Spent Coffee Grounds Valorization as Bioactive Phenolic Source Acquired Antifungal, Anti-Mycotoxigenic, and Anti-Cytotoxic Activities
Abstract
:1. Introduction
2. Results
2.1. Characterization of SCGs Phenolic and Flavonoids
2.2. Cytotoxic Impact of SCGs Isopropanol Extract
2.3. Isopropanol Bacterial (Diffusion Assay)
- The results are represented as means ± SEM, where (n = 3; (p ≤ 0.05); SEM: standard error means);
- IDZ: inhibition diameter of zone record. LSD: (0.577); letters are significantly different at p ≤ 0.05 level: capitals for disk diffusion and small for the well diffusion assays.
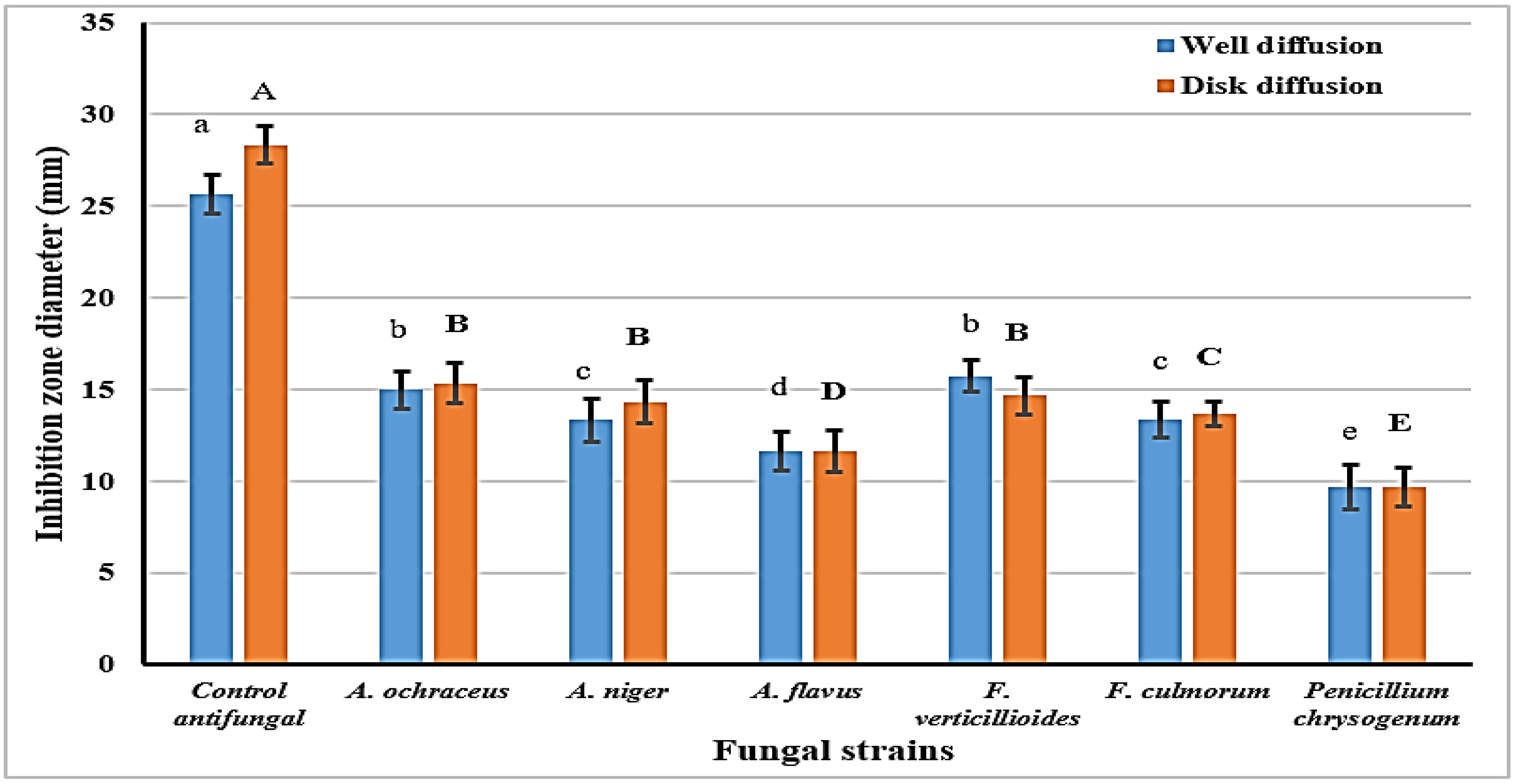
2.4. Isopropanol Extract Antifungal (Diffusion Assay)
- Results are represented as means ± SEM, (n = 3; p ≤ 0.05; LSD-0.701; SEM: standard error means).
- Letters are significantly different at p ≤ 0.05 level: capitals for disk diffusion and small for the well diffusion assays.
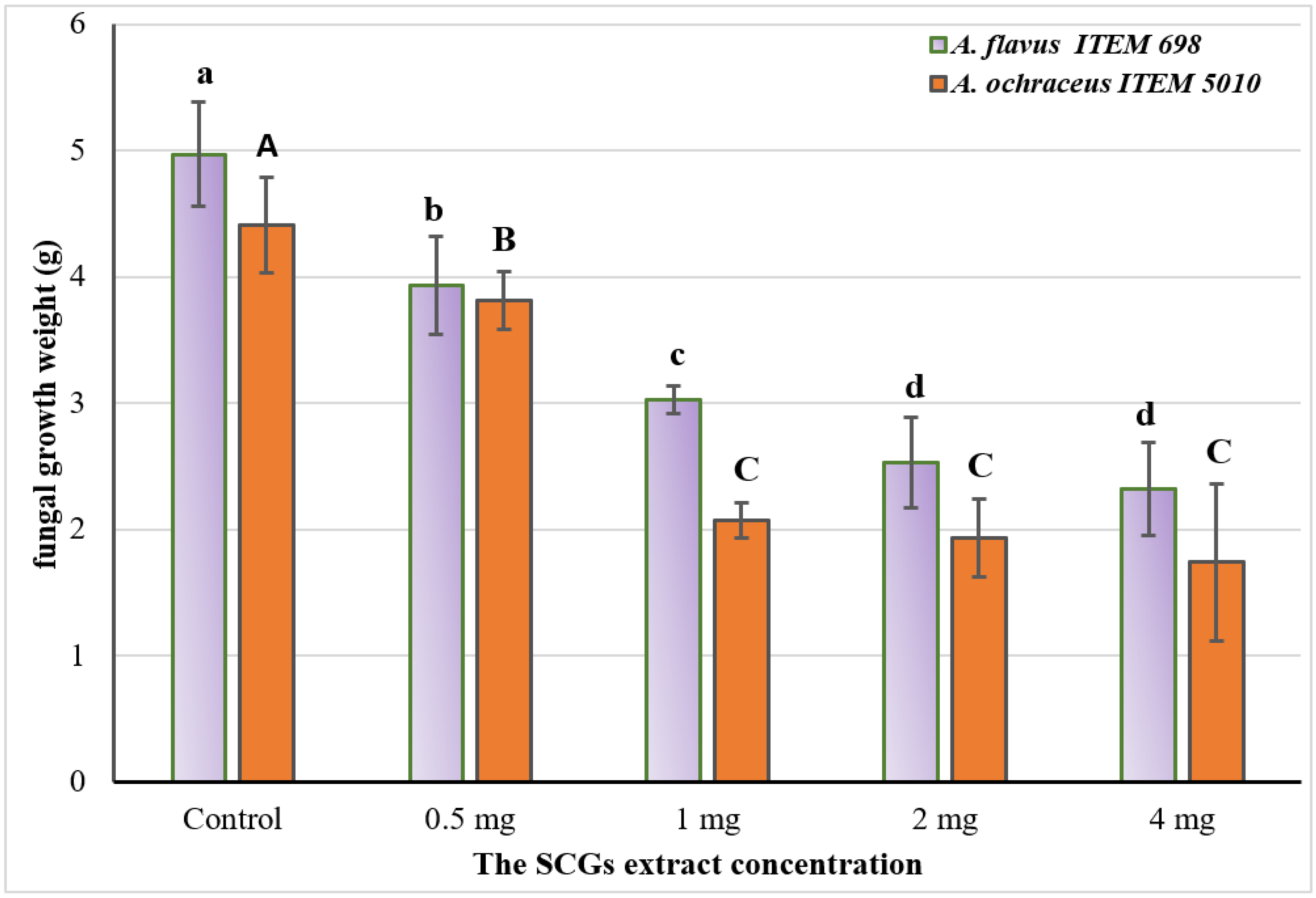
2.5. Simulation Growth Inhibition for Fungal-Producing Toxin Strains on Liquid Media
- The results are represented as means ± SEM, where (n = 3; p ≤ 0.05); LSD = 0.397; SEM: standard error means).
- Values labeled with different letters are significantly different at p ≤ 0.05 level: capitals for A. ochraceus and smalls for A. flavus fungi. Upper and lower cases are for significant response of each strain to the treatment.
2.6. Simulation Degradation of Aflatoxins and Ochratoxin Production on Liquid Media
2.7. Application of the Spent on Brownies
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials, Chemicals, and Microorganisms
5.2. Preparation of Spent Coffee Extract
5.3. Determination of Phenolic Acids and Flavonoids
5.4. Determination of Antibacterial and Antifungal Effects
5.5. Determination of SCGs Cytotoxic Effect Using the Tetrazolium-Based (MTT) Assay
- where; OD sample: optical density of the sample.
- OD blank: optical density of the blank (DMSO).
- OD control: optical density of the control.
5.6. Determination of the SCGs Cytotoxic Effect Using Sulforhodamine B (SRB) Assay
5.7. Selectivity Index (SI)
5.8. Simulated Experiment to Evaluate the Anti-Mycotoxigenic Impact
5.9. Application of Spent Coffee in Brownies for Ochratoxin A Evaluation
5.10. Mycotoxin Determination
5.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, P.R.; Fawcett, D.; Sharma, S.B.; Poinern, G.E.J. Progress towards Sustainable Utilization and Management of Food Wastes in the Global Economy. Int. J. Food Sci. 2016, 2016, 3563478. [Google Scholar] [CrossRef] [Green Version]
- Adejumo, I.O.; Adebiyi, O.A. Agricultural Solid Wastes: Causes, Effects, and Effective Management. In Strategies of Sustainable Solid Waste Management; Saleh, H., Ed.; IntechOpen: London, UK, 2021; pp. 1–19. [Google Scholar] [CrossRef]
- Wu, C.-T.; Agrawal, D.C.; Huang, W.-Y.; Hsu, H.-C.; Yang, S.-J.; Huang, S.-L.; Lin, Y.-S. Functionality Analysis of Spent Coffee Ground Extracts Obtained by the Hydrothermal Method. J. Chem. 2019, 2019, 4671438. [Google Scholar] [CrossRef] [Green Version]
- ICO. ICO, the Coffee Market Report, in Monthly Coffee Market Report (2021/22); ICO: London, UK, 2021; pp. 1–9. Available online: https://www.ico.org/trade_statistics.asp (accessed on 28 February 2021).
- Mitraka, G.-C.; Kontogiannopoulos, K.N.; Batsioula, M.; Banias, G.F.; Assimopoulou, A.N. Spent Coffee Grounds’ Valorization towards the Recovery of Caffeine and Chlorogenic Acid: A Response Surface Methodology Approach. Sustainability 2021, 13, 8818. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of spent coffee grounds: A review. Food Bioprod. Processing 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef] [Green Version]
- López-Barrera, D.M.; Vázquez-Sánchez, K.; Loarca-Piña, M.G.F.; Campos-Vega, R. Spent coffee grounds, an innovative source of colonic fermentable compounds, inhibit inflammatory mediators in vitro. Food Chem. 2016, 212, 282–290. [Google Scholar] [CrossRef]
- Huang, H.-C.; Wei, C.-M.; Siao, J.-H.; Tsai, T.-C.; Ko, W.-P.; Chang, K.-J.; Hii, C.-H.; Chang, T.-M. Supercritical Fluid Extract of Spent Coffee Grounds Attenuates Melanogenesis through Downregulation of the PKA, PI3K/Akt, and MAPK Signaling Pathways. eCAM 2016, 2016, 5860296. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Pujo, D.; Olivella, M.À.; de la Torre, F.; Fiol, N.; Poch, J.; Villaescusa, I. The Role of Exhausted Coffee Compounds on Metal Ions Sorption. Water Air Soil Pollut. 2015, 226, 289. [Google Scholar] [CrossRef]
- Seo, H.S.; Park, B.H. Phenolic compound extraction from spent coffee grounds for antioxidant recovery. Korean J. Chem. Eng. 2019, 36, 186–190. [Google Scholar] [CrossRef]
- Karahalil, E. Principles of halal-compliant fermentations: Microbial alternatives for the halal food industry. Trends Food Sci. Technol. 2020, 98, 1–9. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilke, M.A.; Robert, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential Use of Phenolic Acids as Anti-Candida Agents: A Review. Front. Microbiol. 2015, 6, 1420. [Google Scholar] [CrossRef] [Green Version]
- Evensen, N.A.; Braun, P.C. The effects of tea polyphenols on Candida albicans: Inhibition of biofilm formation and proteasome inactivation. Can. J. Microbiol. 2009, 55, 1033–1039. [Google Scholar] [CrossRef]
- Shahzad, M.; Sherry, L.; Rajendran, R.; Edwards, C.A.; Combet, E.; Ramage, G. Utilizing polyphenols for the clinical management of Candida albicans biofilms. Int. J. Antimicrob. Agents 2014, 44, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Powers, C.N.; Satyal, P.; Mayo, J.A.; McFeeters, H.; McFeeters, R.L. Bigger Data Approach to Analysis of Essential Oils and Their Antifungal Activity against Aspergillus niger, Candida albicans, and Cryptococcus Neoformans. Molecules 2019, 24, 2868. [Google Scholar] [CrossRef] [Green Version]
- Makhuvele, R.; Naidu, K.; Gbashi, S.; Thipe, V.C.; Adebo, O.A.; Njobeh, P.B. The use of plant extracts and their phytochemicals for control of toxigenic fungi and mycotoxins. Heliyon 2020, 6, e05291. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Nepovimova, E.; Wang, Y.; Yang, H.; Li, L.; Zhang, X.; Kuca, K. Antioxidant agents against trichothecenes: New hints for oxidative stress treatment. Oncotarget 2017, 8, 110708–110726. [Google Scholar] [CrossRef] [Green Version]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant Bioactive Compounds in Pre- and Postharvest Management for Aflatoxins Reduction. Front. Microbiol. 2020, 11, 243. [Google Scholar] [CrossRef]
- Wong, S.S.W.; Kao, R.Y.T.; Yuen, K.Y.; Wang, Y.; Yang, D.; Samaranayake, L.P.; Seneviratne, C.J. In Vitro and In Vivo Activity of a Novel Antifungal Small Molecule against Candida Infections. PLoS ONE 2014, 9, e85836. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ami, R.; Garcia-Effron, G.; Lewis, R.E.; Gamarra, S.; Leventakos, K.; Perlin, D.S.; Kontoyiannis, D.P. Fitness and Virulence Costs of Candida albicans FKS1 Hot Spot Mutations Associated with Echinocandin Resistance. J. Infect. Dis. 2011, 204, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Clancy, C.J.; Nguyen, M.H. At What Cost Echinocandin Resistance? J. Infect. Dis. 2011, 204, 499–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negri, M.; Salci, T.P.; Shinobu-Mesquita, C.S.; Capoci, I.R.G.; Svidzinski, T.I.E.; Kioshima, E.S. Early State Research on Antifungal Natural Products. Molecules 2014, 19, 2925–2956. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ahn, D.U.; Eu, J.B.; Moo, S.H. Antioxidant Effect of Extracts from the Coffee Residue in Raw and Cooked Meat. Antioxidants 2016, 5, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murthy, P.S.; Naidu, M.M. Recovery of Phenolic Antioxidants and Functional Compounds from Coffee Industry By-Products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Smiljkovic, M.; Stanisavljevic, D.; Stojkovic, D.; Petrovic, I.; Vicentic, J.M.; Popovic, J.; Grdadolnik, S.G.; Markovic, D.; Sankovic-Babice, S.; Glamoclija, J.; et al. Apigenin-7-O-glucoside versus apigenin: Insight into the modes of anticandidal and cytotoxic actions. EXCLI J. 2017, 16, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Güez, C.M.; de Souza, R.O.; Fischer, P.; Leão, M.F.M.; Duarte, J.A.; Boligon, A.A.; Athayd, M.L.; Zuravsk, L.; de Oliveir, L.F.S.; Machado, M.M. Evaluation of basil extract (Ocimum basilicum L.) on oxidative, anti-genotoxic, and anti-inflammatory effects in human leukocytes cell cultures exposed to challenging agents. Braz. J. Pharm. Sci. 2017, 53, e15098. [Google Scholar] [CrossRef] [Green Version]
- Hall, S.; Desbrow, B.; Anoopkumar-Dukie, S.; Davey, A.K.; Arora, D.; McDermott, C.; Schubert, M.M.; Perkins, A.V.; Kiefel, M.J.; Grant, G.D. A review of the bioactivity of coffee, caffeine and key coffee constituents on inflammatory responses linked to depression. Food Res. Int. 2015, 76, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, C.; Beaman, H.T.; Monroe, M.B.B. Characterization of Phenolic Acid Antimicrobial and Antioxidant Structure-Property Relationships. Pharmaceutics 2020, 12, 419. [Google Scholar] [CrossRef]
- Ouerghemmi, I.; Rebey, I.B.; Rahali, F.Z.; Bourgou, S.; Pistelli, L.; Ksouri, R.; Marzouk, B.; Tounsi, M.S. Antioxidant and antimicrobial phenolic compounds from extracts of cultivated and wild-grown Tunisian Ruta chalepensis. J. Food Drug Anal. 2017, 25, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Mandal, S.M.; Dias, R.O.; Franco, O.L. Phenolic Compounds in Antimicrobial Therapy. J. Med. Food 2017, 20, 1031–1038. [Google Scholar] [CrossRef]
- Badr, A.N.; Ali, H.S.; Abdel-Razek, A.G.; Shehata, M.G.; Albaridi, N.A. Bioactive Components of Pomegranate Oil and Their Influence on Mycotoxin Secretion. Toxins 2020, 12, 748. [Google Scholar] [CrossRef] [PubMed]
- Shahat, M.S.; Badr, A.N.; Hegaziy, A.I.; Ramzy, S.; Samie, M.A. Reducing the histopathological and biochemical toxicity of aflatoxins contaminated soybean using ozone treatment. Ann. Res. Rev. Biol. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Yu, J.J. Genetics and biochemistry of mycotoxin synthesis. Fungal Biotechnol. Agric. Food Environ. Appl. 2004, 2004, 343–361. [Google Scholar]
- Safari, N.; Ardakani, M.M.; Hemmati, R.; Parroni, A.; Beccaccioli, M.; Reverberi, M. The Potential of Plant-Based Bioactive Compounds on Inhibition of Aflatoxin B1 Biosynthesis and Down-regulation of aflR, aflM, and aflP Genes. Antibiotics 2020, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, N.; Molyneux, R.J. Phytochemical Inhibition of Aflatoxigenicity in Aspergillus flavus by Constituents of Walnut (Juglans regia). J. Agri. Food Chem. 2004, 52, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, A.G.; Badr, A.N.; Alharthi, S.S.; Selim, K.A. Efficacy of Bottle Gourd Seeds’ Extracts in Chemical Hazard Reduction Secreted as Toxigenic Fungi Metabolites. Toxins 2021, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Gigliobianco, M.R.; Campisi, B.; Peregrina, D.V.; Censi, R.; Khamitova, G.; Angeloni, S.; Caprioli, G.; Zannotti, M.; Ferraro, S.; Giovannetti, R.; et al. Optimization of the Extraction from Spent Coffee Grounds Using the Desirability Approach. Antioxidants 2020, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Bułdak, R.J.; Hejmo, T.; Osowski, M.; Bułdak, Ł.; Kukl, M.; Polania, R.; Birkner, E. The Impact of Coffee and Its Selected Bioactive Compounds on the Development and Progression of Colorectal Cancer In Vivo and In Vitro. Molecules 2018, 23, 3309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Salam, A.M.; ABadr, N.; Zaghloul, A.H.; Farrag, A.R.H. Functional yogurt aims to protect against the aflatoxin B1 toxicity in rats. Toxicol. Rep. 2020, 7, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Stuper-Szablewska, K.; Kurasiak-Popowska, D.; Nawracała, J.; Perkowski, J. Response of non-enzymatic antioxidative mechanisms to stress caused by infection with Fusarium fungi and chemical protection in different wheat genotypes. Chem. Ecol. 2017, 33, 949–962. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Badr, A.N.; El-Messery, T.M.; El-Said, M.M.; Hussein, A.M.S. Micro-nano encapsulation of black seed oil ameliorate its characteristics and its mycotoxin inhibition. Biosci. Res. 2018, 15, 2591–2601. [Google Scholar]
- Aouf, A.; Ali, H.; Al-Khalifa, A.R.; Mahmoud, K.F.; Farouk, A. Influence of Nanoencapsulation Using High-Pressure Homogenization on the Volatile Constituents and Anticancer and Antioxidant Activities of Algerian Saccocalyx satureioides Coss. et Durieu. Molecules 2020, 25, 4756. [Google Scholar] [CrossRef] [PubMed]
- El-Massry, K.F.; Farouk, A.; Mahmoud, K.F.; El-Ghorab, A.H.; M, S.S.; Musa, A.; Mostafa, E.M.; Ghoneim, M.M.; Naguib, I.A.; Abdelgawad, M.A. Chemical characteristics and targeted encapsulated Cordia myxa fruits extracts nanoparticles for antioxidant and cytotoxicity potentials. Saudi J. Biol. Sci. 2021, 28, 5349–5358. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, J.C.; Naesens, L.; Montoya, J. Treating HHV-6 Infections: The Laboratory Efficacy and Clinical Use of Anti-HHV-6 Agents. In Human Herpesviruses HHV-6A, HHV-6B & HHV-7, 3rd ed.; Flamand, L., Lautenschlager, I., Krueger, G., Ablashi, D., Eds.; Elsevier: Boston, MA, USA, 2014; pp. 311–331. [Google Scholar] [CrossRef]
- Badr, A.N.; Gromadzka, K.; Shehata, M.G.; Stuper-Szablewska, K.; Drzewiecka, K.; Abdel-Razek, A.G.; Youssef, M.M. Encapsulated Bioactive Ingredients of grape by-products applicate in fresh-cut fruit and juices diminished the ochratoxins. J. Food Process. Preserv. 2021, 45, e15112. [Google Scholar] [CrossRef]

| Phenolic Acid Contents of the SCGs Extract | |||
| Compound | Quantities (µg/g) | Compound | Quantities (µg/g) |
| Gallic acid | 18.15 ± 0.63 | Sinapic acid | 17.05 ± 0.54 |
| Protocatechuic acid | 0.82 ± 0.04 | (S)-(-)-rosmarinic acid | 0.92 ± 0.02 |
| p-Hydroxybenzoic acid | 3.65 ± 0.09 | Ferulic acid | 3.64 ± 0.17 |
| Gentisic acid | 8.6 ± 0.13 | Salicylic acid | 12.67 ± 0.27 |
| Chlorogenic acid | 7.38 ± 0.31 | p-coumaric acid | 0.19 ± 0.03 |
| Caffeic acid | 7.2 ± 0.73 | Cinnamic acid | 0.495 ± 0.06 |
| Syringic acid | 64.14 ± 0.83 | (R)-(+)-rosmarinic acid | 176.43 ± 1.27 |
| Vanillic acid | 0.47 ± 0.06 | - | - |
| Flavonoid Contents of the SCGs Extract | |||
| Compound | Quantities (µg/g) | Compound | Quantities (µg/g) |
| Catechin | 16.86 ± 0.27 | Quercetin | 1.38 ± 0.05 |
| Epicatechin | 53.83 ± 1.02 | Apigenin-7-glucoside | 1717.04 ± 3.54 |
| Rutin | 4.87 ± 0.11 | Kaempferol | 3.18 ± 0.41 |
| Naringin | 74.49 ± 0.69 | Chrysin | 1.34 ± 0.18 |
| Alkaloid Contents of the SCGs Extract | |||
| Compound | (µg/g) | ||
| Caffeine | 208.93 ± 2.05 | ||
| Extract | Cell Lines | IC50 (μg/mL) | SI |
|---|---|---|---|
| Cisplatin | HepG2 | 66.69 | 1.3 |
| OEC | 87.69 | - | |
| Isoprpanol extract (MTT) | HepG2 | 112 | 1.2 |
| OEC | 133.7 | - | |
| Isopropanol extract (SRB) | HepG2 | 94.03 | 1.85 |
| OEC | 174.1 | - |
| Concentration | AFB1 | AFB2 | AFG1 | AFG2 | OCA |
|---|---|---|---|---|---|
| Control | 258.0 ±1 4.55 a | 184.0 ± 15.17 a | 208.3 ± 14.82 a | 175.7 ± 12.37 a | 910.7 ± 16.77 a |
| 0.5 | 194.3 ± 13.09 b | 138.3 ± 13.81 b | 174.3 ± 14.26 b | 148.0 ± 12.19 b | 612.6 ± 17.05 b |
| 1 mg | 141.4 ± 13.14 c | 103.7 ± 13.69 c | 118.3 ± 13.37 c | 94.6 ± 10.57 c | 257.7 ± 15.22 c |
| 2 mg | 132.0 ± 12.88 cd | 99.0 ± 11.74 cd | 103.6 ± 12.07 d | 86.3 ± 11.77 cd | 226.8 ± 14.14 d |
| 4 mg | 119.3 ± 16.23 d | 88.3 ± 11.81 d | 98.7 ± 12.54 d | 79.0 ± 11.41 d | 201.0 ± 13.79 e |
| Control | 1 mg * | 3 mg * | 5 mg * | 7 mg * | 10 mg * | |
|---|---|---|---|---|---|---|
| Zero-time | 850 ± 13.14 a | 837 ± 18.54 a | 817 ± 17.63 a | 811 ± 19.05 a | 796 ± 17.49 a | 779 ± 15.17 a |
| 2 h incubation | 852 ± 14.56 a | 742 ± 13.79 b | 711 ± 19.13 b | 671 ± 18.21 b | 524 ± 17.71 b | 319 ± 27.64 b |
| 4 h incubation | 851 ± 12.81 a | 737 ± 11.27 b | 691 ± 16.23 b | 588 ± 28.34 c | 502 ± 26.25 b | 307 ± 37.57 b |
| After cooking | 836 ± 17.37 a | 731 ± 10.91 b | 689 ± 15.84 b | 574 ± 27.77 c | 495 ± 26.84 b | 282 ± 33.93 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badr, A.N.; El-Attar, M.M.; Ali, H.S.; Elkhadragy, M.F.; Yehia, H.M.; Farouk, A. Spent Coffee Grounds Valorization as Bioactive Phenolic Source Acquired Antifungal, Anti-Mycotoxigenic, and Anti-Cytotoxic Activities. Toxins 2022, 14, 109. https://doi.org/10.3390/toxins14020109
Badr AN, El-Attar MM, Ali HS, Elkhadragy MF, Yehia HM, Farouk A. Spent Coffee Grounds Valorization as Bioactive Phenolic Source Acquired Antifungal, Anti-Mycotoxigenic, and Anti-Cytotoxic Activities. Toxins. 2022; 14(2):109. https://doi.org/10.3390/toxins14020109
Chicago/Turabian StyleBadr, Ahmed Noah, Marwa M. El-Attar, Hatem S. Ali, Manal F. Elkhadragy, Hany M. Yehia, and Amr Farouk. 2022. "Spent Coffee Grounds Valorization as Bioactive Phenolic Source Acquired Antifungal, Anti-Mycotoxigenic, and Anti-Cytotoxic Activities" Toxins 14, no. 2: 109. https://doi.org/10.3390/toxins14020109
APA StyleBadr, A. N., El-Attar, M. M., Ali, H. S., Elkhadragy, M. F., Yehia, H. M., & Farouk, A. (2022). Spent Coffee Grounds Valorization as Bioactive Phenolic Source Acquired Antifungal, Anti-Mycotoxigenic, and Anti-Cytotoxic Activities. Toxins, 14(2), 109. https://doi.org/10.3390/toxins14020109







