM-41 zeolite. J. Nanoparticle Res. 2020, 22, 288. [Google Scholar] [CrossRef]
- Idris, S.A.; Davidson, C.M.; McManamon, C.; Morris, M.A.; Anderson, P.; Gibson, L.T. Large pore diameter MCM-41 and its application for lead removal from aqueous media. J. Hazard. Mater. 2011, 185, 898–904. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, W.; Chen, B.; Zhao, Y.; Liu, D.; Sun, Y.; Gong, B. Removal of tetracycline from aqueous solution by MCM-41-zeolite A loaded nano zero valent iron: Synthesis, characteristic, adsorption performance and mechanism. J. Hazard. Mater. 2017, 339, 22–32. [Google Scholar] [CrossRef]
- Zhai, Y.; Pan, L.; Luo, X.; Zhang, Y.; Wang, R.; Chen, Z. Effect of electron beam irradiation on storage, moisture and eating properties of high-moisture rice during storage. J. Cereal Sci. 2022, 103, 103407. [Google Scholar] [CrossRef]
- Chen, J.-P.; Song, G.; Liu, Z.; Kong, Q.-Q.; Zhang, S.-C.; Chen, C.-M. Preparation of SiC whiskers using graphene and rice husk ash and its photocatalytic property. J. Alloys Compd. 2020, 833, 155072. [Google Scholar] [CrossRef]
- Javaheri, F.; Kheshti, Z.; Ghasemi, S.; Altaee, A. Enhancement of Cd2+ removal from aqueous solution by multifunctional mesoporous silica: Equilibrium isotherms and kinetics study. Sep. Purif. Technol. 2019, 224, 199–208. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Chen, S.-S.; Nguyen, N.C.; Nguyen, H.T.; Tsai, H.H.; Chang, C.T. Adsorption of Methyl Blue on Mesoporous Materials Using Rice Husk Ash as Silica Source. J. Nanosci. Nanotechnol. 2016, 16, 4108–4114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wu, D.; Chu, H.; Wang, C.; Wei, Y. Magnetic mesoporous nanoparticles modified with poly(ionic liquids) with multi-functional groups for enrichment and determination of pyrethroid residues in apples. J. Sep. Sci. 2019, 42, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jia, L.; Cheng, J.; Sun, Z. Magnetic ordered mesoporous carbon materials for adsorption of minocycline from aqueous solution: Preparation, characterization and adsorption mechanism. J. Hazard. Mater. 2019, 362, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, R.; Chen, Z.; Zhao, X.; Luo, X.; Wang, L.; Li, Y.; Teng, F. Preparation of magnetic mesoporous silica from rice husk for aflatoxin B1 removal: Optimum process and adsorption mechanism. PLoS ONE 2020, 15, e0238837. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.; Liu, X.; Wang, Y.; Guo, Y.; Guo, Y.; Lu, G. Facile synthesis of ordered magnetic mesoporous γ-Fe2O3/SiO2 nanocomposites with diverse mesostructures. J. Colloid Interface Sci. 2008, 326, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M. Textural zeolites and ordered mesoporous materials by physical adsorption. In Introduction to Zeolite Science and Practice, 3rd ed.; Cejka, J., van Bekkum, H., Corma, A., Schüth, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 168, pp. 495–523. ISBN 978-0-444-53063-9. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press Inc.: New York, NY, USA, 1982; pp. 163–169. ISBN 7-5025-0380-3. [Google Scholar]
- Thommes, M.; Köhn, R.; Fröba, M. Sorption and pore condensation behavior of pure fluids in mesoporous MCM-48 silica, MCM-41 silica, SBA-15 silica and controlled-pore glass at temperatures above and below the bulk triple point. Appl. Surf. Sci. 2002, 196, 239–249. [Google Scholar] [CrossRef]
- Alkafajy, A.M.; Albayati, T.M. High performance of magnetic mesoporous modification for loading and release of meloxicam in drug delivery implementation. Mater. Today Commun. 2020, 23, 100890. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z. Advanced drug delivery systems: Nanotechnology of health design—A review. J. Saudi Chem. Soc. 2014, 18, 85–99. [Google Scholar] [CrossRef]
- Abbas, S.H.; Adam, F.; Muniandy, L. Green synthesis of MCM-41 from rice husk and its functionalization with nickel(II) salen complex for the rapid catalytic oxidation of benzyl alcohol. Microporous Mesoporous Mater. 2020, 305, 110192. [Google Scholar] [CrossRef]
- Artkla, S.; Kim, W.; Choi, W.; Wittayakun, J. Highly enhanced photocatalytic degradation of tetramethylammonium on the hybrid catalyst of titania and MCM-41 obtained from rice husk silica. Appl. Catal. B Environ. 2009, 91, 157–164. [Google Scholar] [CrossRef]
- Phillips, T.D.; Sarr, A.B.; Grant, P.G. Selective chemisorption and detoxification of aflatoxins by phyllosilicate clay. Nat. Toxins 1995, 3, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Molu, Z.B.; Yurdakoç, K. Preparation and characterization of aluminum pillared K10 and KSF for adsorption of trimethoprim. Microporous Mesoporous Mater. 2010, 127, 50–60. [Google Scholar] [CrossRef]
- Usgodaarachchi, L.; Thambiliyagodage, C.; Wijesekera, R.; Bakker, M.G. Synthesis of mesoporous silica nanoparticles derived from rice husk and surface-controlled amine functionalization for efficient adsorption of methylene blue from aqueous solution. Curr. Res. Green Sustain. Chem. 2021, 4, 100116. [Google Scholar] [CrossRef]
- Samuel, M.S.; Kirankumar, V.; Selvarajan, E. Fabrication of a metal-organic framework composite for removal of Aflatoxin B1 from water. J. Environ. Chem. Eng. 2020, 9, 104966. [Google Scholar] [CrossRef]
- Trouvé, A.; Batonneau-Gener, I.; Valange, S.; Bonne, M.; Mignard, S. Tuning the hydrophobicity of mesoporous silica materials for the adsorption of organic pollutant in aqueous solution. J. Hazard. Mater. 2012, 201–202, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Seliem, M.K.; Komarneni, S.; Abu Khadra, M.R. Phosphate removal from solution by composite of MCM-41 silica with rice husk: Kinetic and equilibrium studies. Microporous Mesoporous Mater. 2016, 224, 51–57. [Google Scholar] [CrossRef]


| Adsorbents | Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| MONT | 235 | 0.34 | 1.15 |
| MCM-41 | 1246 | 1.75 | 3.11 |
| CA | 453 | 0.52 | 1.87 |
| Adsorbents | qe (exp) (ng/mg) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| R2 | qe (cal) (ng/mg) | k1 (min−1) | R2 | qe (cal) (ng/mg) | k2 (mg/(ng·min)) | ||
| MONT | 192.42 | 0.9940 | 166.00 | 0.00484 | 0.9960 | 197.58 | 2.596 × 10−5 |
| MCM-41 | 217.22 | 0.9918 | 188.93 | 0.00487 | 0.9981 | 224.02 | 2.335 × 10−5 |
| CA | 229.96 | 0.9898 | 199.67 | 0.00524 | 0.9974 | 234.95 | 2.441 × 10−5 |
| Adsorbents | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| R2 | qmax (ng/mg) | KL | R2 | KF | 1/n | |
| MONT | 0.9978 | 199.41 | 0.0391 | 0.9106 | 31.475 | 0.324 |
| MCM-41 | 0.9965 | 215.93 | 0.128 | 0.8638 | 58.807 | 0.247 |
| CA | 0.9949 | 248.93 | 0.0701 | 0.8876 | 50.889 | 0.293 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, R.; Luo, X.; Chen, Z.; Wang, L.; Zhou, Y.; Liu, W.; Cheng, M.; Zhang, C. Synthesis of Rice Husk-Based MCM-41 for Removal of Aflatoxin B1 from Peanut Oil. Toxins 2022, 14, 87. https://doi.org/10.3390/toxins14020087
Li Y, Wang R, Luo X, Chen Z, Wang L, Zhou Y, Liu W, Cheng M, Zhang C. Synthesis of Rice Husk-Based MCM-41 for Removal of Aflatoxin B1 from Peanut Oil. Toxins. 2022; 14(2):87. https://doi.org/10.3390/toxins14020087
Chicago/Turabian StyleLi, Ya’nan, Ren Wang, Xiaohu Luo, Zhengxing Chen, Li Wang, Yunyu Zhou, Weizhi Liu, Miaomiao Cheng, and Chen Zhang. 2022. "Synthesis of Rice Husk-Based MCM-41 for Removal of Aflatoxin B1 from Peanut Oil" Toxins 14, no. 2: 87. https://doi.org/10.3390/toxins14020087
APA StyleLi, Y., Wang, R., Luo, X., Chen, Z., Wang, L., Zhou, Y., Liu, W., Cheng, M., & Zhang, C. (2022). Synthesis of Rice Husk-Based MCM-41 for Removal of Aflatoxin B1 from Peanut Oil. Toxins, 14(2), 87. https://doi.org/10.3390/toxins14020087





