Botulinum Neurotoxin Therapy in the Clinical Management of Laryngeal Dystonia
Abstract
1. Introduction
1.1. Pathophysiology
1.2. Mechanism of Action of Botulinum Toxin in LD
2. Clinical Presentation of Laryngeal Dystonia
2.1. Classification of Laryngeal Dystonia
2.2. Clinical Assessment and Diagnosis
2.3. The Role of Laryngeal Electromyography (LEMG)
3. Clinical Application of Botulinum Toxin in Laryngeal Dystonia
3.1. Adductor Spasmodic Dysphonia (ADLD)
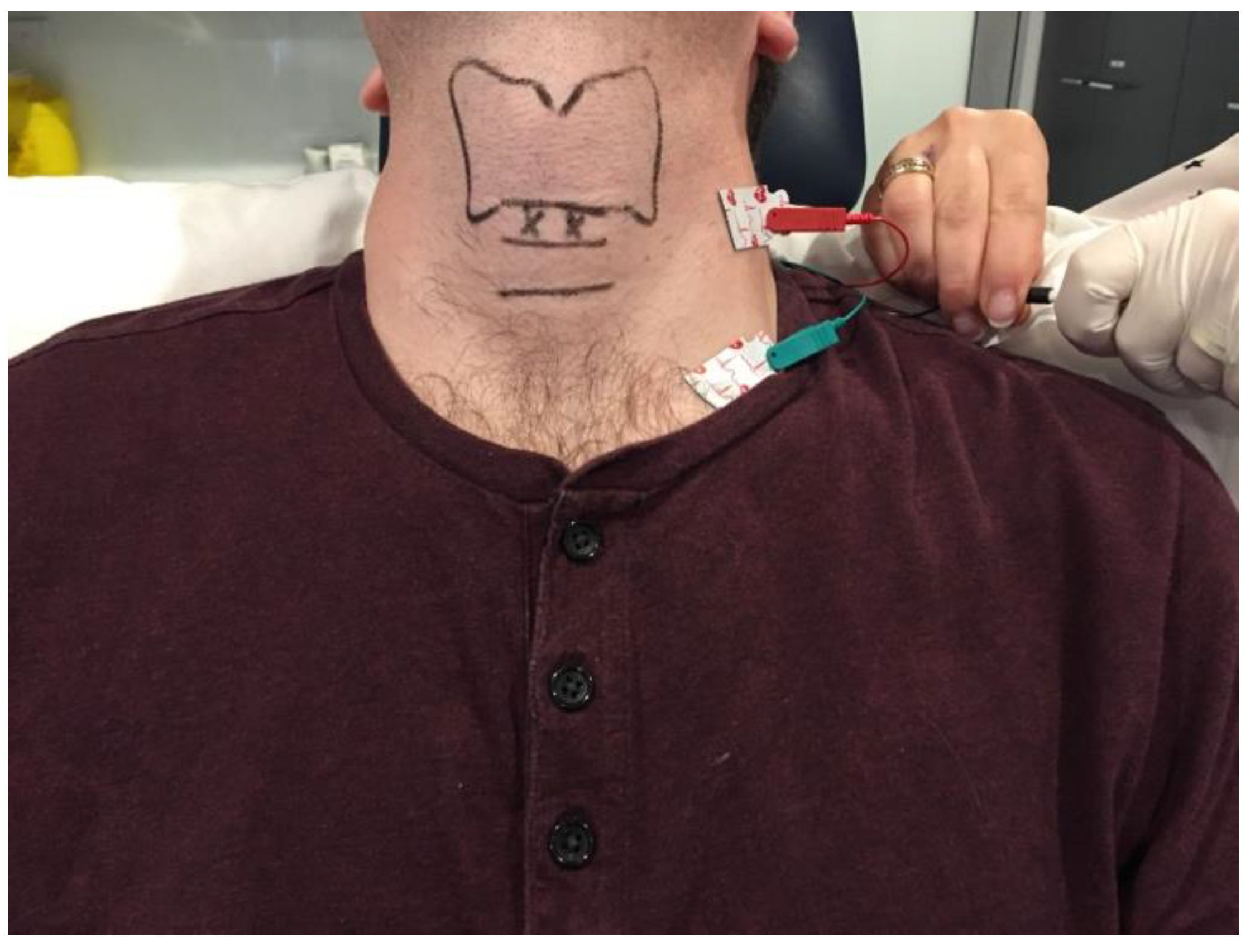
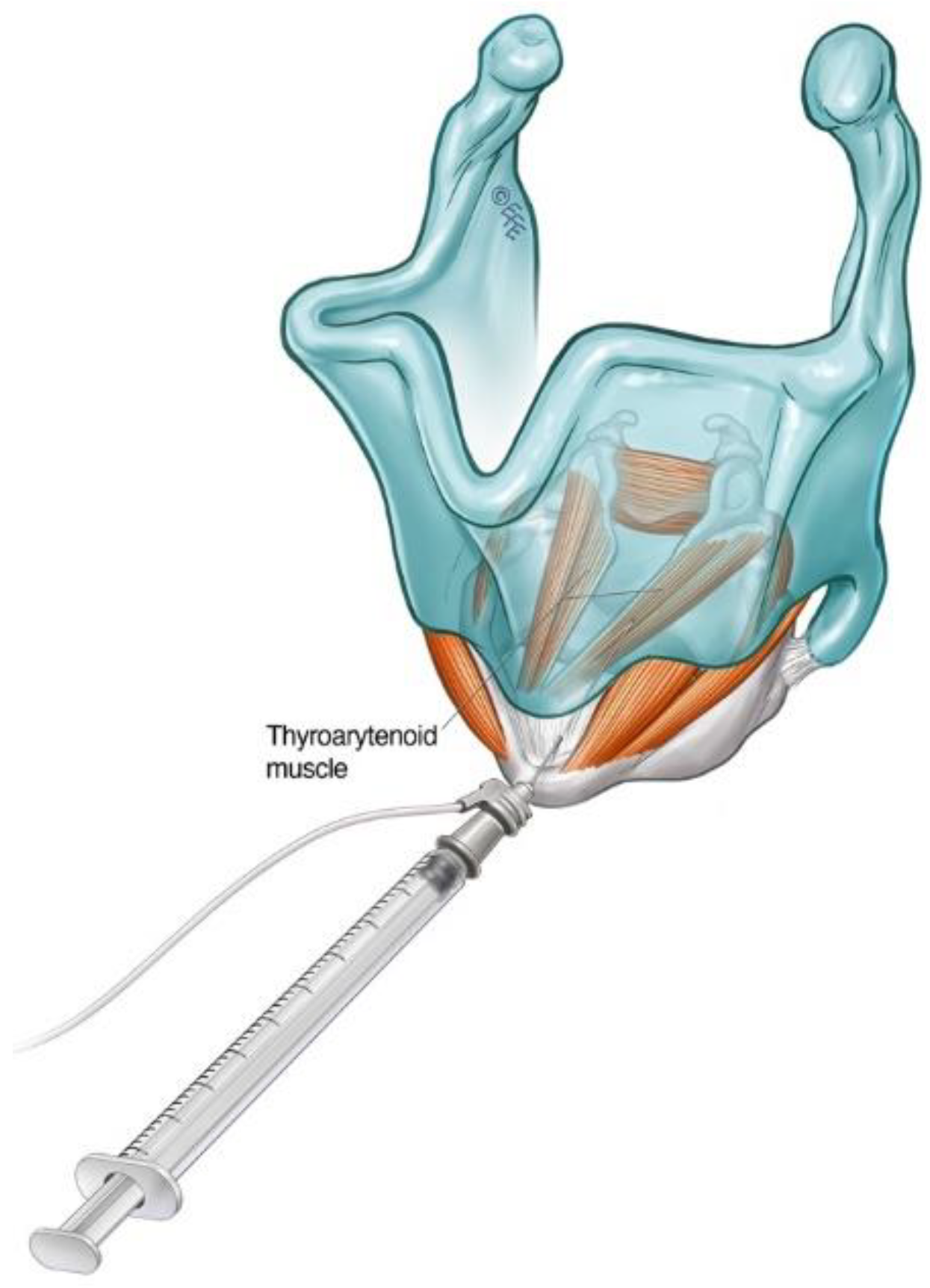
3.1.1. Cricothyroid Membrane Approach to Adductor Muscle Complex
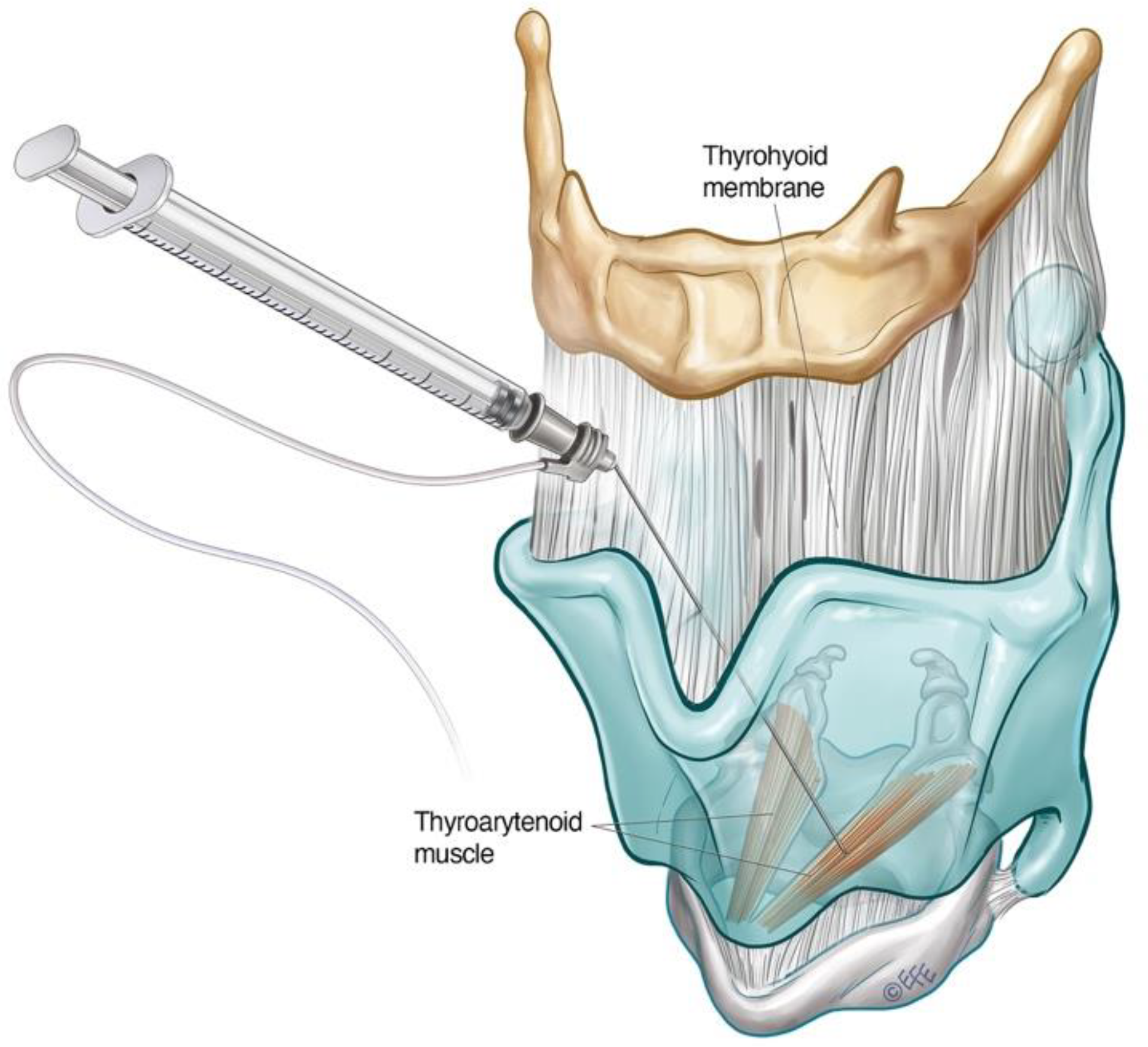
3.1.2. Transthyrohyoid Approaches to the Larynx
3.1.3. Interarytenoid BoNT-A Injections for ADLD
3.1.4. Dosing and Laterality Considerations in ADLD
3.2. Abductor Spasmodic Dysphonia (ABLD)
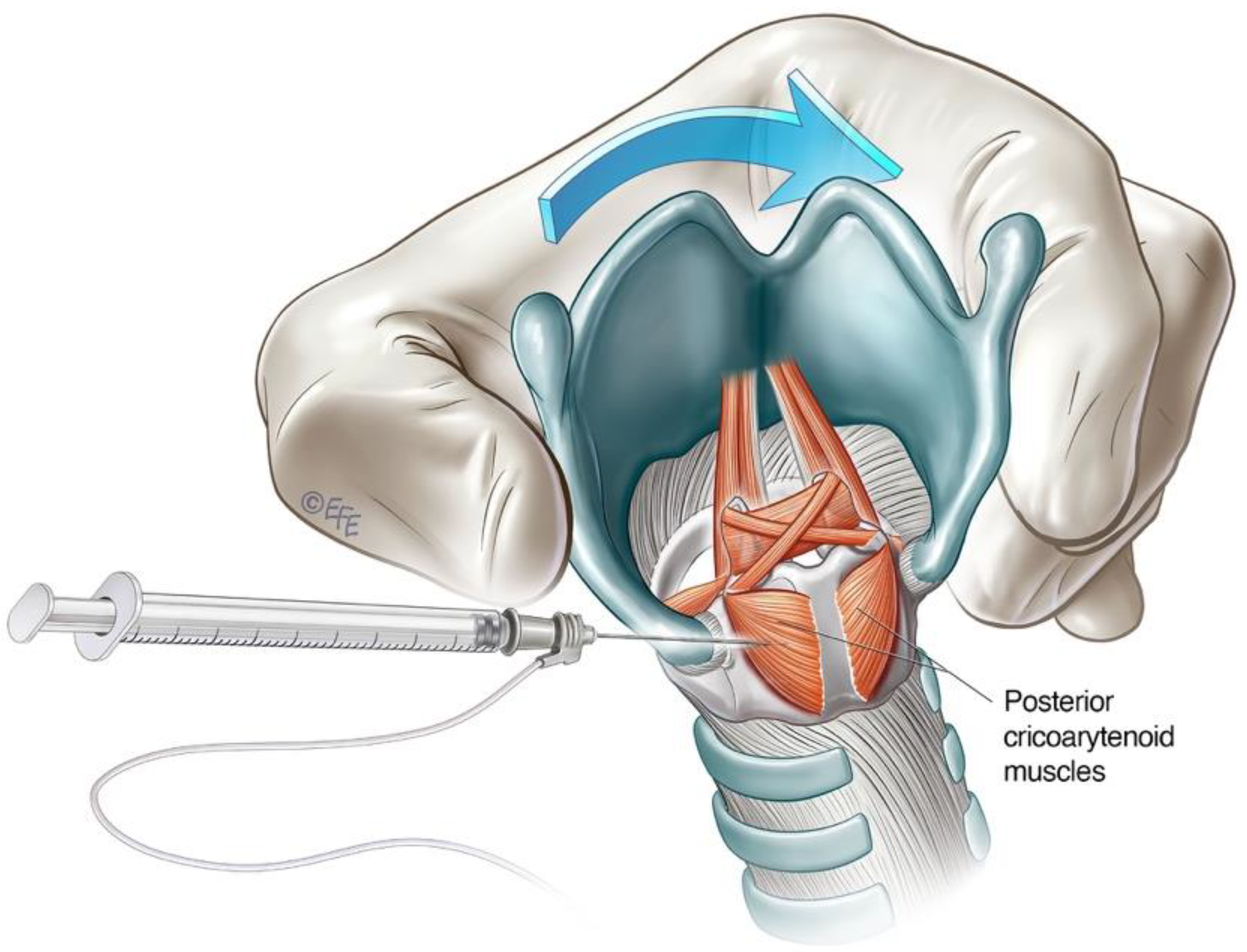
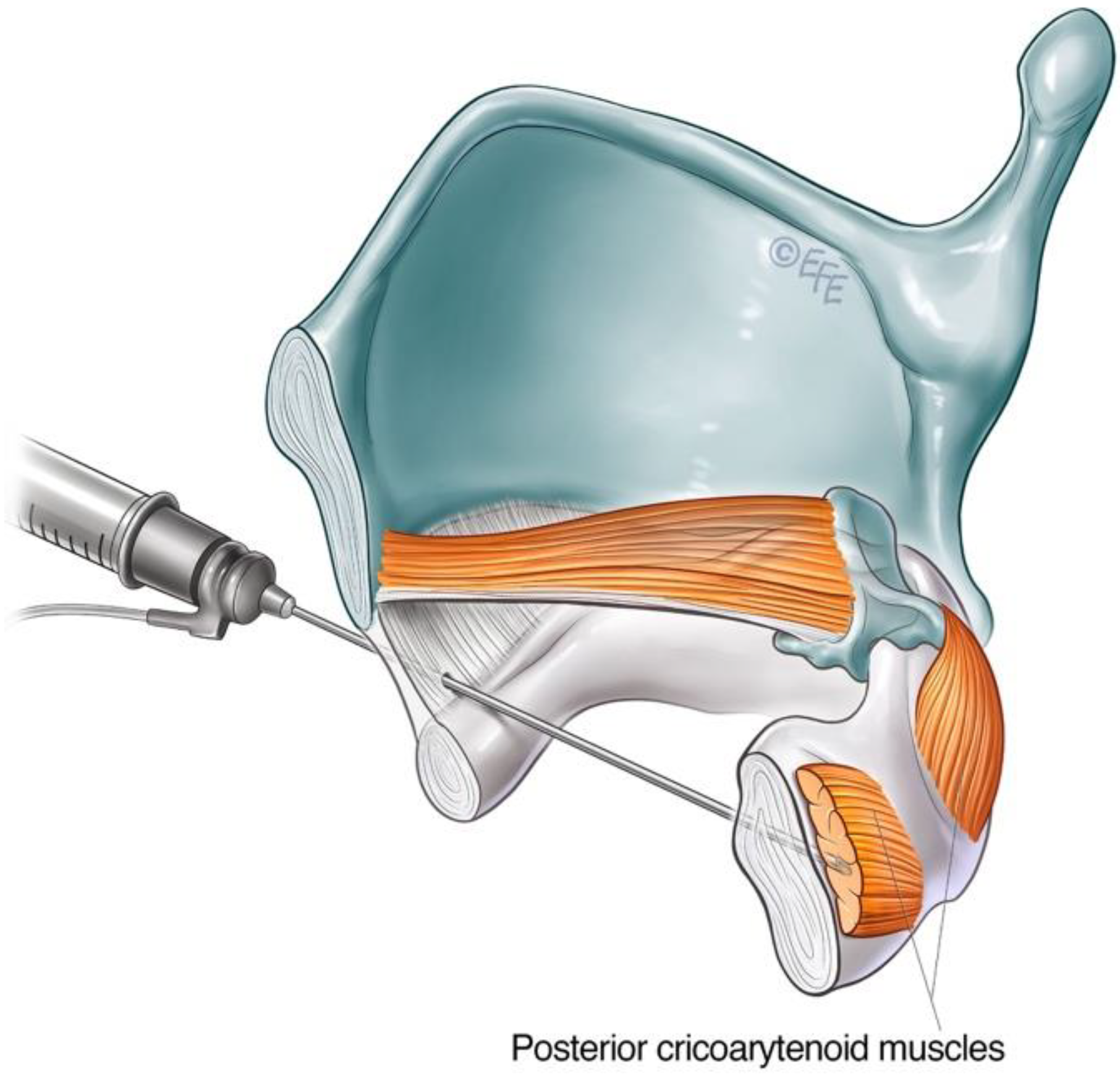
3.2.1. Lateral Rotation Approach to PCA Muscle
3.2.2. Anterior Trans-Airway Approach to PCA Muscle
3.2.3. Cricothyroid (CT) Muscle Injection for ABLD
3.2.4. Dosing and Laterality Considerations for ABLD
3.3. Mixed Laryngeal Dystonia (Mixed LD)
3.4. Other Types of Laryngeal Dystonia
3.4.1. Adductor Laryngeal Breathing Dystonia (ALBD)
3.4.2. Singer’s Dystonia
4. Adverse Effects and Development of Resistance to BoNT-A
5. Assessment of Treatment Outcomes
6. Discussion and Future Perspectives
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BoNT | Botulinum neurotoxin |
| ABLD | Abductor laryngeal dystonia |
| ADLD | Adductor laryngeal dystonia |
| ALBD | Adductor laryngeal breathing dystonia |
| LD | Laryngeal dystonia |
| RLN | Recurrent laryngeal nerve |
| SLN | Superior laryngeal nerve |
| LEMG | Laryngeal electromyography |
| CNS | Central nervous system |
References
- Lorch, M.P.; Whurr, R. Tracing Spasmodic Dysphonia: The Source of Ludwig Traube's Priority. Ann. Otol. Rhinol. Laryngol. 2016, 125, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.; Brin, M.F.; Stewart, C.F. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): A 12-year experience in more than 900 patients. Laryngoscope 1998, 108, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Brin, M.F.; Blitzer, A.; Stewart, C. Laryngeal dystonia (spasmodic dysphonia): Observations of 901 patients and treatment with botulinum toxin. Adv. Neurol. 1998, 78, 237–252. [Google Scholar] [PubMed]
- Simonyan, K.; Barkmeier-Kraemer, J.; Blitzer, A.; Hallett, M.; Houde, J.F.; Jacobson Kimberley, T.; Ozelius, L.J.; Pitman, M.J.; Richardson, R.M.; Sharma, N.; et al. Laryngeal Dystonia. Multidiscip. Update Terminol. Pathophysiol. Res. Priorities 2021, 96, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, M.; Hisa, Y.; Nishizawa, N.; Omori, K.; Shiromoto, O.; Yumoto, E.; Sanuki, T.; Nagao, A.; Hirose, K.; Kobayashi, T.; et al. The prevalence and clinical features of spasmodic dysphonia: A review of epidemiological surveys conducted in Japan. Auris Nasus Larynx 2021, 48, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.G.; Duffey, P.O.; Hawthorne, M.R.; Barnes, M.P. An epidemiologic survey of dystonia within the entire population of northeast England over the past nine years. Adv. Neurol. 2004, 94, 95–99. [Google Scholar] [PubMed]
- Hintze, J.M.; Ludlow, C.L.; Bansberg, S.F.; Adler, C.H.; Lott, D.G. Spasmodic Dysphonia: A Review. Part 1: Pathogenic Factors. Otolaryngol. Head Neck Surg. 2017, 157, 551–557. [Google Scholar] [CrossRef]
- Blitzer, A.; Brin, M.F.; Simonyan, K.; Ozelius, L.J.; Frucht, S.J. Phenomenology, genetics, and CNS network abnormalities in laryngeal dystonia: A 30-year experience. Laryngoscope 2018, 128, S1–S9. [Google Scholar] [CrossRef]
- Blitzer, A.; Brin, M.F.; Fahn, S.; Lovelace, R.E. Clinical and laboratory characteristics of focal laryngeal dystonia: Study of 110 cases. Laryngoscope 1988, 98, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Soland, V.L.; Bhatia, K.P.; Marsden, C.D. Sex prevalence of focal dystonias. J. Neurol. Neurosurg. Psychiatry 1996, 60, 204–205. [Google Scholar] [CrossRef] [PubMed]
- Enver, N.; Pitman, M.J. What Is New in Laryngeal Dystonia: Review of Novel Findings of Pathophysiology and Novel Treatment Options. Curr. Otorhinolaryngol. Rep. 2020, 8, 209–215. [Google Scholar] [CrossRef]
- Schweinfurth, J.M.; Billante, M.; Courey, M.S. Risk factors and demographics in patients with spasmodic dysphonia. Laryngoscope 2002, 112, 220–223. [Google Scholar] [CrossRef] [PubMed]
- de Lima Xavier, L.; Simonyan, K. The extrinsic risk and its association with neural alterations in spasmodic dysphonia. Park. Relat. Disord. 2019, 65, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Brin, M.F.; Fahn, S.; Moskowitz, C.; Friedman, A.; Shale, H.M.; Greene, P.E.; Blitzer, A.; List, T.; Lange, D.; Lovelace, R.E.; et al. Localized injections of botulinum toxin for the treatment of focal dystonia and hemifacial spasm. Mov. Disord. 1987, 2, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.; Brin, M.F. Laryngeal Dystonia: A Series with Botulinum Toxin Therapy. Ann. Otol. Rhinol. Laryngol. 1991, 100, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.; Brin, M.F.; Stewart, C.; Aviv, J.E.; Fahn, S. Abductor laryngeal dystonia: A series treated with botulinum toxin. Laryngoscope 1992, 102, 163–167. [Google Scholar] [CrossRef]
- Robe, E.; Brumlik, J.; Moore, P. A study of spastic dysphonia. Neurologic and electroencephalographic abnormalities. Laryngoscope 1960, 70, 219–245. [Google Scholar] [CrossRef] [PubMed]
- Lungu, C.; Ozelius, L.; Standaert, D.; Hallett, M.; Sieber, B.A.; Swanson-Fisher, C.; Berman, B.D.; Calakos, N.; Moore, J.C.; Perlmutter, J.S.; et al. Defining research priorities in dystonia. Neurology 2020, 94, 526–537. [Google Scholar] [CrossRef]
- Bianchi, S.; Battistella, G.; Huddleston, H.; Scharf, R.; Fleysher, L.; Rumbach, A.F.; Frucht, S.J.; Blitzer, A.; Ozelius, L.J.; Simonyan, K. Phenotype- and genotype-specific structural alterations in spasmodic dysphonia. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Hanekamp, S.; Simonyan, K. The large-scale structural connectome of task-specific focal dystonia. Hum. Brain Mapp. 2020, 41, 3253–3265. [Google Scholar] [CrossRef]
- Kirke, D.N.; Battistella, G.; Kumar, V.; Rubien-Thomas, E.; Choy, M.; Rumbach, A.; Simonyan, K. Neural correlates of dystonic tremor: A multimodal study of voice tremor in spasmodic dysphonia. Brain Imaging Behav. 2017, 11, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Summers, R.L.S.; Prudente, C.N.; Goding, G.S.; Samargia-Grivette, S.; Ludlow, C.L.; Kimberley, T.J. Transcranial magnetic stimulation and functional magnet resonance imaging evaluation of adductor spasmodic dysphonia during phonation. Brain Stimul. 2020, 13, 908–915. [Google Scholar] [CrossRef]
- Samargia, S.; Schmidt, R.; Kimberley, T.J. Cortical Silent Period Reveals Differences Between Adductor Spasmodic Dysphonia and Muscle Tension Dysphonia. Neurorehabil. Neural Repair 2016, 30, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Simonyan, K.; Ludlow, C.L. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: An fMRI study. Cereb Cortex 2010, 20, 2749–2759. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Rocchi, L.; Ferrazzano, G.; Leodori, G.; Bologna, M.; Li Voti, P.; Nardella, A.; Berardelli, A. Primary somatosensory cortical plasticity and tactile temporal discrimination in focal hand dystonia. Clin. Neurophysiol. 2014, 125, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Termsarasab, P.; Ramdhani, R.A.; Battistella, G.; Rubien-Thomas, E.; Choy, M.; Farwell, I.M.; Velickovic, M.; Blitzer, A.; Frucht, S.J.; Reilly, R.B.; et al. Neural correlates of abnormal sensory discrimination in laryngeal dystonia. Neuroimage Clin. 2016, 10, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Konczak, J.; Aman, J.E.; Chen, Y.W.; Li, K.Y.; Watson, P.J. Impaired Limb Proprioception in Adults With Spasmodic Dysphonia. J. Voice 2015, 29, 777.e17–777.e23. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, R.A.; Yoneda, Y.; Shipman, J.M.; Sagar, H.J. Idiopathic focal dystonia: A disorder of muscle spindle afferent processing? Brain 1997, 120, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, Y.; Rome, S.; Sagar, H.J.; Grünewald, R.A. Abnormal perception of the tonic vibration reflex in idiopathic focal dystonia. Eur. J. Neurol. 2000, 7, 529–533. [Google Scholar] [CrossRef]
- Tinazzi, M.; Zarattini, S.; Valeriani, M.; Stanzani, C.; Moretto, G.; Smania, N.; Fiaschi, A.; Abbruzzese, G. Effects of transcutaneous electrical nerve stimulation on motor cortex excitability in writer's cramp: Neurophysiological and clinical correlations. Mov. Disord. 2006, 21, 1908–1913. [Google Scholar] [CrossRef]
- Dressler, D.; Adib Saberi, F. Botulinum Toxin: Mechanisms of Action. Eur. Neurol. 2005, 53, 3–9. [Google Scholar] [CrossRef]
- Brin, M.F. Botulinum toxin: Chemistry, pharmacology, toxicity, and immunology. Muscle Nerve Suppl. 1997, 6, S146–S168. [Google Scholar] [CrossRef]
- Nigam, P.K.; Nigam, A. Botulinum toxin. Indian J. Derm. 2010, 55, 8–14. [Google Scholar] [CrossRef]
- Sellin, L.C. The pharmacological mechanism of botulism. Trends Pharmacol. Sci. 1985, 6, 80–82. [Google Scholar] [CrossRef]
- Anandan, C.; Jankovic, J. Botulinum Toxin in Movement Disorders: An Update. Toxins 2021, 13, 42. [Google Scholar] [CrossRef]
- Jankovic, J. An update on new and unique uses of botulinum toxin in movement disorders. Toxicon 2018, 147, 84–88. [Google Scholar] [CrossRef]
- Burgen, A.S.; Dickens, F.; Zatman, L.J. The action of botulinum toxin on the neuro-muscular junction. J. Physiol. 1949, 109, 10–24. [Google Scholar] [CrossRef]
- Jankovic, J. Botulinum toxin: State of the art. Mov. Disord. 2017, 32, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.D.; Jost, W.H. Botulinum toxin: Clinical use. Park. Relat. Disord. 2006, 12, 331–355. [Google Scholar] [CrossRef] [PubMed]
- Kaye, R.; Blitzer, A. Chemodenervation of the Larynx. Toxins 2017, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Weise, D.; Weise, C.M.; Naumann, M. Central Effects of Botulinum Neurotoxin-Evidence from Human Studies. Toxins 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Hok, P.; Veverka, T.; Hluštík, P.; Nevrlý, M.; Kaňovský, P. The Central Effects of Botulinum Toxin in Dystonia and Spasticity. Toxins 2021, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Trompetto, C.; Currà, A.; Buccolieri, A.; Suppa, A.; Abbruzzese, G.; Berardelli, A. Botulinum toxin changes intrafusal feedback in dystonia: A study with the tonic vibration reflex. Mov. Disord. 2006, 21, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Wohlfarth, K.; Schubert, M.; Rothe, B.; Elek, J.; Dengler, R. Remote F-wave changes after local botulinum toxin application. Clin. Neurophysiol. 2001, 112, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Marchand-Pauvert, V.; Aymard, C.; Giboin, L.-S.; Dominici, F.; Rossi, A.; Mazzocchio, R. Beyond muscular effects: Depression of spinal recurrent inhibition after botulinum neurotoxin A. J. Physiol. 2013, 591, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Baumann, A.O.; Sheean, G.; Passingham, R.E.; Marsden, C.D.; Brooks, D.J. Botulinum toxin does not reverse the cortical dysfunction associated with writer's cramp. A PET study. Brain 1997, 120, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.O.; Thomassen, M.; Schulz, G.M.; Hosey, L.A.; Varga, M.; Ludlow, C.L.; Braun, A.R. Alterations in CNS activity induced by botulinum toxin treatment in spasmodic dysphonia: An H215O PET study. J. Speech Lang. Hear. Res. 2006, 49, 1127–1146. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Suzuki, Y.; Kiyosawa, M.; Wakakura, M.; Ishii, K.; Ishiwata, K.; Mochizuki, M. Glucose hypermetabolism in the thalamus of patients with hemifacial spasm. Mov. Disord. 2012, 27, 519–525. [Google Scholar] [CrossRef]
- Karp, B.I. Botulinum Toxin Physiology in Focal Hand and Cranial Dystonia. Toxins 2012, 4, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchio, R.; Caleo, M. More than at the Neuromuscular Synapse:Actions of Botulinum Neurotoxin A in the Central Nervous System. Neuroscientist 2015, 21, 44–61. [Google Scholar] [CrossRef]
- Hallett, M. Mechanism of action of botulinum neurotoxin: Unexpected consequences. Toxicon 2018, 147, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Bielamowicz, S.; Ludlow, C.L. Effects of botulinum toxin on pathophysiology in spasmodic dysphonia. Ann. Otol. Rhinol. Laryngol. 2000, 109, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, C.L. Spasmodic dysphonia: A laryngeal control disorder specific to speech. J. Neurosci. 2011, 31, 793–797. [Google Scholar] [CrossRef] [PubMed]
- O'Flynn, L.C.; Simonyan, K. Short- and Long-term Central Action of Botulinum Neurotoxin Treatment in Laryngeal Dystonia. Neurology 2022, 99, e1178–e1190. [Google Scholar] [CrossRef]
- Leis, A.A.; Dimitrijevic, M.R.; Delapasse, J.S.; Sharkey, P.C. Modification of cervical dystonia by selective sensory stimulation. J. Neurol. Sci. 1992, 110, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.D.; Sapienza, C.M.; Bidus, K.; Ludlow, C.L. Acoustic measures of symptoms in abductor spasmodic dysphonia. J. Voice 2001, 15, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Cannito, M.P.; Johnson, J.P. Spastic dysphonia: A continuum disorder. J. Commun. Disord. 1981, 14, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Cannito, M.P.; Chorna, L.B.; Kahane, J.C.; Dworkin, J.P. Influence of consonant voicing characteristics on sentence production in abductor versus adductor spasmodic dysphonia. J. Voice 2014, 28, 394.e13–394.e22. [Google Scholar] [CrossRef] [PubMed]
- Tisch, S.H.; Brake, H.M.; Law, M.; Cole, I.E.; Darveniza, P. Spasmodic dysphonia: Clinical features and effects of botulinum toxin therapy in 169 patients-an Australian experience. J. Clin. Neurosci. 2003, 10, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Chitkara, A.; Meyer, T.; Keidar, A.; Blitzer, A. Singer's dystonia: First report of a variant of spasmodic dysphonia. Ann. Otol. Rhinol. Laryngol. 2006, 115, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, C.L.; Domangue, R.; Sharma, D.; Jinnah, H.A.; Perlmutter, J.S.; Berke, G.; Sapienza, C.; Smith, M.E.; Blumin, J.H.; Kalata, C.E.; et al. Consensus-Based Attributes for Identifying Patients With Spasmodic Dysphonia and Other Voice Disorders. JAMA Otolaryngol Head Neck Surg. 2018, 144, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Hillel, A.D. The study of laryngeal muscle activity in normal human subjects and in patients with laryngeal dystonia using multiple fine-wire electromyography. Laryngoscope 2001, 111, 1–47. [Google Scholar] [CrossRef]
- Grillone, G.A.; Blitzer, A.; Brin, M.F.; Annino, D.J., Jr.; Saint-Hilaire, M.H. Treatment of adductor laryngeal breathing dystonia with botulinum toxin type A. Laryngoscope 1994, 104, 30–32. [Google Scholar] [CrossRef]
- Tierney, W.S.; Bryson, P.C.; Nelson, R.; Kaplan, S.E.; Benninger, M.S.; Milstein, C.F. Respiratory Laryngeal Dystonia: Characterization and Diagnosis of a Rare Neurogenic Disorder. Laryngoscope 2020, 130, 2843–2846. [Google Scholar] [CrossRef]
- Ludlow, C.L.; Naunton, R.F.; Terada, S.; Anderson, B.J. Successful treatment of selected cases of abductor spasmodic dysphonia using botulinum toxin injection. Otolaryngol. Head Neck Surg. 1991, 104, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Cannito, M.P.; Woodson, G.E.; Murry, T.; Bender, B. Perceptual analyses of spasmodic dysphonia before and after treatment. Arch. Otolaryngol.-Head Neck Surg. 2004, 130, 1393–1399. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kirke, D.N.; Frucht, S.J.; Simonyan, K. Alcohol responsiveness in laryngeal dystonia: A survey study. J. Neurol. 2015, 262, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Simonyan, K.; Frucht, S.J.; Blitzer, A.; Sichani, A.H.; Rumbach, A.F. A novel therapeutic agent, sodium oxybate, improves dystonic symptoms via reduced network-wide activity. Sci. Rep. 2018, 8, 16111. [Google Scholar] [CrossRef]
- Termsarasab, P.; Tanenbaum, D.R.; Frucht, S.J. The phenomenology and natural history of idiopathic lower cranial dystonia. J. Clin. Mov. Disord. 2014, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Aronson, A.E.; Hartman, D.E. Adductor spastic dysphonia as a sign of essential (voice) tremor. J. Speech Hear. Disord. 1981, 46, 52–58. [Google Scholar] [CrossRef]
- Blitzer, A.; Lovelace, R.E.; Brin, M.F.; Fahn, S.; Fink, M.E. Electromyographic findings in focal laryngeal dystonia (spastic dysphonia). Ann. Otol. Rhinol. Laryngol. 1985, 94, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, C.M.; Murry, T.; Brown, W.S., Jr. Variations in adductor spasmodic dysphonia: Acoustic evidence. J. Voice 1998, 12, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, C.M.; Walton, S.; Murry, T. Acoustic variations in adductor spasmodic dysphonia as a function of speech task. J. Speech Lang. Hear. Res. 1999, 42, 127–140. [Google Scholar] [CrossRef]
- Daraei, P.; Villari, C.R.; Rubin, A.D.; Hillel, A.T.; Hapner, E.R.; Klein, A.M.; Johns, M.M., 3rd. The role of laryngoscopy in the diagnosis of spasmodic dysphonia. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Young, N.; Blitzer, A. Management of supraglottic squeeze in adductor spasmodic dysphonia: A new technique. Laryngoscope 2007, 117, 2082–2084. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.B.; Lee, C.T.; Hatcher, J.L.; Michalek, J. Botulinum toxin treatment of false vocal folds in adductor spasmodic dysphonia: Functional outcomes. Laryngoscope 2016, 126, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Hintze, J.M.; Ludlow, C.L.; Bansberg, S.F.; Adler, C.H.; Lott, D.G. Spasmodic Dysphonia: A Review. Part 2: Characterization of Pathophysiology. Otolaryngol. Head Neck Surg. 2017, 157, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Klotz, D.A.; Maronian, N.C.; Waugh, P.F.; Shahinfar, A.; Robinson, L.; Hillel, A.D. Findings of multiple muscle involvement in a study of 214 patients with laryngeal dystonia using fine-wire electromyography. Ann. Otol. Rhinol. Laryngol. 2004, 113, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Sataloff, R.T.; Mandel, S.; Mann, E.A.; Ludlow, C.L. Laryngeal electromyography: An evidence-based review. Muscle Nerve 2003, 28, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, W.; Li, Y.; Cheng, L. Value of Laryngeal Electromyography in Spasmodic Dysphonia Diagnosis and Therapy. Ann. Otol. Rhinol. Laryngol. 2015, 124, 579–583. [Google Scholar] [CrossRef]
- Kohli, N.; Lerner, M.; Rashty, J.; Kirke, D.; Stewart, T.; Blitzer, A. IncobotulinimtoxinA (Xeomin) for the treatment of adductor laryngeal dystonia: A prospective, open-label clinical trial. Am. J. Otolaryngol. 2022, 43, 103613. [Google Scholar] [CrossRef] [PubMed]
- Elmiyeh, B.; Prasad, V.M.; Upile, T.; Saunders, N.; Youl, B.D.; Epstein, R.; Rubin, J.S. A single-centre retrospective review of unilateral and bilateral Dysport injections in adductor spasmodic dysphonia. Logoped. Phoniatr. Vocol. 2010, 35, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.H.; Bansberg, S.F.; Krein-Jones, K.; Hentz, J.G. Safety and efficacy of botulinum toxin type B (Myobloc) in adductor spasmodic dysphonia. Mov. Disord. 2004, 19, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Sataloff, R.T.; Heman-Ackah, Y.D.; Simpson, L.L.; Park, J.B.; Zwislewski, A.; Sokolow, C.; Mandel, S. Botulinum toxin type B for treatment of spasmodic dysphonia: A case report. J. Voice 2002, 16, 422–424. [Google Scholar] [CrossRef]
- Blitzer, A. Botulinum toxin A and B: A comparative dosing study for spasmodic dysphonia. Otolaryngol.-Head Neck. Surg. 2005, 133, 836–838. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.R. Thyrohyoid approach for vocal fold augmentation. Ann. Otol. Rhinol. Laryngol. 2006, 115, 699–702. [Google Scholar] [CrossRef]
- Schönweiler, R.; Wohlfarth, K.; Dengler, R.; Ptok, M. Supraglottal injection of botulinum toxin type A in adductor type spasmodic dysphonia with both intrinsic and extrinsic hyperfunction. Laryngoscope 1998, 108, 55–63. [Google Scholar] [CrossRef]
- Hillel, A.D.; Maronian, N.C.; Waugh, P.F.; Robinson, L.; Klotz, D.A. Treatment of the interarytenoid muscle with botulinum toxin for laryngeal dystonia. Ann. Otol. Rhinol. Laryngol. 2004, 113, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kendall, K.A.; Leonard, R.J. Interarytenoid muscle botox injection for treatment of adductor spasmodic dysphonia with vocal tremor. J. Voice 2011, 25, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, D.; Waters, H.H.; D'Elia, J.B.; Blitzer, A. Botulinum toxin treatment of adductor spasmodic dysphonia: Longitudinal functional outcomes. Laryngoscope 2011, 121, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Lerner, M.Z.; Lerner, B.A.; Patel, A.A.; Blitzer, A. Gender differences in onabotulinum toxin A dosing for adductor spasmodic dysphonia. Laryngoscope 2017, 127, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, S.; Birkent, H.; Sardesai, M.G.; Merati, A.L.; Hillel, A.D. Influence of age and gender on dose and effectiveness of botulinum toxin for laryngeal dystonia. Laryngoscope 2009, 119, 2004–2007. [Google Scholar] [CrossRef] [PubMed]
- Shoffel-Havakuk, H.; Rosow, D.E.; Lava, C.X.; Hapner, E.R.; Johns, M.M., 3rd. Common practices in botulinum toxin injection for spasmodic dysphonia treatment: A national survey. Laryngoscope 2019, 129, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Watts, C.C.; Whurr, R.; Nye, C. Botulinum toxin injections for the treatment of spasmodic dysphonia. Cochrane Database Syst. Rev. 2004, 2010, CD004327. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Asano, K.; Sakaguchi, M.; Nagao, A.; Nakahira, M.; Doi, N.; Kobayashi, T.; Hyodo, M. Post-treatment clinical course following botulinum toxin injection therapy for adductor spasmodic dysphonia: Analysis of data from a placebo-controlled, randomized, double-blinded clinical trial in Japan. Laryngoscope Investig. Otolaryngol. 2021, 6, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A. Spasmodic dysphonia and botulinum toxin: Experience from the largest treatment series. Eur. J. Neurol. 2010, 17, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kang, M.S.; Choi, H.S.; Lim, J.Y. Alternating Unilateral Versus Bilateral Injections of Botulinum Toxin for the Treatment of Adductor Spasmodic Dysphonia. Otolaryngol. Head Neck Surg. 2021, 164, 815–820. [Google Scholar] [CrossRef]
- Dharia, I.; Bielamowicz, S. Unilateral versus bilateral botulinum toxin injections in adductor spasmodic dysphonia in a large cohort. Laryngoscope 2020, 130, 2659–2662. [Google Scholar] [CrossRef] [PubMed]
- Dewan, K.; Berke, G.S. Bilateral Vocal Fold Medialization: A Treatment for Abductor Spasmodic Dysphonia. J. Voice 2019, 33, 45–48. [Google Scholar] [CrossRef]
- Venkatesan, N.N.; Johns, M.M.; Hapner, E.R.; DelGaudio, J.M. Abductor paralysis after botox injection for adductor spasmodic dysphonia. Laryngoscope 2010, 120, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Rontal, M.; Rontal, E.; Rolnick, M.; Merson, R.; Silverman, B.; Truong, D.D. A method for the treatment of abductor spasmodic dysphonia with botulinum toxin injections: A preliminary report. Laryngoscope 1991, 101, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Meleca, R.J.; Hogikyan, N.D.; Bastian, R.W. A comparison of methods of botulinum toxin injection for abductory spasmodic dysphonia. Otolaryngol. Head Neck Surg. 1997, 117, 487–492. [Google Scholar] [CrossRef]
- Rodriquez, A.A.; Ford, C.N.; Bless, D.M.; Harmon, R.L. Electromyographic assessment of spasmodic dysphonia patients prior to botulinum toxin injection. Electromyogr. Clin. Neurophysiol. 1994, 34, 403–407. [Google Scholar] [PubMed]
- Woodson, G.; Hochstetler, H.; Murry, T. Botulinum toxin therapy for abductor spasmodic dysphonia. J. Voice 2006, 20, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Bielamowicz, S.; Squire, S.; Bidus, K.; Ludlow, C.L. Assessment of posterior cricoarytenoid botulinum toxin injections in patients with abductor spasmodic dysphonia. Ann. Otol. Rhinol. Laryngol. 2001, 110, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Stong, B.C.; DelGaudio, J.M.; Hapner, E.R.; Johns, M.M., 3rd. Safety of simultaneous bilateral botulinum toxin injections for abductor spasmodic dysphonia. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.M.; Stong, B.C.; Wise, J.; DelGaudio, J.M.; Hapner, E.R.; Johns, M.M., 3rd. Vocal outcome measures after bilateral posterior cricoarytenoid muscle botulinum toxin injections for abductor spasmodic dysphonia. Otolaryngol. Head Neck Surg. 2008, 139, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, D. Chapter 32: Atypical Spasmodic Dysphonia with Tremor. In Laryngology: A Case-Based Approach; Allen, J.E., Nouraei, S.A., Sandhu, G., Eds.; Plural Publishing Inc.: San Diego, CA, USA, 2020; Volume 1, pp. 327–338. [Google Scholar]
- Payne, S.; Tisch, S.; Cole, I.; Brake, H.; Rough, J.; Darveniza, P. The clinical spectrum of laryngeal dystonia includes dystonic cough: Observations of a large series. Mov. Disord. 2014, 29, 729–735. [Google Scholar] [CrossRef]
- Halstead, L.A.; McBroom, D.M.; Bonilha, H.S. Task-specific singing dystonia: Vocal instability that technique cannot fix. J. Voice 2015, 29, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Yershov, D.; Partridge, R. Life Threatening Delayed Complication of Botulinum Toxin Injection for Treatment of Spasmodic Dysphonia. Prague Med. Rep. 2020, 121, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Young, D.L.; Halstead, L.A. Pyridostigmine for reversal of severe sequelae from botulinum toxin injection. J. Voice 2014, 28, 830–834. [Google Scholar] [CrossRef]
- Bellows, S.; Jankovic, J. Immunogenicity Associated with Botulinum Toxin Treatment. Toxins 2019, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Boo, L.M.; Ackerman, A.H.; Gallagher, C.J. Immunogenicity of botulinum toxins. J. Neural Transm. 2013, 120, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Guillaud, M.; Hu, A. Factors Associated with Failure of Botulinum Toxin Injection in Adductor Spasmodic Dysphonia. Ann. Otol. Rhinol. Laryngol. 2020, 129, 996–1002. [Google Scholar] [CrossRef]
- Lange, O.; Bigalke, H.; Dengler, R.; Wegner, F.; de Groot, M.; Wohlfarth, K. Neutralizing Antibodies and Secondary Therapy Failure After Treatment With Botulinum Toxin Type A: Much Ado About Nothing? Clin. Neuropharmacol. 2009, 32, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Hanna, P.A.; Jankovic, J. Mouse bioassay versus Western blot assay for botulinum toxin antibodies: Correlation with clinical response. Neurology 1998, 50, 1624–1629. [Google Scholar] [CrossRef]
- Brin, M.F.; Comella, C.L.; Jankovic, J.; Lai, F.; Naumann, M. Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov. Disord. 2008, 23, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Lundy, D.S.; Lu, F.L.; Casiano, R.R.; Xue, J.W. The effect of patient factors on response outcomes to Botox treatment of spasmodic dysphonia. J. Voice 1998, 12, 460–466. [Google Scholar] [CrossRef]
- Altman, K.W.; Schaefer, S.D.; Yu, G.P.; Hertegard, S.; Lundy, D.S.; Blumin, J.H.; Maronian, N.C.; Heman-Ackah, Y.D.; Abitbol, J.; Casiano, R.R. The voice and laryngeal dysfunction in stroke: A report from the Neurolaryngology Subcommittee of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol. Head Neck Surg. 2007, 136, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Wingate, J.M.; Ruddy, B.H.; Lundy, D.S.; Lehman, J.; Casiano, R.; Collins, S.P.; Woodson, G.E.; Sapienza, C. Voice handicap index results for older patients with adductor spasmodic dysphonia. J. Voice 2005, 19, 124–131. [Google Scholar] [CrossRef]
- Morzaria, S.; Damrose, E.J. A comparison of the VHI, VHI-10, and V-RQOL for measuring the effect of botox therapy in adductor spasmodic dysphonia. J. Voice 2012, 26, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Paniello, R.C.; Barlow, J.; Serna, J.S. Longitudinal follow-up of adductor spasmodic dysphonia patients after botulinum toxin injection: Quality of life results. Laryngoscope 2008, 118, 564–568. [Google Scholar] [CrossRef]
- Shoffel-Havakuk, H.; Marks, K.L.; Morton, M.; Johns, M.M., 3rd; Hapner, E.R. Validation of the OMNI vocal effort scale in the treatment of adductor spasmodic dysphonia. Laryngoscope 2019, 129, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.D.; Wodchis, W.P.; Spak, C.; Kileny, P.R.; Hogikyan, N.D. Longitudinal effects of Botox injections on voice-related quality of life (V-RQOL) for patients with adductory spasmodic dysphonia: Part II. Arch. Otolaryngol.-Head Neck Surg. 2004, 130, 415–420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whurr, R.; Nye, C.; Lorch, M. Meta-analysis of botulinum toxin treatment of spasmodic dysphonia: A review of 22 studies. Int J. Lang. Commun. Disord. 1998, 33, 327–329. [Google Scholar] [CrossRef]
- Faham, M.; Ahmadi, A.; Silverman, E.; Harouni, G.G.; Dabirmoghaddam, P. Quality of Life After Botulinum Toxin Injection in Patients With Adductor Spasmodic Dysphonia; a Systematic Review and Meta-analysis. J. Voice 2021, 35, 271–283. [Google Scholar] [CrossRef]
- Watts, C.; Nye, C.; Whurr, R. Botulinum toxin for treating spasmodic dysphonia (laryngeal dystonia): A systematic Cochrane review. Clin. Rehabil. 2006, 20, 112–122. [Google Scholar] [CrossRef]
- Rumbach, A.; Aiken, P.; Novakovic, D. Treatment Outcome Measures for Spasmodic Dysphonia: A Systematic Review. J. Voice 2022. [Google Scholar] [CrossRef]
- Dedo, H.H. Recurrent laryngeal nerve section for spastic dysphonia. Ann. Otol. Rhinol. Laryngol. 1976, 85, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Berke, G.S.; Blackwell, K.E.; Gerratt, B.R.; Verneil, A.; Jackson, K.S.; Sercarz, J.A. Selective laryngeal adductor denervation-reinnervation: A new surgical treatment for adductor spasmodic dysphonia. Ann. Otol. Rhinol. Laryngol. 1999, 108, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Sanuki, T.; Isshiki, N. Overall evaluation of effectiveness of type II thyroplasty for adductor spasmodic dysphonia. Laryngoscope 2007, 117, 2255–2259. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, N.; Sanuki, T. Surgical tips for type II thyroplasty for adductor spasmodic dysphonia: Modified technique after reviewing unsatisfactory cases. Acta Otolaryngol. 2010, 130, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, D.H.; Takahashi, M.T.; Imamura, R.; Hachiya, A.; Sennes, L.U. Endoscopic laser thyroarytenoid myoneurectomy in patients with adductor spasmodic dysphonia: A pilot study on long-term outcome on voice quality. J. Voice 2012, 26, 666.e7–666.e12. [Google Scholar] [CrossRef] [PubMed]
- Remacle, M.; Plouin-Gaudon, I.; Lawson, G.; Abitbol, J. Bipolar radiofrequency-induced thermotherapy (rfitt) for the treatment of spasmodic dysphonia. A report of three cases. Eur. Arch. Otorhinolaryngol. 2005, 262, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.G.; Novakovic, D.; Mor, N.; Blitzer, A. Onabotulinum toxin A dosage trends over time for adductor spasmodic dysphonia: A 15-year experience. Laryngoscope 2016, 126, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, M.; Asano, K.; Nagao, A.; Hirose, K.; Nakahira, M.; Yanagida, S.; Nishizawa, N. Botulinum Toxin Therapy: A Series of Clinical Studies on Patients with Spasmodic Dysphonia in Japan. Toxins 2021, 13, 840. [Google Scholar] [CrossRef]
- Rumbach, A.F.; Blitzer, A.; Frucht, S.J.; Simonyan, K. An open-label study of sodium oxybate in Spasmodic dysphonia. Laryngoscope 2017, 127, 1402–1407. [Google Scholar] [CrossRef]
- Poologaindran, A.; Ivanishvili, Z.; Morrison, M.D.; Rammage, L.A.; Sandhu, M.K.; Polyhronopoulos, N.E.; Honey, C.R. The effect of unilateral thalamic deep brain stimulation on the vocal dysfunction in a patient with spasmodic dysphonia: Interrogating cerebellar and pallidal neural circuits. J. Neurosurg. 2018, 128, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Honey, C.R.; Krüger, M.T.; Almeida, T.; Rammage, L.A.; Tamber, M.S.; Morrison, M.D.; Poologaindran, A.; Hu, A. Thalamic Deep Brain Stimulation for Spasmodic Dysphonia: A Phase I Prospective Randomized Double-Blind Crossover Trial. Neurosurgery 2021, 89, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Khosravani, S.; Mahnan, A.; Yeh, I.L.; Aman, J.E.; Watson, P.J.; Zhang, Y.; Goding, G.; Konczak, J. Laryngeal vibration as a non-invasive neuromodulation therapy for spasmodic dysphonia. Sci. Rep. 2019, 9, 17955. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, C.L. Treatment for spasmodic dysphonia: Limitations of current approaches. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 160–165. [Google Scholar] [CrossRef] [PubMed]


| Laryngeal Dystonia Type | Muscle Group(s) Predominantly Affected | Main Clinical Features |
|---|---|---|
| Adductor laryngeal dystonia (~80%) | Adductors (Predominantly TA, but can also affect LCA, IA and CT) | Tight, strained voice with characteristic adductor pitch breaks |
| Abductor laryngeal dystonia (~15%) | Abductors (PCA) | Breathy, asthenic voice with characteristic abductor pitch breaks |
| Mixed laryngeal dystonia (~5%) | Adductors and abductors | Features of both ADLD and ABLD, challenging to diagnose and manage |
| Adductor respiratory laryngeal dystonia (Uncommon) | Adductors | Persistent stridor due to paradoxical vocal fold motion from abnormal inspiratory adductor activity, speech unaffected |
| Singer’s dystonia (Rare) | Adductors, when singing | Adductor pitch breaks on singing only, speech unaffected |
| ADLD: Voice-Weight Sentences (Vowel Sounds: a, e, i, o, u) |
|---|
| ‘We eat eels every Easter.’ |
| ‘Tom wants to be in the army.’ ‘We mow our lawn all year.’ ‘I hurt my arm on the iron bar.’ ‘Ada and Eve ate oysters at the oyster bar.’ |
| ABLD: Voice-Less Weighted Sentences (Consonant Sounds: h, s, p, t, k) |
|---|
| ‘Harry hung his hat on the hook.’ |
| ‘Cake and ice-cream are tasty treats.’ ‘Patty helped Kathy carve the turkey.’ ‘A mahogany highboy isn’t heavy.’ ‘Potato soup tastes fine with crackers.’ |
| Laryngeal Dystonia Type | Target Muscle | Suggested Botox® Dosing (Each Side)—Bilateral Treatment. In Units | Suggested Botox® Dosing—Unilateral Treatment. In Units (U) |
|---|---|---|---|
| Adductor laryngeal dystonia | TA/LCA | 0.6–1.3 | 2.5–3.75 |
| Supraglottis | 7.5 | - | |
| IA | - | 2 | |
| Abductor laryngeal dystonia | PCA | 1.25–2.5 | 3.75–10 |
| CT | 3.75–5 | - | |
| Adductor breathing laryngeal dystonia | TA/LCA | 0.625–3.75 | 2.5–5 |
| Singer’s dystonia | TA | 0.25–0.5 | 0.5–1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeung, W.; Richards, A.L.; Novakovic, D. Botulinum Neurotoxin Therapy in the Clinical Management of Laryngeal Dystonia. Toxins 2022, 14, 844. https://doi.org/10.3390/toxins14120844
Yeung W, Richards AL, Novakovic D. Botulinum Neurotoxin Therapy in the Clinical Management of Laryngeal Dystonia. Toxins. 2022; 14(12):844. https://doi.org/10.3390/toxins14120844
Chicago/Turabian StyleYeung, Winnie, Amanda L. Richards, and Daniel Novakovic. 2022. "Botulinum Neurotoxin Therapy in the Clinical Management of Laryngeal Dystonia" Toxins 14, no. 12: 844. https://doi.org/10.3390/toxins14120844
APA StyleYeung, W., Richards, A. L., & Novakovic, D. (2022). Botulinum Neurotoxin Therapy in the Clinical Management of Laryngeal Dystonia. Toxins, 14(12), 844. https://doi.org/10.3390/toxins14120844





