BoNT/A1 Secondary Failure for the Treatment of Neurogenic Detrusor Overactivity: An Ex Vivo Functional Study
Abstract
:1. Introduction
2. Results
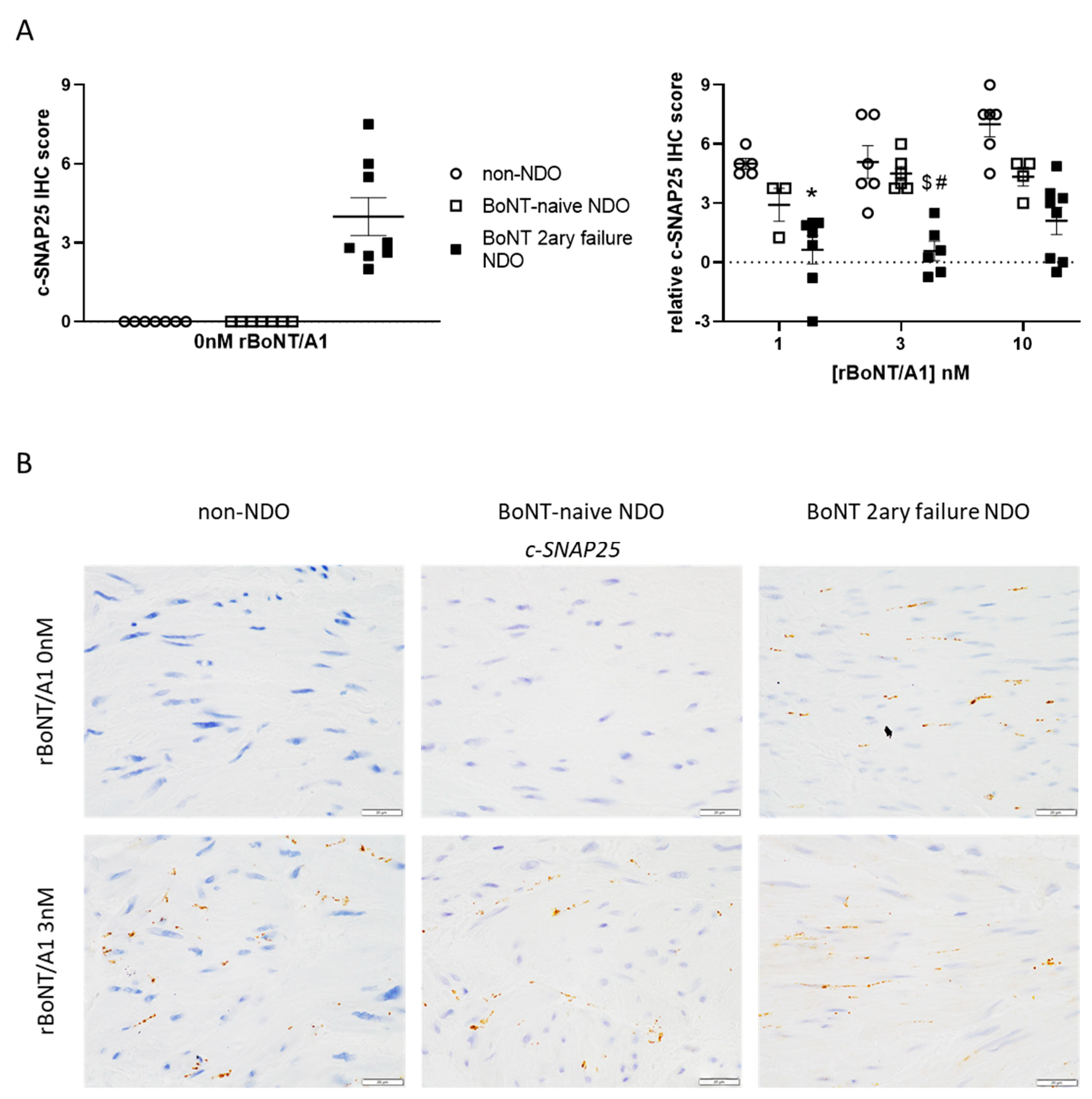
2.1. Immunohistochemistry
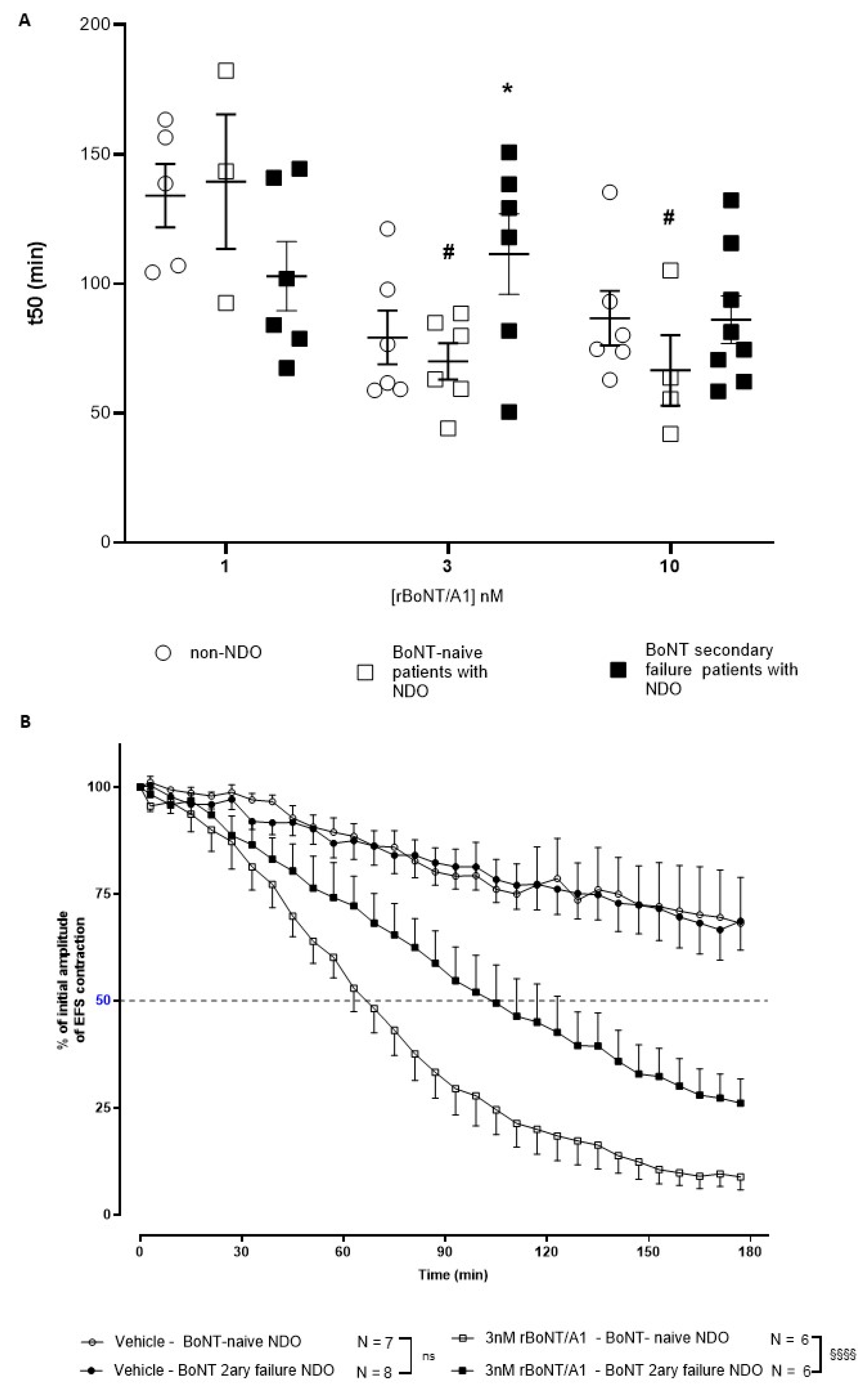
2.2. Ex Vivo Detrusor Strip Assay
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Patients Details and Medical History
5.2. Histopathology and Immunohistochemistry
5.3. Ex Vivo Detrusor Strip Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, Y.; Ding, H.; Zhou, H.; Wei, Z.; Liu, L.; Pan, D.; Feng, S. Epidemiology of worldwide spinal cord injury: A literature review. J. Neurorestoratol. 2018, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar]
- Albert, T.; Ravaud, J.-F.; Tetrafigap Group. Rehabilitation of spinal cord injury in France: A nationwide multicentre study of incidence and regional disparities. Spinal Cord. 2005, 43, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nambiar, A.; Lucas, M. Chapter 4: Guidelines for the diagnosis and treatment of overactive bladder (OAB) and neurogenic detrusor overactivity (NDO). Neurourol. Urodyn. 2014, 33, S21–S25. [Google Scholar] [CrossRef]
- Schurch, B.; Stohrer, M.; Kramer, G.; Schmid, D.M.; Gaul, G.; Hauri, D. Botulinum—A toxin for treating detrusor hyper-reflexia in spinal cord injured patients: A new alternative to anticholinergic drugs? Preliminary results. J. Urol. 2000, 164, 692–697. [Google Scholar] [CrossRef]
- Cruz, F.; Herschorn, S.; Aliotta, P.; Brin, M.; Thompson, C.; Lam, W.; Daniell, G.; Heesakkers, J.; Haag-Molkenteller, C. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: A randomized, double-blind, placebo-controlled trial. Eur. Urol. 2011, 60, 742–750. [Google Scholar] [CrossRef]
- Ginsberg, D.; Gousse, A.; Keppenne, V.; Sievert, K.D.; Thompson, C.; Lam, W.; Brin, M.F.; Jenkins, B.; Haag-Molkenteller, C. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J. Urol. 2012, 187, 2131–2139. [Google Scholar] [CrossRef]
- Kennelly, M.; Dmochowski, R.; Schulte-Baukloh, H.; Ethans, K.; Del Popolo, G.; Moore, C.; Jenkins, B.; Guard, S.; Zheng, Y.; Karsenty, G. Efficacy and safety of onabotulinumtoxinA therapy are sustained over 4 years of treatment in patients with neurogenic detrusor overactivity: Final results of a long-term extension study. Neurourol. Urodyn. 2015, 36, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Humeau, Y.; Doussau, F.; Grant, N.J.; Poulain, B. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie 2000, 82, 427–446. [Google Scholar] [CrossRef]
- Purkiss, J.; Welch, M.; Doward, S.; Foster, K. Capsaicin-stimulated release of substance P from cultured dorsal root ganglion neurons: Involvement of two distinct mechanisms. Biochem. Pharmacol. 2000, 59, 1403–1406. [Google Scholar] [CrossRef]
- Rapp, D.E.; Turk, K.W.; Bales, G.T.; Cook, S.P. Botulinum toxin type a inhibits calcitonin gene-related peptide release from isolated rat bladder. J. Urol. 2006, 175, 1138–1142. [Google Scholar] [CrossRef]
- Apostolidis, A.; Popat, R.; Yiangou, Y.; Cockayne, D.; Ford, A.; Davis, J.; Dasgupta, P.; Fowler, C.; Anand, P. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J. Urol. 2005, 174, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Khera, M.; Somogyi, G.T.; Kiss, S.; Boone, T.B.; Smith, C.P. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem. Int. 2004, 45, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Chancellor, M.B.; Kuo, H.C. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur. Urol. 2009, 56, 700–706. [Google Scholar] [CrossRef]
- Baron, M.; Peyronnet, B.; Aublé, A.; Hascoet, J.; Castel-Lacanal, E.; Miget, G.; Le Doze, S.; Prudhomme, T.; Manunta, A.; Cornu, J.-N.; et al. Long-Term Discontinuation of Botulinum Toxin A Intradetrusor Injections for Neurogenic Detrusor Overactivity: A Multicenter Study. J. Urol. 2018, 201, 769–776. [Google Scholar] [CrossRef]
- Joussain, C.; Popoff, M.; Phé, V.; Even, A.; Bosset, P.O.; Pottier, S.; Falcou, L.; Levy, J.; Vaugier, I.; Chartier Kastler, E.; et al. Long-term outcomes and risks factors for failure of intradetrusor onabotulinumtoxin A injections for the treatment of refractory neurogenic detrusor overactivity. Neurourol. Urodyn. 2018, 37, 799–806. [Google Scholar] [CrossRef]
- Dominique, I.; Tremblais, B.; Charvier, K.; Nogueira, M.C.S.; Paparel, P.; Journel, N.M.; Ruffion, A. How long does the effect of botulinum toxin in neurogenic patients last? An analysis of the subset of “good responders”. Low. Urin. Tract Symptoms 2019, 12, 155–161. [Google Scholar] [CrossRef]
- Traini, C.; Del Popolo, G.; Faussone-Pellegrini, M.S.; Guasti, D.; Catarinicchia, S.; Vannucchi, M.G. Nerve sprouting and neurogenic inflammation characterize the neurogenic detrusor overactive bladder of patients no longer responsive to drug therapies. J. Cell. Mol. Med. 2019, 23, 4076–4087. [Google Scholar] [CrossRef]
- Traini, C.; Vannucchi, M.G. The Botulinum Treatment of Neurogenic Detrusor Overactivity: The Double-Face of the Neurotoxin. Toxins 2019, 11, 614. [Google Scholar] [CrossRef] [Green Version]
- Peyronnet, B.; Sanson, S.; Amarenco, G.; Castel-Lacanal, E.; Chartier-Kastler, E.; Charvier, K.; Damphousse, M.; Denys, P.; de Seze, M.; Egon, G.; et al. Definition of botulinum toxin failure in neurogenic detrusor overactivity: Preliminary results of the DETOX survey. Prog. Urol. 2015, 25, 1219–1224. (In French) [Google Scholar] [CrossRef]
- Poulain, B.; Baux, G.; Tauc, L. Presynaptic transmitter content controls the number of quanta released at a neuro-neuronal cholinergic synapse. Proc. Natl. Acad. Sci. USA 1986, 83, 170–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haferkamp, A.; Schurch, B.; Reitz, A.; Krengel, U.; Grosse, J.; Kramer, G.; Schumacher, S.; Bastian, P.J.; Büttner, R.; Müller, S.C.; et al. Lack of ultrastructural detrusor changes following endoscopic injection of botulinum toxin type a in overactive neurogenic bladder. Eur. Urol. 2004, 46, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Baukloh, H.; Zurawski, T.H.; Knispel, H.H.; Miller, K.; Haferkamp, A.; Dolly, J.O. Persistence of the synaptosomal-associated protein-25 cleavage product after intradetrusor botulinum toxin A injections in patients with myelomeningocele showing an inadequate response to treatment. BJU Int. 2007, 100, 1075–1080. [Google Scholar] [CrossRef]
- Stevens, L.A.; Chapple, C.R.; Chess-Williams, R. Human idiopathic and neurogenic overactive bladders and the role of M2 muscarinic receptors in contraction. Eur Urol. 2007, 52, 531–538. [Google Scholar] [CrossRef]
- German, K.; Bedwani, J.; Davies, J.; Brading, A.F.; Stephenson, T.P. Physiological and morphometric studies into the pathophysiology of detrusor hyperreflexia in neuropathic patients. J. Urol. 1995, 153, 1678–1683. [Google Scholar] [CrossRef]
- Andersson, K.E.; Campeau, L.; Olshansky, B. Cardiac effects of muscarinic receptor antagonists used for voiding dysfunction. Br. J. Clin. Pharmacol. 2011, 72, 186–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottet, F.; Peyronnet, B.; Boissier, R.; Reiss, B.; Previnaire, J.G.; Manunta, A.; Kerdraon, J.; Ruffion, A.; Lenormand, L.; Verbe, B.P.; et al. Switch to Abobotulinum toxin A may be useful in the treatment of neurogenic detrusor overactivity when intradetrusor injections of Onabotulinum toxin A failed. Neurourol. Urodyn. 2017, 37, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Field, M.; Splevins, A.; Picaut, P.; van der Schans, M.; Langenberg, J.; Noort, D.; Snyder, D.; Foster, K. AbobotulinumtoxinA (Dysport®), OnabotulinumtoxinA (Botox®), and IncobotulinumtoxinA (Xeomin®) Neurotoxin Content and Potential Implications for Duration of Response in Patients. Toxins 2018, 10, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquenazi, A.; Stoquart, G.; Hedera, P.; Jacinto, L.J.; Dimanico, U.; Constant-Boyer, F.; Brashear, A.; Grandoulier, A.S.; Vilain, C.; Picaut, P.; et al. Efficacy and Safety of AbobotulinumtoxinA for the Treatment of Hemiparesis in Adults with Lower Limb Spasticity Previously Treated with Other Botulinum Toxins: A Secondary Analysis of a Randomized Controlled Trial. PM&R 2020, 12, 853–860. [Google Scholar] [CrossRef]
- Collins, V.M.; Daly, D.M.; Liaskos, M.; McKay, N.G.; Sellers, D.; Chapple, C.; Grundy, D. OnabotulinumtoxinA significantly attenuates bladder afferent nerve firing and inhibits ATP release from the urothelium. BJU Int. 2013, 112, 1018–1026. [Google Scholar] [CrossRef] [Green Version]
- Papagiannopoulou, D.; Vardouli, L.; Dimitriadis, F.; Apostolidis, A. Retrograde transport of radiolabelled botulinum neurotoxin type A to the CNS after intradetrusor injection in rats. BJU Int. 2016, 117, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Périer, C.; Martin, V.; Cornet, S.; Favre-Guilmard, C.; Rocher, M.; Bindler, J.; Wagner, S.; Andriambeloson, E.; Rudkin, B.B.; Marty, R.; et al. Recombinant botulinum neurotoxin serotype A1 in vivo characterization. Pharmacol. Res. Perspect. 2021, 9, e00857. [Google Scholar] [CrossRef] [PubMed]

| Groups | Gender | Age (Year) | Low Bladder Compliance * | Neurologic Disease | Seniority of the Neurologic Disease (year) | Antimuscarinic (yes = 1; no = 0) | Number of BoNT/A Injection before Surgery | Last Intradetrusorial Injection of BoNT/A (Days) |
|---|---|---|---|---|---|---|---|---|
| BoNT/A-naïve patients | F | 55 | 0 | MS | 21 | 0 | NA | NA |
| M | 33 | 0 | SCI | 12 | 0 | NA | NA | |
| F | 67 | 0 | SCI | 4 | 0 | NA | NA | |
| F | 71 | 0 | SCI | 45 | 1 | NA | NA | |
| F | 52 | 1 (10) | SCI | 38 | 0 | NA | NA | |
| M | 50 | 0 | SCI | 24 | 0 | NA | NA | |
| M | 64 | 0 | SCI | 33 | 0 | NA | NA | |
| BoNT/A- secondary failure patients | F | 18 | 0 | SCI | 10 | 1 | 6 | 141 |
| M | 32 | 0 | SCI | 10 | 1 | 5 | 79 | |
| M | 27 | 0 | SCI | 10 | 1 | 16 | 147 | |
| F | 28 | 1 (13) | SB | 28 | 1 | 5 | 122 | |
| M | 31 | 0 | SCI | 8 | 1 | 6 | 37 | |
| F | 60 | Na | EM | 14 | 1 | 14 | 256 | |
| M | 21 | Na | SCI | 8 | 1 | 5 | 412 | |
| M | 37 | 1 (10) | SCI | 5 | 1 | 4 | 163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maignel, J.; Martin, V.; Assaly, R.; Vogt, M.L.; Retailleau, K.; Hornby, F.; Laugerotte, A.; Lezmi, S.; Denys, P.; Krupp, J.; et al. BoNT/A1 Secondary Failure for the Treatment of Neurogenic Detrusor Overactivity: An Ex Vivo Functional Study. Toxins 2022, 14, 77. https://doi.org/10.3390/toxins14020077
Maignel J, Martin V, Assaly R, Vogt ML, Retailleau K, Hornby F, Laugerotte A, Lezmi S, Denys P, Krupp J, et al. BoNT/A1 Secondary Failure for the Treatment of Neurogenic Detrusor Overactivity: An Ex Vivo Functional Study. Toxins. 2022; 14(2):77. https://doi.org/10.3390/toxins14020077
Chicago/Turabian StyleMaignel, Jacquie, Vincent Martin, Rana Assaly, Mathieu L. Vogt, Kevin Retailleau, Fraser Hornby, Alexandra Laugerotte, Stéphane Lezmi, Pierre Denys, Johannes Krupp, and et al. 2022. "BoNT/A1 Secondary Failure for the Treatment of Neurogenic Detrusor Overactivity: An Ex Vivo Functional Study" Toxins 14, no. 2: 77. https://doi.org/10.3390/toxins14020077
APA StyleMaignel, J., Martin, V., Assaly, R., Vogt, M. L., Retailleau, K., Hornby, F., Laugerotte, A., Lezmi, S., Denys, P., Krupp, J., & Joussain, C. (2022). BoNT/A1 Secondary Failure for the Treatment of Neurogenic Detrusor Overactivity: An Ex Vivo Functional Study. Toxins, 14(2), 77. https://doi.org/10.3390/toxins14020077





