Cytotoxicity of Aspergillus Section Fumigati Isolates Recovered from Protection Devices Used on Waste Sorting Industry
Abstract
:1. Introduction
2. Materials and Methods
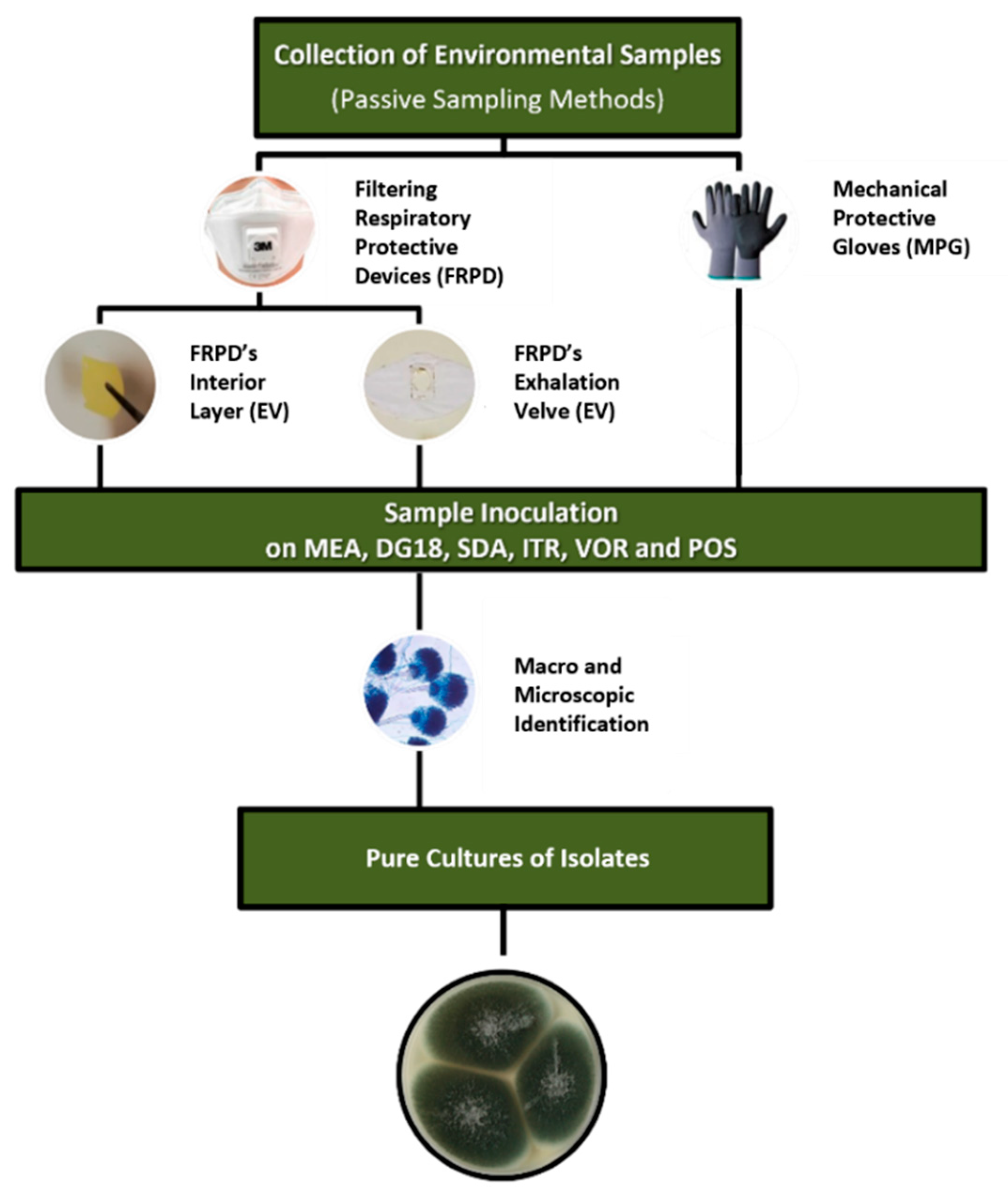
2.1. Protection Devices’ Sampling and Sample Preparation
2.2. Microbiological Characterization and Cytotoxicity Evaluation of FRPD e MPG
2.3. Collection of Aspergillus Section Fumigati Isolates
2.4. Cell Lines and Culture Conditions
2.5. Cytotoxicity Evaluation
2.6. Statistical Analysis
3. Results
3.1. Aspergillus Section Fumigati Cytotoxicity
3.2. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- European Union. DIRECTIVE 2008/98/EC of the European Parliament and of the Council. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32008L0098 (accessed on 5 January 2022).
- Park, D.U.; Ryu, S.H.; Kim, S.-B.; Yoon, C.-S. An assessment of dust, endotoxin, and microorganism exposure during waste collection and sorting. J. Air Waste Manag. Assoc. 2011, 61, 461–468. [Google Scholar] [CrossRef]
- Schlosser, O.; Déportes, I.Z.; Facon, B.; Fromont, E. Extension of the sorting instructions for household plastic packaging and changes in exposure to bioaerosols at materials recovery facilities. Waste Manag. 2015, 46, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Faria, T.; dos Santos, M.; Carolino, E.; Gomes, A.Q.; Sabino, R.; Viegas, S. Fungal burden in waste industry: An occupational risk to be solved. Environ. Monit. Assess. 2015, 187, 199. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; Frederiksen, M.W.; Jacobsen, M.H.; Tendal, K. Towards a risk evaluation of workers’ exposure to handborne and airborne microbial species as exemplified with waste collection workers. Environ. Res. 2020, 183, 109177. [Google Scholar] [CrossRef]
- Poole, C.J.M.; Basu, S. Systematic Review: Occupational illness in the waste and recycling sector. Occup. Med. 2017, 67, 626–636. [Google Scholar] [CrossRef]
- Viegas, S.; Caetano, L.A.; Korkalainen, M.; Faria, T.; Pacífico, C.; Carolino, E.; Quintal Gomes, A.; Viegas, C. Cytotoxic and Inflammatory Potential of Air Samples from Occupational Settings with Exposure to Organic Dust. Toxics 2017, 5, 8. [Google Scholar] [CrossRef]
- Ladeira, C.; Gajski, G.; Meneses, M.; Gerić, M.; Viegas, S. The genotoxicity of an organic solvent mixture: A human biomonitoring study and translation of a real-scenario exposure to in vitro. Regul. Toxic. Pharmacol. 2020, 116, 104726. [Google Scholar] [CrossRef] [PubMed]
- Degois, J.; Simon, X.; Clerc, F.; Bontemps, C.; Leblond, P.; Duquenne, P. One-year follow-up of microbial diversity in bioaerosols emitted in a waste sorting plant in France. Waste Manag 2021, 120, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Dias, M.; Almeida, B.; Aranha Caetano, L.; Carolino, E.; Quintal Gomes, A.; Twaruzek, M.; Kosicki, R.; Grajewski, J.; Marchand, G.; et al. Are workers from waste sorting industry really protected by wearing Filtering Respiratory Protective Devices? The gap between the myth and reality. Waste Manag. 2020, 102, 856–867. [Google Scholar] [CrossRef]
- Viegas, C.; Twarużek, M.; Dias, M.; Almeida, B.; Carolino, E.; Kosicki, R.; Soszczyńska, E.; Grajewski, J.; Aranha Caetano, L.; Viegas, S. Assessment of the microbial contamination of mechanical protection gloves used on waste sorting industry: A contribution for the risk characterization. Environ. Res. 2020, 189, 109881. [Google Scholar] [CrossRef]
- Varga, J.; Baranyi, N.; Chandrasekaran, M.; Vágvölgyi, C.; Kocsubé, S. Mycotoxin producers in the Aspergillus genus: An update. Acta Biol. Szeged. 2015, 59, 151–167. [Google Scholar]
- Sabino, R. Exposure to Fungi in Health Care Facilities. In Encyclopedia of Mycology; Zaragoza, O., Casadevall, A., Balestrini, R., Nosanchuk, J., Viegas, C., Vizzini, A., de Vries, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Nature Microbiology. Stop neglecting fungi. Nat. Microbiol. 2017, 2, 17120. [Google Scholar] [CrossRef]
- Anyanwu, E.C.; Campbell, A.W.; Jones, J.; Ehiri, J. The neurological significance of abnormal natural killer cell activity in chronic toxigenic mold exposures. Scien. World J. 2003, 13, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Sutton, P.; Newcombe, N.R.; Waring, P.; Müllbacher, A. In vivo immunosuppressive activity of gliotoxin, a metabolite produced by human pathogenic fungi. Infect. Immun. 2003, 62, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Cramer, R.A.; Rivera, A.; Hohl, T.M. Immune responses against Aspergillus fumigatus: What have we learned? Curr. Opin. Infect. Dis. 2011, 24, 315–322. [Google Scholar] [CrossRef]
- Viegas, C.; Caetano, L.A.; Viegas, S. Occupational Exposure to Aspergillus Section Fumigati: Tackling the Knowledge Gap in Portugal. Environ. Res. 2021, 194, 110674. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, T.K.; Choi, E.J.; Yang, J.; Shukla, S.K.; Hwang, S.-I.; Park, J. Molecular Approaches for the Detection and Monitoring of Microbial Communities in Bioaerosols: A Review. J. Environ. Sci. 2017, 51, 234–247. [Google Scholar] [CrossRef]
- Viegas, C.; Gomes, B.; Dias, M.; Carolino, E.; Aranha Caetano, L. Aspergillus Section Fumigati in Firefighter Headquarters. Microorganisms 2021, 9, 2112. [Google Scholar] [CrossRef]
- Pandey, S.; Hoselton, S.A.; Schuh, J.M. The Impact of Aspergillus Fumigatus Viability and Sensitization to Its Allergens on the Murine Allergic Asthma Phenotype. BioMed Res. Int. 2013, 619614. [Google Scholar] [CrossRef]
- Clinical & Laboratory Standards Institute: CLSI Guidelines. Available online: https://clsi.org/ (accessed on 5 January 2022).
- Kozel, T.R.; Wickes, B. Fungal Diagnostics. Cold Spring Harb. Perspect. Med. 2014, 4, a019299. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Twarużekd, M.; Dias, M.; Almeida, B.; Carolino, E.; Soszczyńska, E.; Viegas, S.; Caetano, L.A. Cytotoxicity of filtering respiratory protective devices from the waste sorting industry: A comparative study between interior layer and exhalation valve. Environ. Inter. 2021, 155, 106603. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Aranha Caetano, L.; Afanou, A.; Straumfors, A.; Veríssimo, C.; Gonçalves, P.; Sabino, R. Algorithm to assess the presence of Aspergillus fumigatus resistant strains: The case of Norwegian sawmills. Inter. J. Environ. Health Res. 2020. [Google Scholar] [CrossRef]
- Cetin, Y.; Bullerman, L.B. Cytotoxicity of Fusarium mycotoxins to mammalian cell cultures as determined by the MTT bioassay. Food Chem Toxicol. 2005, 43, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Swain, R.J.; Kemp, S.J.; Goldstraw, P.; Tetley, T.D.; Stevens, M.M. Assessment of Cell Line Models of Primary Human Cells by Raman Spectral Phenotyping. Biophys. J. 2010, 98, 1703–1711. [Google Scholar] [CrossRef]
- Heussner, A.H.; Dietrich, D.R. Primary porcine proximal tubular cells as an alternative to human primary renal cells in vitro: An initial characterization. BMC Cell Biol. 2013, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Fornelli, F.; Minervini, F.; Logrieco, A. Cytotoxicity of fungal metabolites to lepidopteran (Spodoptera frugiperda) cell line (SF-9). J. Invertebr. Pathol. 2004, 85, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Prinsloo, S.; Pieters, R.; Bezuidenhout, C.C. A cell viability assay to determine the cytotoxic effects of water contaminated by microbes. S. Afr. J. Sci. 2013, 109, 7–8. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, W.; Xu, J.; Miler, D. Characterization of 2 related exoantigens from the biodeteriogenic fungus Aspergillus versicolor. Int. Biodeterior Biodegrad. 2011, 65, 217–226. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, L.; Alexander, B.D.; Kett, D.H.; Vazquez, J.; Pappas, P.G.; Saeki, F. Multicenter clinical evaluation of the (1→3) β-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 2005, 41, 654–659. [Google Scholar] [CrossRef]
- Gutarowska, B. Hyphate fungi in building materials. Increase and production of mycotoxins and allergens. Sci. Bull Tech. Uni. Lodz. 2010, 1074, 1–164. [Google Scholar]
- Schulz, T.; Senkpiel, K.; Ohgke, H. Comparison of toxicity of reference mycotoxins and spore extracts of common indoor moulds. Int. J. Hyg. Environ. Health 2004, 207, 267–277. [Google Scholar] [CrossRef]
- Fairs, A.; Agbetile, J.; Bourne, M.; Hargadon, B.; Monteiro, W.R.; Morley, J.P. Isolation of Aspergillus fumigatus from sputum is associated with elevated airborne levels in homes of patients with asthma. Indoor Air 2013, 23, 275–284. [Google Scholar] [CrossRef]
- Viegas, C.; Twarużek, M.; Dias, M.; Almeida, B.; Carolino, E.; Soszczyńska, E.; Ałtyn, I.; Viegas, S.; Aranha Caetano, L. Cytotoxic effect of filtering respiratory protective devices from the waste sorting industry: Is in vitro toxicology useful for risk characterization? Environ. Res. 2020, 191, 110134. [Google Scholar] [CrossRef]
- Grant, K.; Goldizen, F.C.; Sly, P.D. Health consequences of exposure to e-waste: Asystematic review. Lancet. Glob. Heal. 2013, 1, e350–e361. [Google Scholar] [CrossRef]
- Thakur, P.; Ganguly, R.; Dhulia, A. Occupational health hazard exposure among municipalsolid waste workers in Himachal Pradesh, India. Waste Manag. 2018, 78, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Gniadek, A.; Krzyściak, P.; Twarużek, M.; Macura, A.B. Occurrence of fungi and cytotoxicity of the species: Aspergillus ochraceus, Aspergillus niger and Aspergillus flavus isolated from the air of hospital wards. Int. J. Occup. Med. Environ. Health 2017, 30, 231–239. [Google Scholar] [CrossRef]
- Smith, J.E.; Anderson, J.G.; Lewis, C.W.; Murad, Y.M. Cytotoxic fungal spores in the indoor atmosphere of the damp domestic environment. FEMS Microbiol. Lett. 1992, 100, 337–343. [Google Scholar] [CrossRef]
- Gniadek, A.; Krzyściak, P.; Hawryszuk, A.; Macura, A.B.; Brzostek, T.; Składzień, J. Mycobiota of the air in hospital rooms and the fungal colonisation of tracheostomy tubes used by patients diagnosed with larynx cancer–Preliminary research. Ann. Parasitol. 2013, 59, 69–71. [Google Scholar]
- Kamei, K.; Watanabe, A.; Nishimura, K.; Miyaji, M. Cytotoxicity of Aspergillus fumigatus culture filtrate against macrophages. Nihon Ishinkin Gakkai Zasshi 2002, 43, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, R.; Basnet, B.B.; Bao, L.; Han, J.; Wang, L.; Liu, H. New phenolic bisabolane sesquiterpenoid derivatives with cytotoxicity from Aspergillus tennesseensis. J. Antibiot. 2018, 71, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.; Melo, A.; Dias, M.; Almeida, B.; Caetano, L.A.; Veríssimo, C.; Viegas, C.; Sabino, R. Azole-Resistant Aspergillus fumigatus Harboring the TR34/L98H Mutation: First Report in Portugal in Environmental Samples. Microorganisms 2021, 9, 57. [Google Scholar] [CrossRef] [PubMed]

| Source | MEA | DG18 | SAB | ITR | VOR | POS | Total |
|---|---|---|---|---|---|---|---|
| FRPD-IL | 24 | 11 | 22 | 2 | 2 | 0 | 61 |
| FRPD-EV | 21 | 14 | 13 | 2 | 0 | 0 | 50 |
| MPG | 8 | 1 | 13 | 0 | 0 | 0 | 22 |
| TOTAL | 53 | 26 | 48 | 4 | 2 | 0 | 133 |
| Dilution Step | IC50 | A549 | SK |
|---|---|---|---|
| N | N | ||
| 1 | 31.250 cm2/mL | 1 | 2 |
| 2 | 15.625 cm2/mL | 1 | 2 |
| 3 | 7.813 cm2/mL | 4 | 3 |
| 4 | 3.906 cm2/mL | 1 | 3 |
| 5 | 1.953 cm2/mL | 0 | 3 |
| 6 | 0.977 cm2/mL | 2 | 6 |
| 7 | 0.488 cm2/mL | 3 | 11 |
| 8 | 0.244 cm2/mL | 4 | 20 |
| 9 | 0.122 cm2/mL | 27 | 31 |
| 10 | 0.061 cm2/mL | 48 | 52 |
| 11 | 3.050 mm2/mL | 25 | 0 |
| 12 | 1.525 mm2/mL | 10 | 0 |
| 13 | 0.7625 mm2/mL | 3 | 0 |
| 14 | 0.3812 mm2/mL | 2 | 0 |
| 15 | 0.1906 mm2/mL | 1 | 0 |
| 16 | 0.0953 mm2/mL | 1 | 0 |
| IC50 in the Sample | IC50 Aspergillus Section Fumigati Isolates | |||
|---|---|---|---|---|
| SK | A549 | SK | ||
| IC50 in the sample | A549 | 0.667 | −0.339 * | −0.157 |
| SK | 0.247 | −0.128 | ||
| IC50 Aspergillus section Fumigati isolates | A549 | 0.076 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viegas, C.; Twarużek, M.; Dias, M.; Carolino, E.; Soszczyńska, E.; Aranha Caetano, L. Cytotoxicity of Aspergillus Section Fumigati Isolates Recovered from Protection Devices Used on Waste Sorting Industry. Toxins 2022, 14, 70. https://doi.org/10.3390/toxins14020070
Viegas C, Twarużek M, Dias M, Carolino E, Soszczyńska E, Aranha Caetano L. Cytotoxicity of Aspergillus Section Fumigati Isolates Recovered from Protection Devices Used on Waste Sorting Industry. Toxins. 2022; 14(2):70. https://doi.org/10.3390/toxins14020070
Chicago/Turabian StyleViegas, Carla, Magdalena Twarużek, Marta Dias, Elisabete Carolino, Ewelina Soszczyńska, and Liliana Aranha Caetano. 2022. "Cytotoxicity of Aspergillus Section Fumigati Isolates Recovered from Protection Devices Used on Waste Sorting Industry" Toxins 14, no. 2: 70. https://doi.org/10.3390/toxins14020070
APA StyleViegas, C., Twarużek, M., Dias, M., Carolino, E., Soszczyńska, E., & Aranha Caetano, L. (2022). Cytotoxicity of Aspergillus Section Fumigati Isolates Recovered from Protection Devices Used on Waste Sorting Industry. Toxins, 14(2), 70. https://doi.org/10.3390/toxins14020070








