An Agro-Climatic Approach to Developing a National Prevention Tool for Deoxynivalenol in French Maize-Growing Areas
Abstract
1. Introduction
2. Results
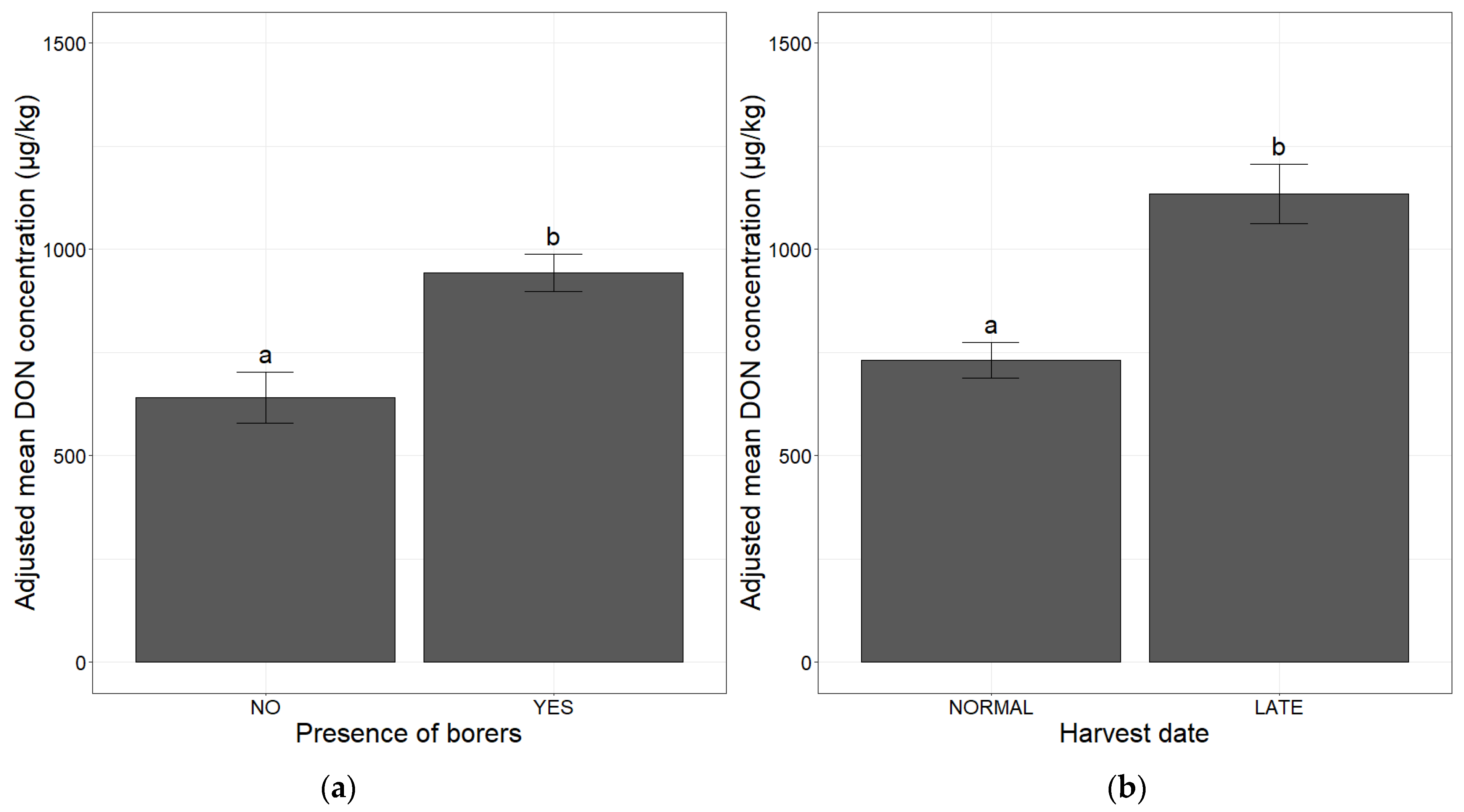
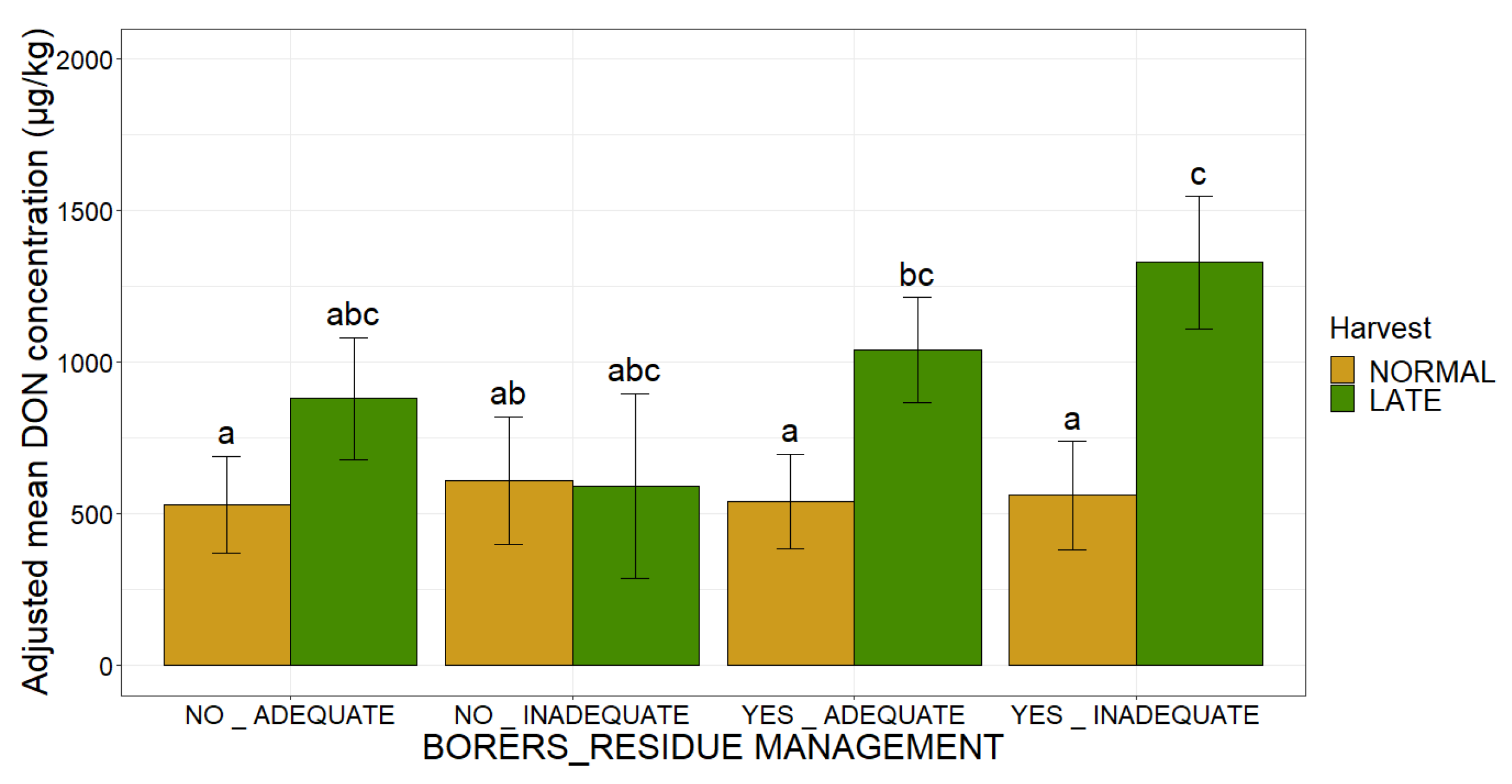
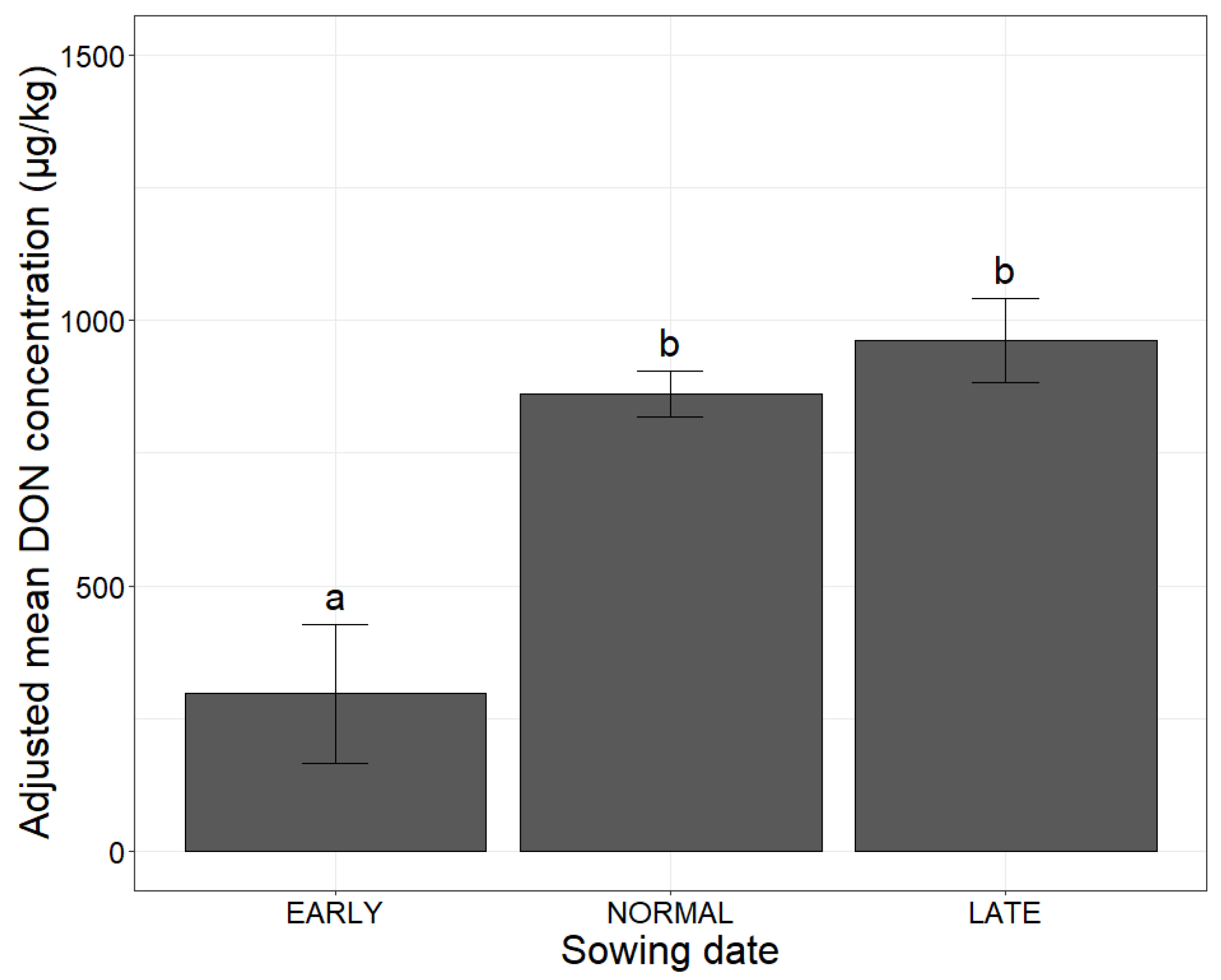
2.1. Association of Agronomic Factors Influencing DON Concentrations
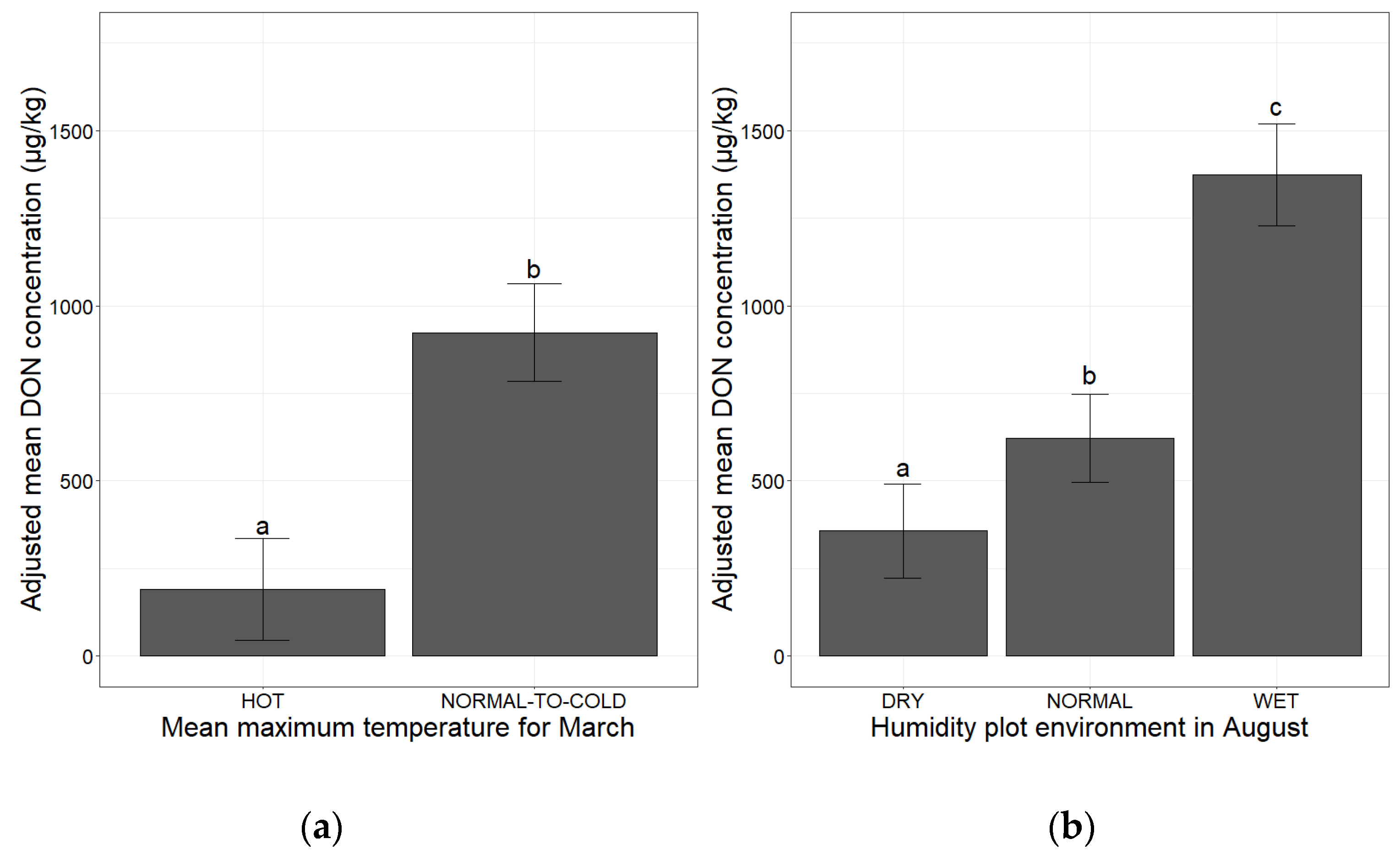
2.2. Association of Climatic Factors at Sensitive Periods Influencing DON Concentrations
2.2.1. Temperature and Humidity during the Pre-Sowing and Post-Flowering Periods
2.2.2. Characterization of a Hot End to the Maize Development Cycle
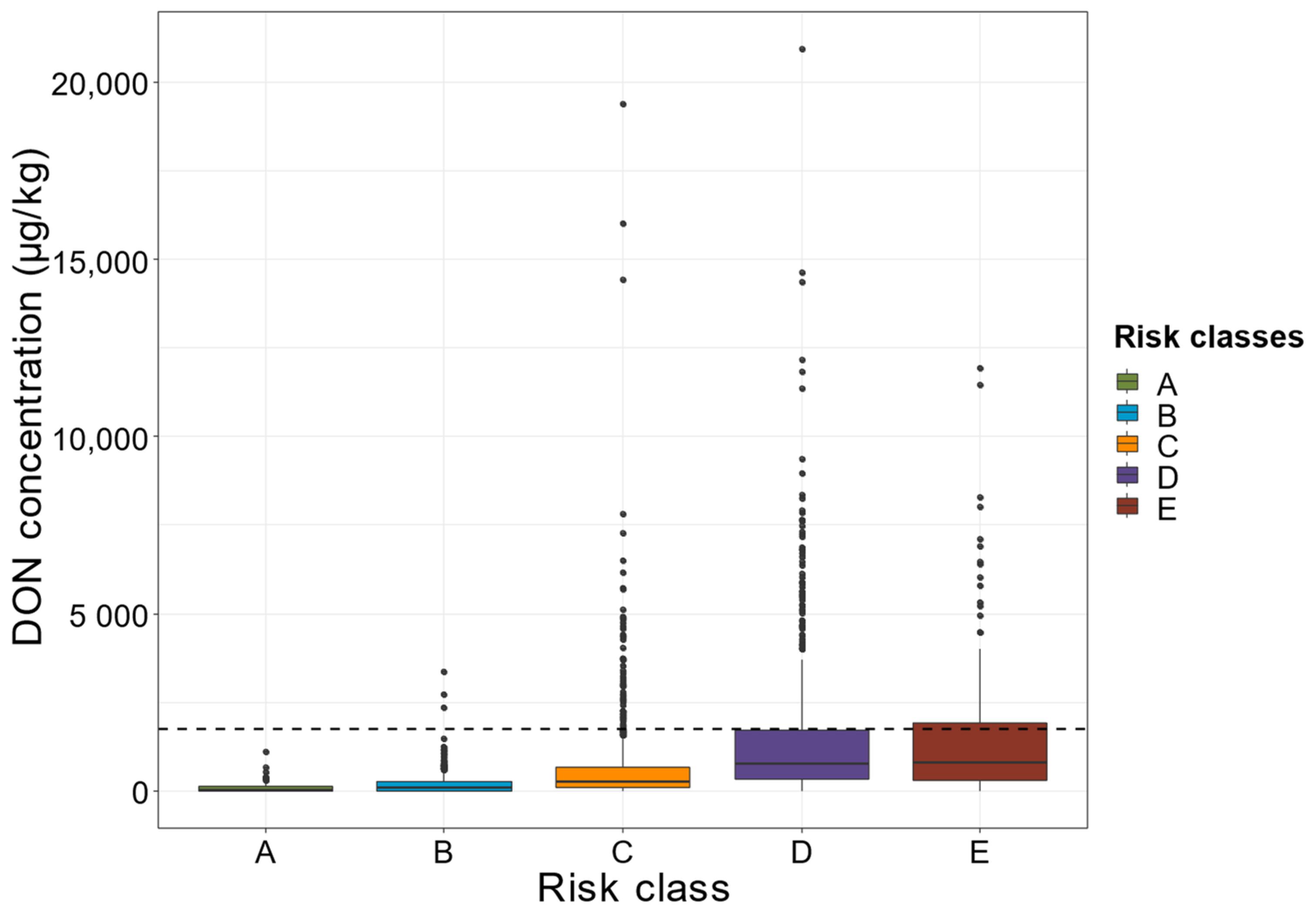
2.3. Combinations of Categories for the Agronomic and Climatic Factors Can Create Definitions Determining Whether the EU Regulatory Limits for DON in Maize Are Respected
3. Discussion
4. Conclusions
5. Materials and Methods
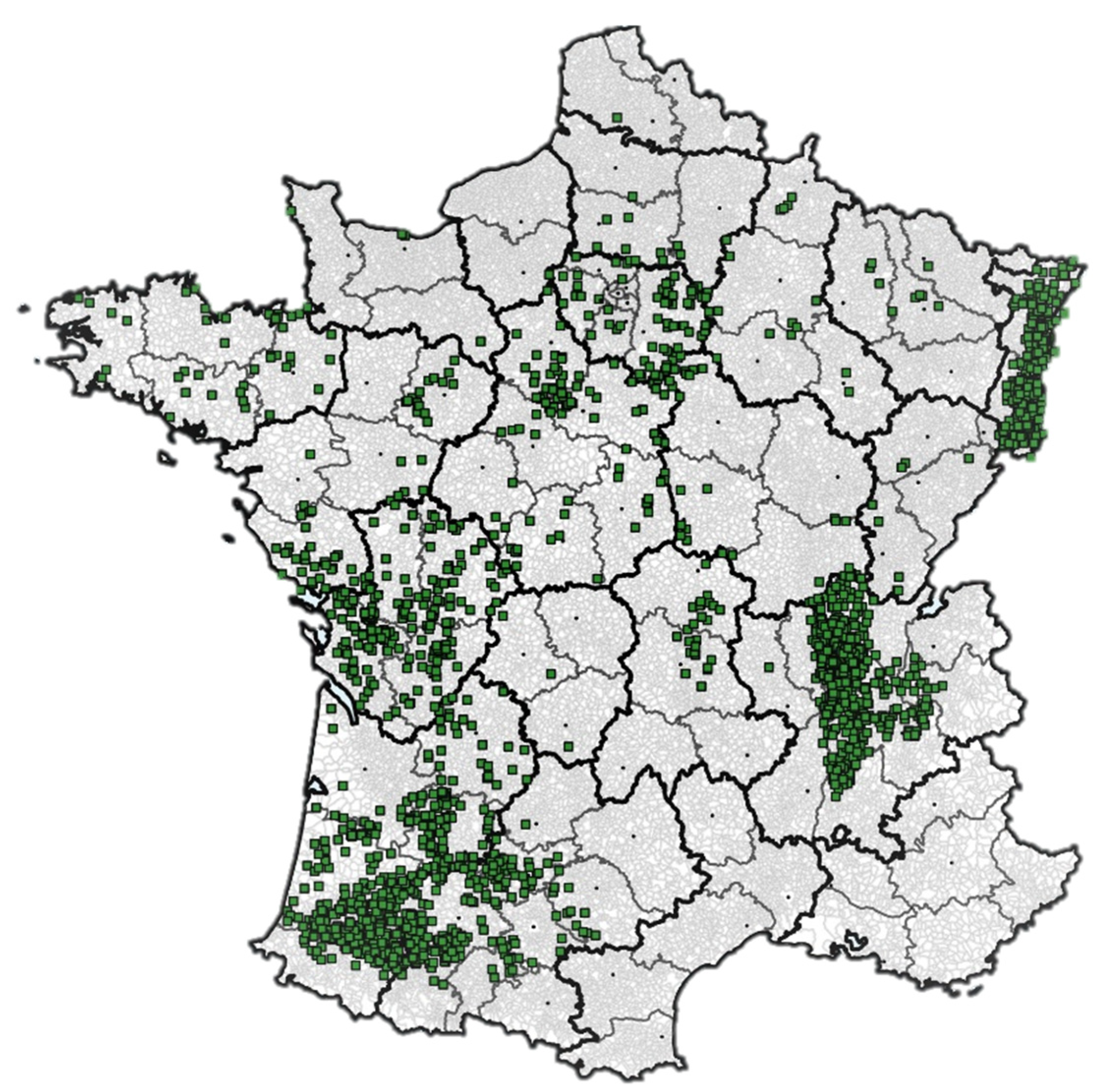
5.1. Multiyear Field Surveys at the French National Scale
5.2. Sample Collection
5.3. Sample Preparation for Analysis
5.4. Deoxynivalenol Quantification
5.5. Agronomic Factors
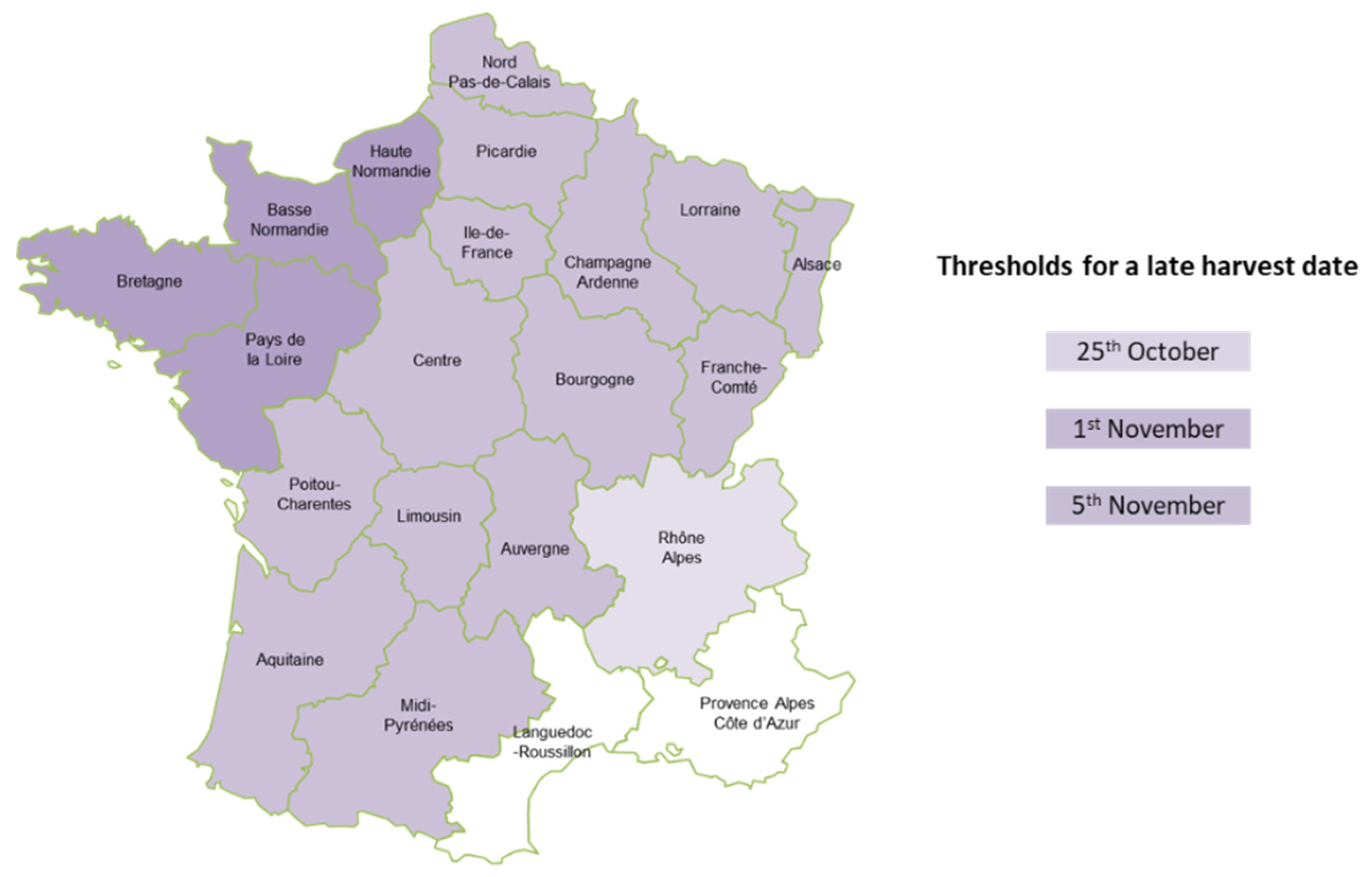
5.6. Climatic Factors
5.6.1. Quantitative Climatic Parameters
5.6.2. Quantitative Climatic Parameters into Single Qualitative Variables
5.7. Statistical Analyses
5.7.1. Selection of Climatic and Agronomic Risk Factors
5.7.2. Conversion of Climatic Quantitative Factors into Categorical Variables
5.7.3. Univariate and Multivariate Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- La Filière Maïs, Maïserie et Maïs Doux en Chiffres. Available online: https://www.passioncereales.fr/dossier-thematique/la-fili%C3%A8re-mais-maiserie-et-mais-doux-en-chiffres (accessed on 24 November 2021).
- Logrieco, A.; Bottalico, A.; Mulé, G.; Moretti, A.; Perrone, G. Epidemiology of Toxigenic Fungi and Their Associated Mycotoxins for Some Mediterranean Crops. In Epidemiology of Mycotoxin Producing Fungi; Xu, X., Bailey, J.A., Cooke, B.M., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 645–667. [Google Scholar]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and Its Toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Vigier, B.; Reid, L.M.; Dwyer, L.M.; Stewart, D.W.; Sinha, R.C.; Arnason, J.T.; Butler, G. Maize Resistance to Gibberella Ear Rot: Symptoms, Deoxynivalenol, and Yield1. Can. J. Plant Pathol. 2001, 23, 99–105. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and Potential Effects on Humans. J. Toxicol. Environ. Health Part B 2005, 8, 39–698. [Google Scholar] [CrossRef] [PubMed]
- Neme, K.; Mohammed, A. Mycotoxin Occurrence in Grains and the Role of Postharvest Management as a Mitigation Strategies. A Review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Srecec, S. Decreasing Deoxynivalenol Concentration in Maize within the Production Chain of Animal Feed. Agro Food Ind. Hi Tech 2013, 24, 4. [Google Scholar]
- Xu, Y.; Huang, Z.-B.; He, Q.-H.; Deng, S.-Z.; Li, L.-S.; Li, Y.-P. Development of an Immunochromatographic Strip Test for the Rapid Detection of Deoxynivalenol in Wheat and Maize. Food Chem. 2010, 119, 834–839. [Google Scholar] [CrossRef]
- Richard, J.L. Some Major Mycotoxins and Their Mycotoxicoses—An Overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P. Epidemiology of Fusarium Diseases and Their Mycotoxins in Maize Ears. In Epidemiology of Mycotoxin Producing Fungi; Xu, X., Bailey, J.A., Cooke, B.M., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 705–713. [Google Scholar]
- Quesada-Ocampo, L.M.; Al-Haddad, J.; Scruggs, A.C.; Buell, C.R.; Trail, F. Susceptibility of Maize to Stalk Rot Caused by Fusarium Graminearum Deoxynivalenol and Zearalenone Mutants. Phytopathology 2016, 106, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Gaikpa, D.S.; Miedaner, T. Genomics-Assisted Breeding for Ear Rot Resistances and Reduced Mycotoxin Contamination in Maize: Methods, Advances and Prospects. Appl. Genet. 2019, 132, 2721–2739. [Google Scholar] [CrossRef]
- Kuntz, M. The GMO Case in France: Politics, Lawlessness and Postmodernism. GM Crops Food 2014, 5, 163–169. [Google Scholar] [CrossRef][Green Version]
- Munkvold, G.P.; Arias, S.; Taschl, I.; Gruber-Dorninger, C. Chapter 9-Mycotoxins in Corn: Occurrence, Impacts, and Management. In Corn, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019; pp. 235–287. [Google Scholar]
- Ostry, V.; Ovesna, J.; Skarkova, J.; Pouchova, V.; Ruprich, J. A Review on Comparative Data Concerning Fusarium Mycotoxins in Bt Maize and Non-Bt Isogenic Maize. Mycotoxin Res. 2010, 26, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Papst, C.; Utz, H.F.; Melchinger, A.E.; Eder, J.; Magg, T.; Klein, D.; Bohn, M. Mycotoxins Produced by Fusarium Spp. in Isogenic Bt vs. Non-Bt Maize Hybrids under European Corn Borer Pressure. Agron. J. 2005, 97, 6. [Google Scholar]
- Miller, J.D. Epidemiology of Fusarium graminearum diseases of wheat and corn. In Mycotoxins in Grain: Compounds Other than Aflatoxin; Miller, J.D., Trenholm, H.M., Eds.; Eagan Press: St Paul, MN, USA, 1994. [Google Scholar]
- Folcher, L.; Weissenberger, A.; Delos, M. Quantitative Relationships between Ostrinia Nubilalis Activity and Deoxynivalenol Contamination in French Maize. Int. J. Pest. Manag. 2012, 58, 302–309. [Google Scholar] [CrossRef]
- Agusti, N.; Bourguet, D.; Spataro, T.; Délos, M.; Eychenne, N.; Folcher, L.; Arditi, R. Detection, Identification and Geographical Distribution of European Corn Borer Larval Parasitoids Using Molecular Markers. Mol. Ecol. 2005, 14, 3267–3274. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, A.W.; Hooker, D.C.; Baute, T.S.; Illincic-Tamburic, L. Effect of Bt -Corn Hybrids on Deoxynivalenol Content in Grain at Harvest. Plant Dis. 2002, 86, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Lauren, D.R.; Smith, W.A.; Di Menna, M.E. Influence of Harvest Date and Hybrid on the Mycotoxin Content of Maize (Zea Mays) Grain Grown in New Zealand. N. Zeal. J. Crop Hortic. Sci. 2007, 35, 331–340. [Google Scholar] [CrossRef]
- Blandino, M.; Reyneri, A.; Vanara, F.; Tamietti, G.; Pietri, A. Influence of Agricultural Practices on Fusarium Infection, Fumonisin and Deoxynivalenol Contamination of Maize Kernels. World Mycotoxin J. 2009, 2, 409–418. [Google Scholar] [CrossRef]
- Eckard, S.; Wettstein, F.E.; Forrer, H.-R.; Vogelgsang, S. Incidence of Fusarium Species and Mycotoxins in Silage Maize. Toxins 2011, 3, 949–967. [Google Scholar] [CrossRef]
- Orlando, B.; Méléard, B.; Coudure, R. Qualité sanitaire du maïs grain: La prévention pour mieux maîtriser les risques. Perspect. Agric. 2015, 425, 30–32. [Google Scholar]
- Champeil, A.; Doré, T.; Fourbet, J.F. Fusarium Head Blight: Epidemiological Origin of the Effects of Cultural Practices on Head Blight Attacks and the Production of Mycotoxins by Fusarium in Wheat Grains. Plant Sci. 2004, 166, 1389–1415. [Google Scholar] [CrossRef]
- Pereyra, S.A.; Dill-Macky, R.; Sims, A.L. Survival and Inoculum Production of Gibberella Zeae in Wheat Residue. Plant Dis. 2004, 88, 724–730. [Google Scholar] [CrossRef]
- Blandino, M.; Pilati, A.; Reyneri, A.; Scudellari, D. Effect of Maize Crop Residue Density on Fusarium Head Blight and on Deoxynivalenol Contamination of Common Wheat Grains. Cereal. Res. Commun. 2010, 38, 550–559. [Google Scholar] [CrossRef]
- Oldenburg, E.; Brunotte, J.; Weinert, J. Strategies to Reduce DON Contamination of Wheat with Different Soil Tillage and Variety Systems. Mycotox Res. 2007, 23, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Champeil, A.; Fourbet, J.F.; Doré, T.; Rossignol, L. Influence of Cropping System on Fusarium Head Blight and Mycotoxin Levels in Winter Wheat. Crop Prot. 2004, 23, 531–537. [Google Scholar] [CrossRef]
- Maiorano, A.; Blandino, M.; Reyneri, A.; Vanara, F. Effects of Maize Residues on the Fusarium Spp. Infection and Deoxynivalenol (DON) Contamination of Wheat Grain. Crop Prot. 2008, 27, 182–188. [Google Scholar] [CrossRef]
- Manstretta, V.; Rossi, V. Effects of Temperature and Moisture on Development of Fusarium Graminearum gerithecia in Maize Stalk Residues. Appl. Environ. Microbiol. 2016, 82, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.C. Epidemiology of Wheat Head Blight and Maize Ear Rot Caused by Fusarium graminearum. Can. J. Plant Pathol. 1982, 4, 195–209. [Google Scholar] [CrossRef]
- Tschanz, A.T.; Horst, R.K.; Nelson, P.E. The Effect of Environment on Sexual Reproduction of Gibberella Zeae. Mycologia 1976, 68, 327–340. [Google Scholar] [CrossRef]
- Booth, C. The Genus Fusarium. In Genus Fusarium; Commonwealth Mycological Institute: Kew, UK, 1971; p. 237. [Google Scholar]
- Mansfield, M.A.; De Wolf, E.D.; Kuldau, G.A. Relationships Between Weather Conditions, Agronomic Practices, and Fermentation Characteristics with Deoxynivalenol Content in Fresh and Ensiled Maize. Plant Dis. 2005, 89, 1151–1157. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Hellmich, R.L.; Showers, W.B. Reduced Fusarium Ear Rot and Symptomless Infection in Kernels of Maize Genetically Engineered for European Corn Borer Resistance. Phytopathology 1997, 87, 1071–1077. [Google Scholar] [CrossRef]
- Pleadin, J.; Vasilj, V.; Petrović, D.; Frece, J.; Vahčić, N.; Jahić, S.; Markov, K. Annual Variations of Fusarium Mycotoxins in Unprocessed Maize, Wheat and Barley from Bosnia and Herzegovina. Croat. J. Food Sci. Technol. 2017, 9, 11–18. [Google Scholar] [CrossRef][Green Version]
- Kos, J.; Hajnal, E.J.; Šarić, B.; Jovanov, P.; Nedeljković, N.; Milovanović, I.; Krulj, J. The Influence of Climate Conditions on the Occurrence of Deoxynivalenol in Maize Harvested in Serbia during 2013–2015. Food Control 2017, 73, 734–740. [Google Scholar] [CrossRef]
- Paul, P.A.; El-Allaf, S.M.; Lipps, P.E.; Madden, L.V. Rain Splash Dispersal of Gibberella Zeae Within Wheat Canopies in Ohio. Phytopathology® 2004, 94, 1342–1349. [Google Scholar] [CrossRef]
- Doohan, F.M.; Brennan, J.; Cooke, B.M. Influence of Climatic Factors on Fusarium Species Pathogenic to Cereals. In Epidemiology of Mycotoxin Producing Fungi: Under the Aegis of COST Action 835 ‘Agriculturally Important Toxigenic Fungi 1998–2003′, EU Project (QLK 1-CT-1998–01380); Xu, X., Bailey, J.A., Cooke, B.M., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 755–768. [Google Scholar]
- Blandino, M.; Scarpino, V.; Giordano, D.; Sulyok, M.; Krska, R.; Vanara, F.; Reyneri, A. Impact of Sowing Time, Hybrid and Environmental Conditions on the Contamination of Maize by Emerging Mycotoxins and Fungal Metabolites. Ital. J. Agron. 2017, 12, 215–224. [Google Scholar] [CrossRef]
- Logrieco, A.; Battilani, P.; Leggieri, M.C.; Jiang, Y.; Haesaert, G.; Lanubile, A.; Mahuku, G.; Mesterházy, A.; Ortega-Beltran, A.; Pasti, M.; et al. Perspectives on Global Mycotoxin Issues and Management From the MycoKey Maize Working Group. Plant Dis. 2021, 105, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.-L.; Richard-Forget, F.; Barreau, C. Natural Mechanisms for Cereal Resistance to the Accumulation of Fusarium Trichothecenes. Eur. J. Plant Pathol. 2008, 121, 411–423. [Google Scholar] [CrossRef]
- Leslie, J.F.; Logrieco, A.F. Mycotoxin Reduction in Grain Chains, 1st ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Degraeve, S.; Madege, R.R.; Audenaert, K.; Kamala, A.; Ortiz, J.; Kimanya, M.; Tiisekwa, B.; De Meulenaer, B.; Haesaert, G. Impact of Local Pre-Harvest Management Practices in Maize on the Occurrence of Fusarium Species and Associated Mycotoxins in Two Agro-Ecosystems in Tanzania. Food Control 2016, 59, 225–233. [Google Scholar] [CrossRef]
- Miller, S.S.; Reid, L.M.; Harris, L.J. Colonization of Maize Silks by Fusarium graminearum, the Causative Organism of Gibberella Ear Rot. Can. J. Bot. 2007, 85, 369–376. [Google Scholar] [CrossRef]
- Marín, P.; Magan, N.; Vázquez, C.; González-Jaén, M.T. Differential Effect of Environmental Conditions on the Growth and Regulation of the Fumonisin Biosynthetic Gene FUM1 in the Maize Pathogens and Fumonisin Producers Fusarium Verticillioides and Fusarium Proliferatum: Ecophysiology of F. Verticillioides and F. Proliferatum. FEMS Microbiol. Ecol. 2010, 72, 303–311. [Google Scholar]
- Pfordt, A.; Romero, L.R.; Schiwek, S.; Karlovsky, P.; von Tiedemann, A. Impact of Environmental Conditions and Agronomic Practices on the Prevalence of Fusarium Species Associated with Ear- and Stalk Rot in Maize. Pathogens 2020, 9, 236. [Google Scholar] [CrossRef]
- Picot, A.; Hourcade-Marcolla, D.; Barreau, C.; Pinson-Gadais, L.; Caron, D.; Richard-Forget, F.; Lannou, C. Interactions between Fusarium Verticillioides and Fusarium graminearum in Maize Ears and Consequences for Fungal Development and Mycotoxin Accumulation: Fusarium Spp. Interactions in Maize Ears. Plant Pathol. 2012, 61, 140–151. [Google Scholar] [CrossRef]
- Fels-klerx, H.J.; van der Booij, C.J.H. Perspectives for geographically oriented management of fusarium mycotoxins in the cereal supply chain. J. Food Prot. 2010, 73, 1153–1159. [Google Scholar] [CrossRef]
- Van Asselt, E.D.; Booij, C.J.H.; Fels-Klerx, H.J. van der Modelling Mycotoxin Formation by Fusarium graminearum in Maize in The Netherlands. Food Addit. Contam. Part A 2012, 29, 1572–1580. [Google Scholar] [CrossRef]
- Hooker, D.C.; Schaafsma, A.W. Agronomic and Environmental Impacts on Concentrations of Deoxynivalenol and Fumonisin B1 in Corn across Ontario. Can. J. Plant Pathol. 2005, 27, 347–356. [Google Scholar] [CrossRef]
- Roucou, A.; Bergez, C.; Méléard, B.; Orlando, B. A Fumonisin Prevention Tool for Targeting and Ranking Agroclimatic Conditions Favoring Exposure in French Maize-Growing Areas. Toxins 2021, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Bhatnagar, D.; Bui-Klimke, T.; Carbone, I.; Hellmich, R.; Munkvold, G.; Paul, P.; Payne, G.; Takle, E. Climate Change Impacts on Mycotoxin Risks in US Maize. World Mycotoxin J. 2011, 4, 79–93. [Google Scholar] [CrossRef]
- Jajić, I.; Jurić, V.; Glamočić, D.; Abramović, B. Occurrence of Deoxynivalenol in Maize and Wheat in Serbia. Int. J. Mol. Sci. 2008, 9, 2114–2126. [Google Scholar] [CrossRef]
- Giorni, P.; Bertuzzi, T.; Battilani, P. Impact of Fungi Co-Occurrence on Mycotoxin Contamination in Maize During the Growing Season. Front. Microbiol. 2019, 10, 2365. [Google Scholar] [CrossRef]
- Vandicke, J.; De Visschere, K.; Croubels, S.; De Saeger, S.; Audenaert, K.; Haesaert, G. Mycotoxins in Flanders’ Fields: Occurrence and Correlations with Fusarium Species in Whole-Plant Harvested Maize. Microorganisms 2019, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Deudon, O. Interest and implementation of a spatialization method of meteorological data used in agricultural decision support tools. In Proceedings of the 2017 EFFITA WCCA Congress, Montpellier, France, 2–6 July 2017. [Google Scholar]
- Hastie, T.; Taylor, J.; Tibshirani, R.; Walther, G. Forward Stagewise Regression and the Monotone Lasso. Electron. J. Stat. 2007, 1, 1–29. [Google Scholar] [CrossRef]
- Anbari, M.E. Regularization and variable selection using penalized lieklihood. Ph.D. Thesis, General Mathematics. Université Paris-Sud Paris IX, Paris, France, Université Cadi Ayyad, Marrakech, Maroc, 2011. [Google Scholar]
- Genuer, R.; Poggi, J.-M.; Tuleau-Malot, C. Variable selection using random forests. Pattern Recognit. Lett. 2010, 31, 2225–2236. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. Multivariate Linear Models in R. In An R Companion to Applied Regression, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models; Version 1.4–1.5; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Lenth, R.V. Using Lsmeans; The University of Iowa: Iowa City, IA, USA, 2017. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for statistical computing: Vienna, Austria, 2020. [Google Scholar]
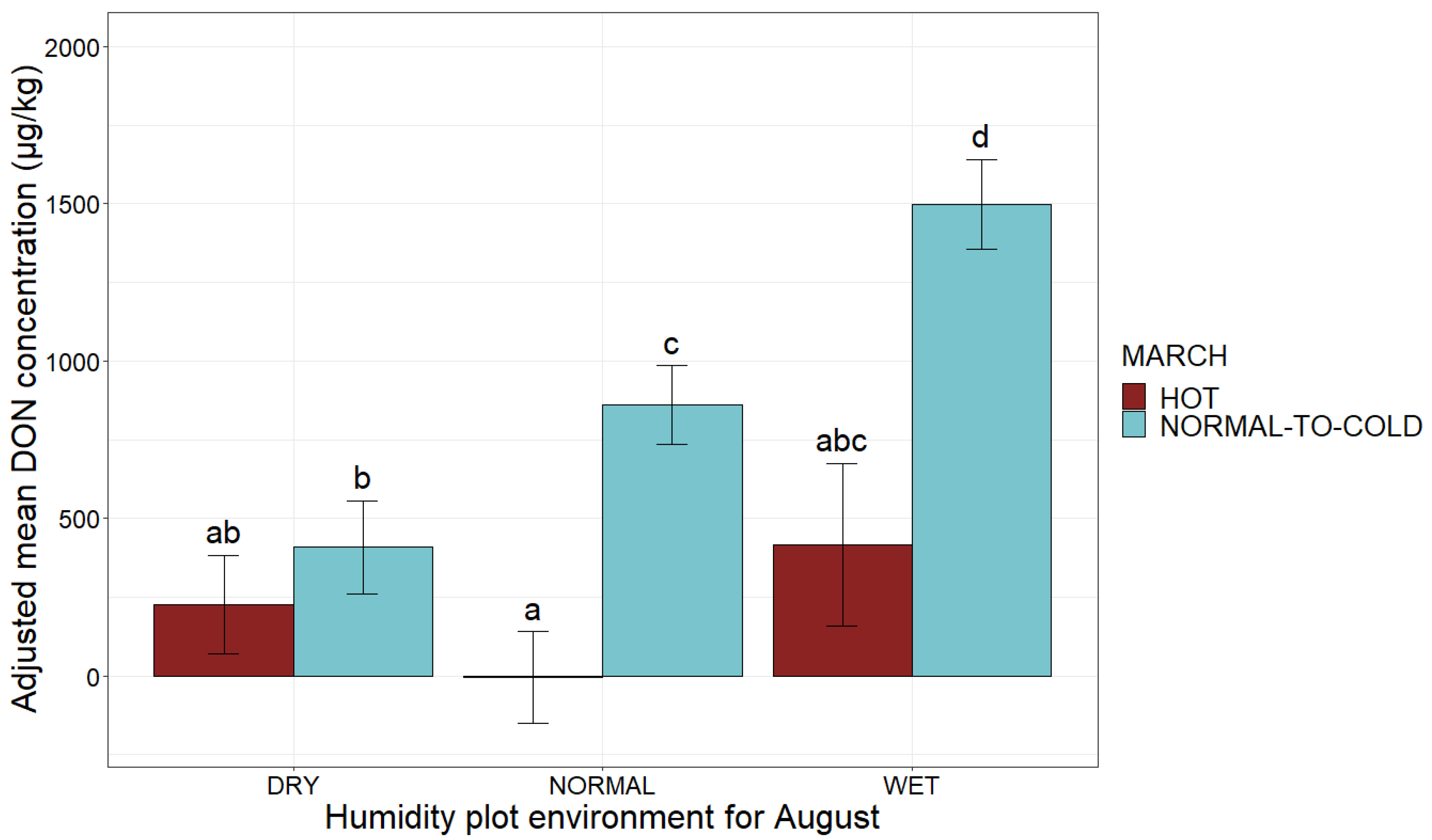
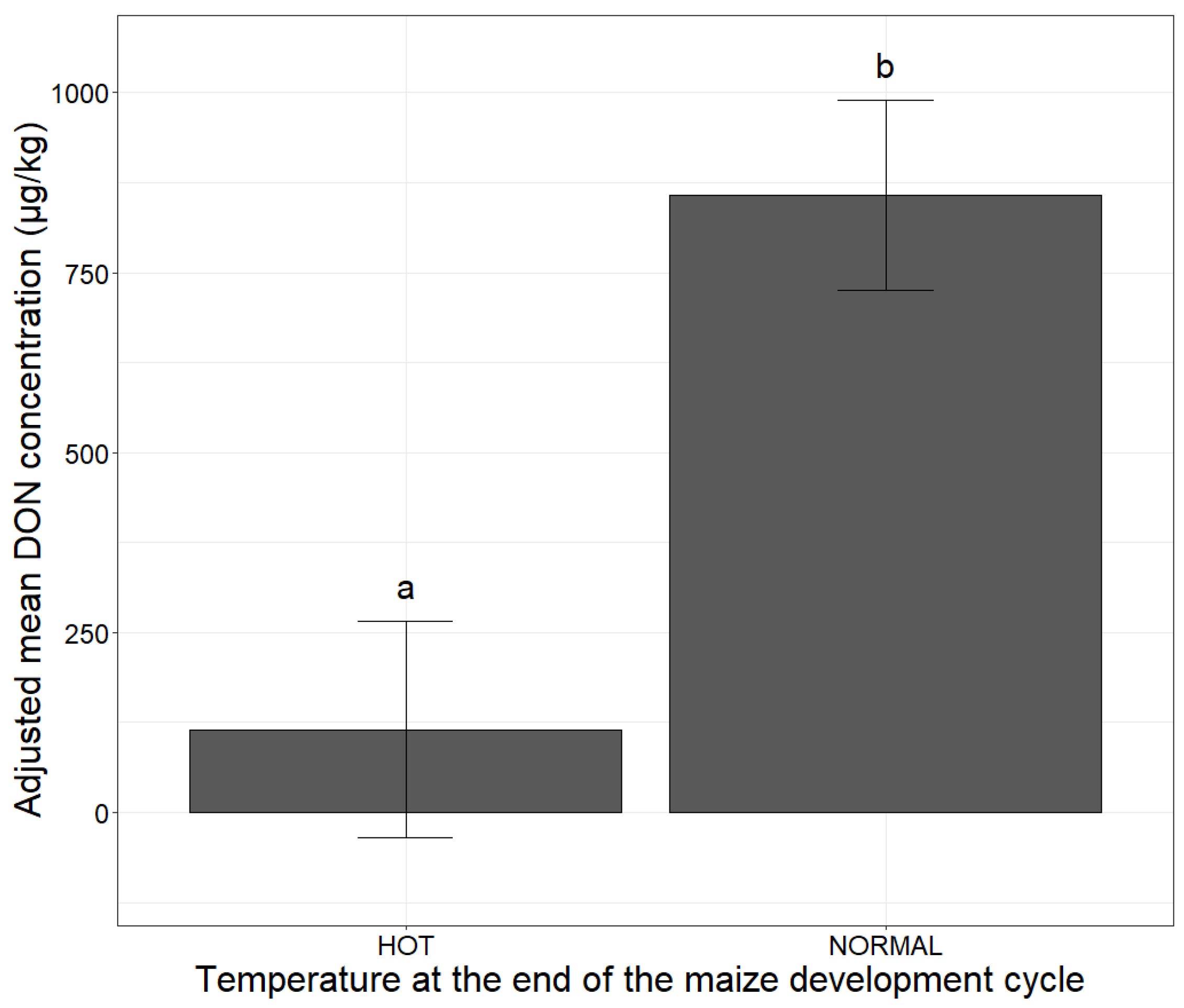
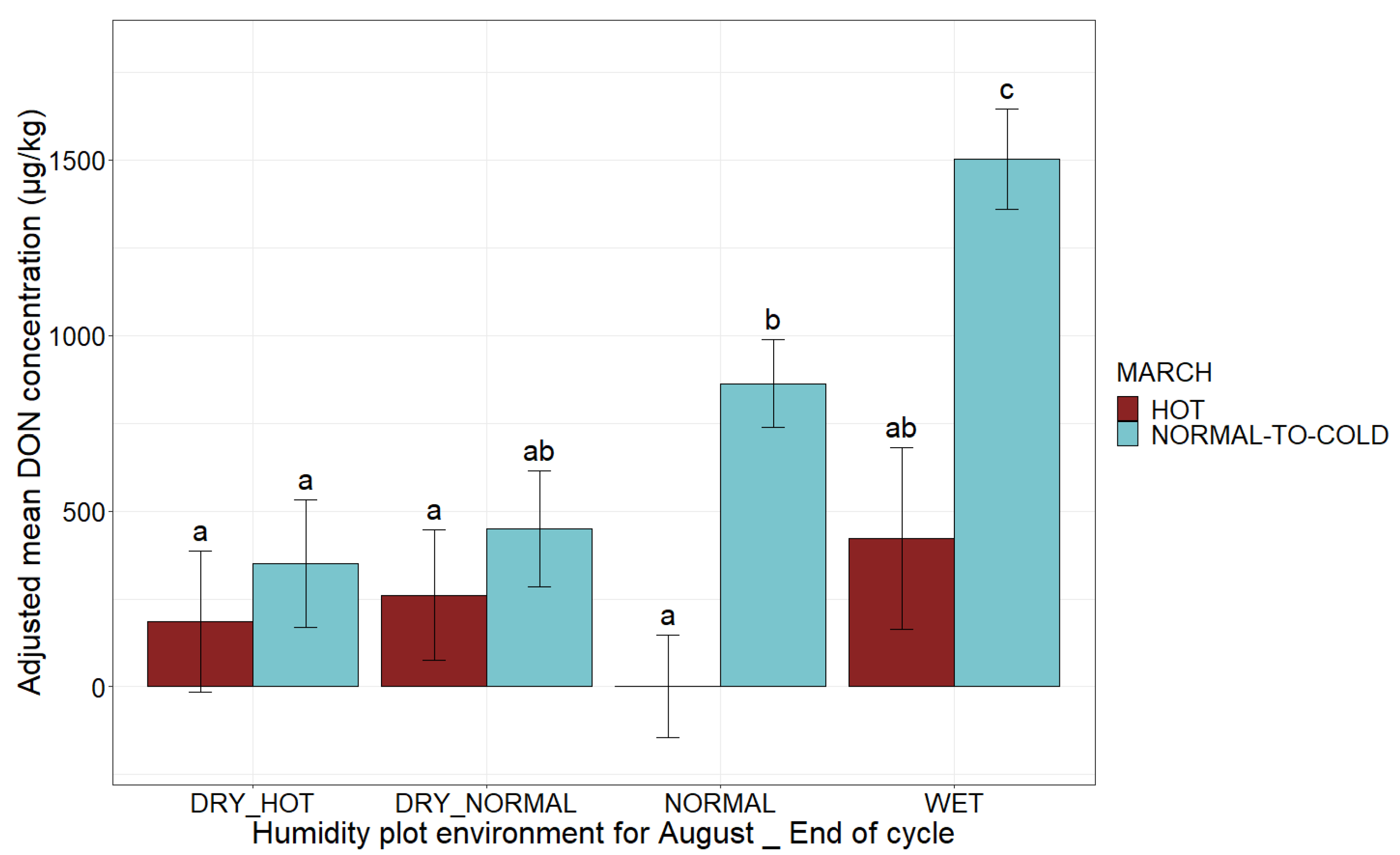
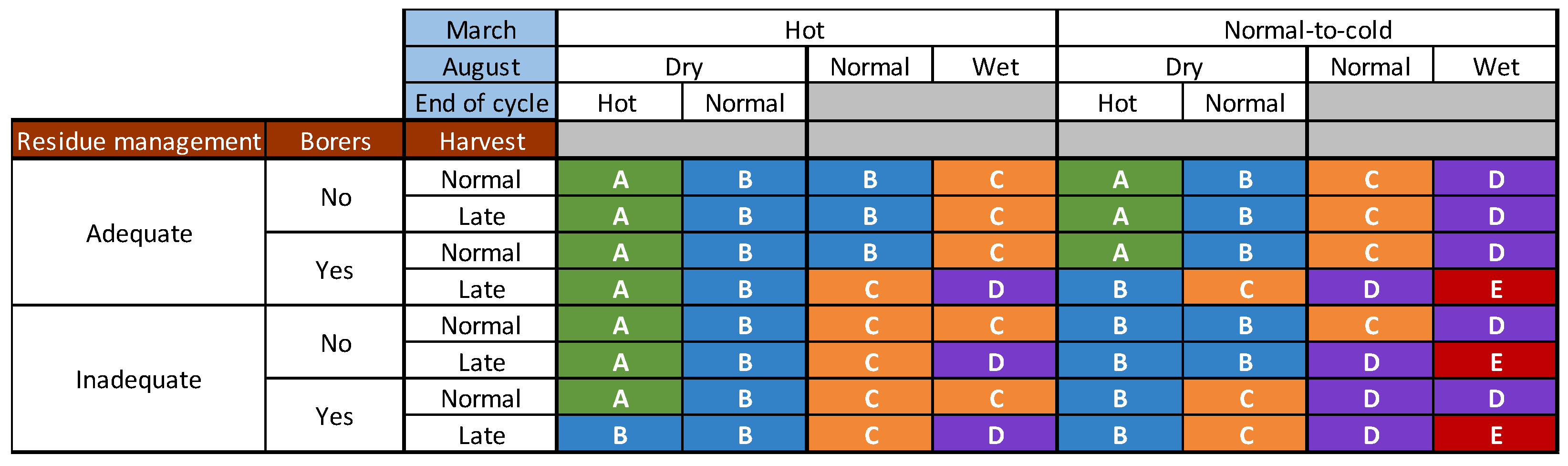
| Climatic Factors | Possible Values | Adjusted Mean DON Concentration (µg/kg) | Comparison of Means |
|---|---|---|---|
| March, mean maximum monthly temperature | Hot | 103 | a 1 |
| Normal-to-cold | 878 | b 1 | |
| August, humidity plot environment | Dry | 365 | a 2 |
| Normal | 616 | b 2 | |
| Wet | 1343 | c 2 |
| End of Cycle | Adjusted Mean DON Concentration (µg/kg) | Comparison of Means |
|---|---|---|
| Hot | 115 | a 1 |
| Normal | 858 | b 1 |
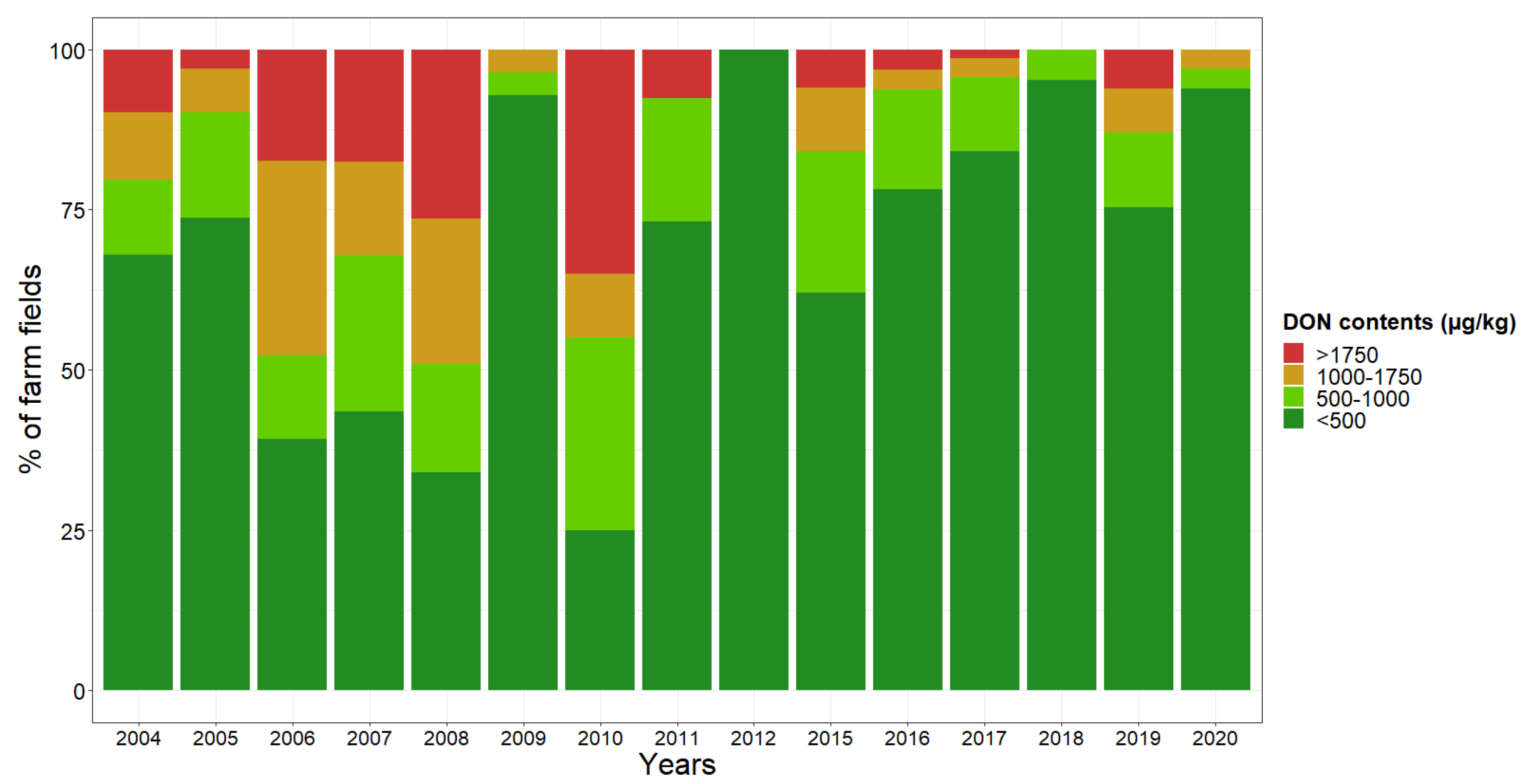
| Harvest Year | n | p-Values | R2 | Percentage of Farm Fields for Each Risk Level | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | ||||
| 2004 | 265 | <0.001 | 0.06 | 3 | 3 | 25 | 41 | 28 |
| 2005 | 426 | <0.001 | 0.08 | 1 | 31 | 52 | 14 | 2 |
| 2006 | 441 | <0.001 | 0.13 | 0.5 | 14 | 32.5 | 48 | 5 |
| 2007 | 177 | >0.05 | 0.02 | 0 | 0 | 24 | 73 | 3 |
| 2008 | 53 | >0.05 | −0.008 | 0 | 2 | 38 | 51 | 9 |
| 2009 | 28 | >0.05 | 0.01 | 36 | 53 | 11 | 0 | 0 |
| 2010 | 20 | >0.05 | −0.01 | 5 | 0 | 85 | 10 | 0 |
| 2011 | 26 | <0.01 | 0.43 | 4 | 31 | 58 | 7 | 0 |
| 2012 | 13 | NA | NA | 0 | 69 | 31 | 0 | 0 |
| 2015 | 50 | <0.001 | 0.26 | 0 | 6 | 64 | 26 | 4 |
| 2016 | 64 | <0.001 | 0.28 | 23 | 45 | 27 | 5 | 0 |
| 2017 | 69 | >0.05 | −0.003 | 10 | 70 | 20 | 0 | 0 |
| 2018 | 125 | >0.05 | 0.04 | 47 | 18 | 32 | 3 | 0 |
| 2019 | 146 | <0.001 | 0.10 | 15 | 46 | 32 | 6 | 1 |
| 2020 | 129 | <0.001 | 0.1 | 50 | 18 | 27 | 5 | 0 |
| 2004–2020 | 2032 | <0.001 | 0.12 | 9 | 21 | 35 | 29 | 6 |
| Years | Samples |
|---|---|
| 2004 | 265 |
| 2005 | 426 |
| 2006 | 441 |
| 2007 | 177 |
| 2008 | 53 |
| 2009 | 28 |
| 2010 | 20 |
| 2011 | 26 |
| 2012 | 13 |
| 2013 | 0 |
| 2014 | 0 |
| 2015 | 50 |
| 2016 | 64 |
| 2017 | 69 |
| 2018 | 125 |
| 2019 | 146 |
| 2020 | 129 |
| Previous Crop | Plowing | Crushing of the Residues | Residue Management |
|---|---|---|---|
| Others | Yes | Yes | Adequate |
| No | Adequate | ||
| No | Yes | Adequate | |
| No | Inadequate | ||
| Maize, Sorghum | Yes | Yes | Adequate |
| No | Adequate | ||
| No | Yes | Inadequate | |
| No | Inadequate |
| Quantitative Climatic Factors | Thresholds | Qualitative Climatic Factors | Possible Values |
|---|---|---|---|
| March, mean maximum monthly temperature | >14.5 °C | March, mean maximum monthly temperature | Hot |
| <14.5 °C | Normal-to-cold | ||
| August, (Rain–ETP 1) | <−93.6 | August, humidity plot environment | Dry |
| −93.6< … <−16.8 | Normal | ||
| >−16.8 | Wet |
| 30–60 Days before Harvest, Mean Maximum Monthly Temperature | 0–30 Days before Harvest, Number of Days with a Mean Maximum Daily Temperature Above 28 °C | End of Cycle |
|---|---|---|
| >26 °C | >3 | Hot |
| >26 °C | <3 | |
| <26 °C | <3 | Normal |
| <26 °C | >3 |
| Linear Mixed Models | Objectives |
|---|---|
| [DON] = Agroi 1/Climj 2 + (1|Year) | Individual effect |
| [DON] = ∑(Agroi1/Climj1 × Agroi2/Climj2) + (1|Year) | Combination of climatic or agronomic effects |
| [DON] = ∑(Agroi × Climj) + (1|Year) | Combination of agronomic and climatic effects |
| [DON] = Risk classes + (1|Year) | Individual effect of the DON risk class |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roucou, A.; Bergez, C.; Méléard, B.; Orlando, B. An Agro-Climatic Approach to Developing a National Prevention Tool for Deoxynivalenol in French Maize-Growing Areas. Toxins 2022, 14, 74. https://doi.org/10.3390/toxins14020074
Roucou A, Bergez C, Méléard B, Orlando B. An Agro-Climatic Approach to Developing a National Prevention Tool for Deoxynivalenol in French Maize-Growing Areas. Toxins. 2022; 14(2):74. https://doi.org/10.3390/toxins14020074
Chicago/Turabian StyleRoucou, Agathe, Christophe Bergez, Benoît Méléard, and Béatrice Orlando. 2022. "An Agro-Climatic Approach to Developing a National Prevention Tool for Deoxynivalenol in French Maize-Growing Areas" Toxins 14, no. 2: 74. https://doi.org/10.3390/toxins14020074
APA StyleRoucou, A., Bergez, C., Méléard, B., & Orlando, B. (2022). An Agro-Climatic Approach to Developing a National Prevention Tool for Deoxynivalenol in French Maize-Growing Areas. Toxins, 14(2), 74. https://doi.org/10.3390/toxins14020074





