Abstract
The widespread fungal toxin Aflatoxin B1 (AFB1) is an inevitable pollutant affecting the health of humans, poultry, and livestock. Although studies indicate that AFB1 is hepatotoxic, there are few studies on AFB1-induced hepatotoxicity in sheep. Thus, this study examined how AFB1 affected sheep liver function 24 h after the animals received 1 mg/kg bw of AFB1 orally (dissolved in 20 mL, 4% v/v ethanol). The acute AFB1 poisoning caused histopathological injuries to the liver and increased total bilirubin (TBIL) and alkaline phosphatase (AKP) levels. AFB1 also markedly elevated the levels of the pro-inflammatory cytokines TNF-α and IL-6 while considerably reducing the expression of antioxidation-related genes (SOD-1 and SOD-2) and the anti-inflammatory gene IL-10 in the liver. Additionally, it caused apoptosis by dramatically altering the expression of genes associated with apoptosis including Bax, Caspase-3, and Bcl-2/Bax. Notably, AFB1 exposure altered the gut microbiota composition, mainly manifested by BF311 spp. and Alistipes spp. abundance, which are associated with liver injury. In conclusion, AFB1 can cause liver injury and liver dysfunction in sheep via oxidative stress, inflammation, apoptosis, and gut-microbiota disturbance.
Key Contribution:
Our experiments confirmed that AFB1 can cause liver injury and liver dysfunction in sheep through oxidative stress, inflammation, apoptosis, and gut-microbiota disturbance.
1. Introduction
Mycotoxins are widespread in all processes of agricultural production and can seriously endanger food and feed safety, threatening human and animal health [1]. Over 4.5 billion people are at high risk of exposure to these food contaminants, and it causes annual economic losses of hundreds of billions of dollars. According to the Food and Agriculture Organization of the United Nations (FAO), about 25% of the world’s crops are polluted by mycotoxins in varying degrees [2,3]. Aflatoxin B1 (AFB1), the most toxic mycotoxin, is hepatotoxic, teratogenic, and mutagenic, and it is classified as a class I carcinogen by the World Health Organization [4,5]. The sensitivity of different livestock species to AFB1 varies [6,7]. Chickens are highly sensitive to AFB1, and poisoning is caused mostly by long-term consumption of AFB1-contaminated feed [5,7]. Although sheep are typically thought to have significant tolerance to AFB1 due to their status as ruminants, poisoning can, nonetheless, occur from prolonged or excessive consumption of an AFB1-containing diet [8].
As a hepatotoxic chemical substance, AFB1 is activated into AFB1 epoxide under the action of the cytochrome P450 (CYP450) enzyme, inducing liver cancer and hepatotoxicity [9]. AFB1 toxicity can lead to oxidative damage, apoptosis, and inflammation, resulting in liver congestion, pale coloration, enlargement, and necrosis, inducing chronic and acute hepatocellular injury [7,10,11,12]. Livestock and poultry can accumulate a certain level of AFB1 through dietary exposure, leading to chronic and acute liver diseases in humans via biological accumulation in the food chain [13].
The liver is an important detoxification center as well as a target organ for the metabolic transformation of AFB1 in the body, and it is crucial for the body’s defense against xenobiotics [14]. Past studies on the hepatotoxic effects of AFB1 exposure in sheep have not explored the association between changes in liver function indicators and oxidative stress, inflammation, apoptosis, or gut microbiota. Therefore, we sought to assess the liver function, oxidation, inflammation, and apoptosis indices and composition of the fecal microbial community to offer theoretical support and experimental data on liver damage caused by AFB1 exposure in sheep.
2. Results
2.1. Effects of AFB1 Exposure on Clinical Symptoms, Body Temperature, Respiration, Heart Rate, and Conjunctival Color
Sheep in the AFB1 group showed poor mental status, foaming at the mouth, and reduced food intake after gavage compared to the control group. Before AFB1 exposure, there were no significant differences between the control and AFB1 group body temperatures, respiration, or heart rates, as indicated in Table S2 (p = 0.280, 0.280, and 0.146, respectively). Nevertheless, 24 h after AFB1 exposure, the respiration and heart rate of the AFB1 group were lower than those of the control group (p = 0.071 and 0.084, respectively), while the body temperature was higher (p = 0.519). The AFB1 group had lower changes (slope) in respiration and heart rate than the control group (p = 0.488 and 0.022, respectively). Furthermore, the temperature change in the AFB1 group was greater than in the control group (p = 0.043).
As can be observed in Figure S1A–C, the conjunctival color L of the AFB1 group dropped considerably (p = 0.005) after acute exposure compared with the control group, although redness (a value) and yellowness (b value) did not differ significantly (p = 0.442 and 0.262, respectively).
2.2. Effects of AFB1 Exposure on Serum Biochemical Indices
As shown in Table S3, serum calcium (Ca) levels in the AFB1 group were considerably lower (p = 0.006) than those in the control group, while serum total protein (TP), albumin (ALB), globulin (GLO), white sphere ratio (A/G), amylase (AMY), phosphorus (P), and glucose (GLU) levels showed insignificant decreasing trends (p > 0.05). Furthermore, serum total bilirubin (TBIL) levels were considerably higher (p = 0.002), and serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CRE), urea nitrogen (BUN), BUN/CRE, and creatine kinase (CK) levels increased in the AFB1 group compared with the control group (p = 0.297, 0.190, 0.306, 0.599, 0.938, and 0.41, respectively). The increased serum indices of AST, ALT, and TBIL suggests that AFB1 exposure could result in liver dysfunction (Table S3).
2.3. Effects of AFB1 Exposure on Liver Tissue Structure and Liver Tissue Function
As shown in Figure 1A–C, the ALT, AST, and AKP levels in the AFB1 group increased when compared with those of the control group. Among them, the AKP level was considerably higher than that in the control group (p = 0.007).
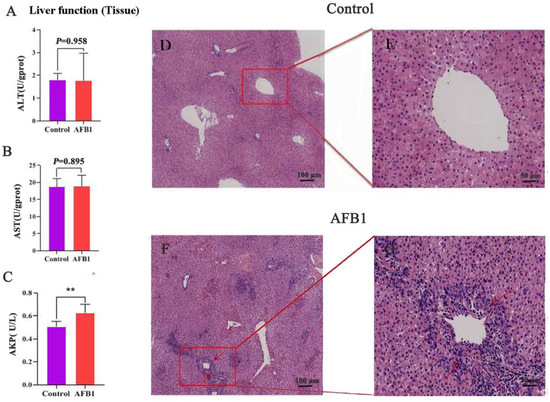
Figure 1.
Effects of AFB1 exposure on liver function and morphological structure of liver tissue. (A–C) Liver function indexes of liver tissue (ALT, AST, and AKP). (D,E) H&E staining reveals normal liver tissues. (F,G) Liver morphology after AFB1 exposure. Note the presence of ruptured cells and inflammatory cell infiltration (arrow). 100 μm (D,F), 50 μm (E,G). ** p < 0.01.
Figure 1D–G depicts liver tissue stained with H&E. The liver lobules of the control group had normal shape; the cords were arranged in an orderly, radial pattern; and the liver cells were complete and uniform in size and cytoplasm. The liver tissue of the AFB1 group, in contrast, exhibited pathological changes, including abnormal hepatic lobule structure, disorganized hepatic cord arrangement, swollen and unevenly sized hepatocytes, severe infiltration of inflammatory cells, vacuolar degeneration, and necrosis in some hepatocytes.
2.4. Effects of AFB1 Exposure on Liver Oxidative Damage
A decrease in liver function is related to oxidative stress [15]. Measurements were taken of the concentrations of MDA and T-AOC and the activities of antioxidant enzymes (CAT, SOD). There was no significant difference in MDA, SOD, CAT, and T-AOC levels between the two groups (p > 0.05; Figure 2A–D). Notably, MDA concentration and SOD activity showed an increasing and decreasing trend compared with the control group, respectively. Protein and mRNA levels typically show reasonable correlation [16], so we estimated the expression of SOD-1 and SOD-2 genes in liver tissues. SOD-1 and SOD-2 levels in the AFB1 group were considerably lower than those in the control group (p < 0.001; Figure 2E,F).
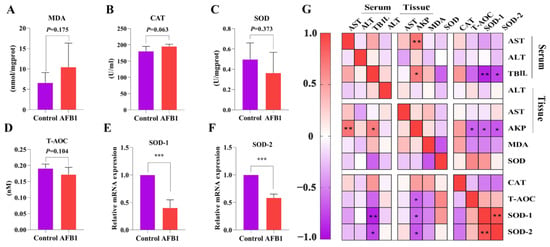
Figure 2.
Effects of AFB1 exposure on liver oxidative damage in sheep. (A–D) Liver tissue MDA, CAT, SOD, and T-AOC levels. (E,F) Relative expressions of the genes SOD-1, and SOD-2 related to oxidative stress in the liver. (G) Pearson correlation analysis of oxidative stress indexes and parameters related to liver function. * p < 0.05, ** p < 0.01, *** p < 0.001.
The relationship between antioxidant indexes and liver function-related indexes was investigated using Pearson correlation analysis (Figure 2G). The levels of AKP (tissue) were negatively correlated with those of T-AOC, SOD-1, and SOD-2 (r = −0.639, −0.175, −0.411, and p = 0.025, 0.03, and 0.002, respectively). SOD-1 (r = −0.893, p = 0.001) and SOD-2 (r = −0.760, p = 0.029) levels were also negatively correlated with TBIL (serum).
2.5. Effects of AFB1 Exposure on the Expression of Inflammation-Related Factors in the Liver
To further investigate the effects of AFB1 on liver inflammation, we examined the expression of the inflammatory cytokines TNF-α, IL-6, and IL-1β and anti-inflammatory cytokine IL-10 in the liver tissues of sheep in each group (Figure 3A–D). The results indicate that the AFB1 group had significantly higher levels of IL-1β and IL-6 expression than those of the control group (p = 0.021 and 0.003, respectively). Furthermore, IL-10 expression was considerably lower in the AFB1 group (p = 0.034), although TNF-α expression did not change significantly (p = 0.641). Pearson correlation analysis revealed that IL-6 gene expression was positively correlated to serum TBIL levels (r = 0.798 and p = 0.01; Figure 3E).
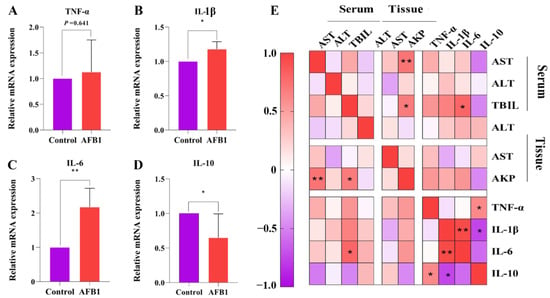
Figure 3.
Effects of AFB1 exposure on inflammation-related indexes in sheep. (A–D) TNF-α, IL-1β, IL-6, and IL-10 mRNA levels detected in liver. (E) Heat map showing the correlation between indicators of liver function and levels of inflammatory gene expression. * p < 0.05, ** p < 0.01.
2.6. Effects of AFB1 Exposure on Liver Cell Apoptosis and Apoptosis-Related Gene Expression
TUNEL staining in the liver of sheep in each group is shown in Figure 4A,B. The AFB1 group had a considerably higher positive rate of TUNEL than the control group. We also detected the mRNA expression of Caspase-3, Bax, and Bcl-2 in the hepatocytes. As seen in Figure 4C,D, the Bcl-2/Bax ratio was reduced dramatically (p < 0.001) in the AFB1 group compared with the control group, whereas Caspase-3 gene expression increased significantly (p = 0.002).
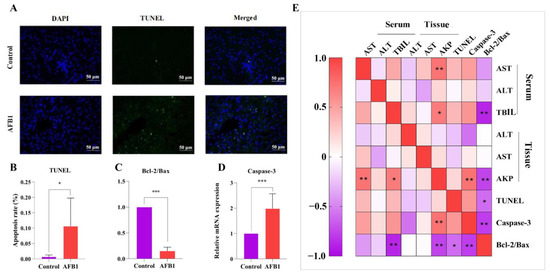
Figure 4.
Effects of AFB1 exposure on hepatocyte apoptosis indexes in sheep. (A) TUNEL staining of sheep liver tissue, with green fluorescence indicating TUNEL positive cells and DAPI staining for nucleus (400×). (B) TUNEL-positive cell percentage. (C) Bcl-2 to Bax ratio. (D) Caspase 3 mRNA level detected in liver using RT-qPCR. (E) Heat map showing the correlation between indicators of liver function and hepatocyte apoptosis indexes. * p < 0.05, ** p < 0.01, *** p < 0.001.
According to the Pearson correlation analysis, AKP (tissue) was negatively correlated with the Bcl-2/Bax ratio (r = −0.762 and p = 0.004) and positively correlated with the Caspase-3 gene (r = 0.730 and p = 0.07; Figure 4E). The Bcl-2/Bax ratio and serum TBIL level had a negative correlation (r = −0.883 and p = 0.002; Figure 4E).
2.7. Changes in Gut Microbiota Induced by AFB1 Exposure
An increasing number of studies show that changes in gut microbiota composition and function are crucial for liver health [17]. To study how the composition of gut microbiota changed after AFB1 exposure, 16S rRNA gene sequencing was performed. The AFB1 and control groups had coverage rates of 98.39% and 96.46%, respectively, indicating that the majority of the gut microbiota diversity was detected (Figure 5A). In comparison to the control group, the observed species, Shannon, Simpson, and Good’s coverage indexes in the AFB1 group were reduced (p = 0.39, 0.021, 0.021, and 0.25, respectively; Figure 5A). The gut microbiota composition showed a trend of relative separation (p = 0.06) for Beta diversity between the control and AFB1 groups (PCo1 contribution of 27.80%, PCo2 contribution of 22.80%; Figure 5B). A total of 2204 ASVs were found in both groups, with 9768 and 6632 ASVs found in the control and AFB1 groups, respectively (Figure 5C). Firmicutes, Bacteroidetes, Spirochaetes, Verrucomicrobia, and Proteobacteria were the top five abundant phyla in both groups, as shown in Figure 5D. Firmicutes, Spirochaetes, Verrucomicrobia, and Proteobacteria were more abundant in the AFB1 group (73.49%, 0.96%, 0.99%, and 0.61%, respectively) than in the control group (72.23%, 0.56%, 0.49%, and 0.56%, respectively), while Bacteroidetes were more abundant in the control group (24.32%) than in the AFB1 group (23.01%; Figure 5D–I). The Firmicutes/Bacteroides ratio (F/B ratio) was higher in the AFB1 group than in the control group, but the difference was not significant (p = 0.564; Figure 5J).
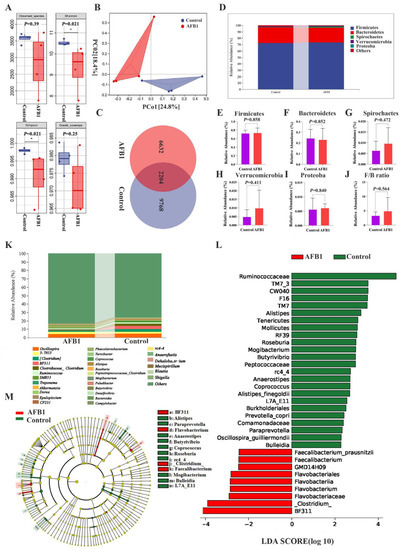
Figure 5.
Effects of AFB1 exposure on gut microbiota structure. (A) Alpha diversity indexes (observed species, Shannon, Simpson, and Good’s coverage) of intestinal microbial. (B) Principal Coordination Analysis (PCoA) of the intestinal microbiome in the AFB1 and control groups (n = 4). (C) ASVs Venn diagram. (D) The top five phylum-level abundances of gut microbiota. (E–J) Difference in the abundances of gut microbiota (Firmicutes, Bacteroidetes, Spirochaetes, Verrucomicrobia, Proteobacteria, and F/B ratio, respectively) at the phylum level. (K) Gut microbiota relative abundance at the genus level (Top 30). (L,M) LDA score and cladogram of LDA effect size (LEfSe). * p < 0.05.
The top 30 genera were also identified. Among them, oscillospira spp., 5-7N15 spp., and (Clostridium) spp. were the most predominant in the control group, and oscillospira spp., 5-7N15 spp., and BF311 spp. accounted for the predominance in the AFB1 group (Figure 5K). LEfSe analysis was utilized to further detect the differential bacterial taxa between the two groups, and 33 discriminative features were identified (genus level; LDA score > 2, p < 0.05; Figure 5L). Among them, BF311 spp., Flavobacterium spp., and (Clostridium) spp. were concentrated in the AFB1 group, while Ruminococcaceae spp., Alistipes spp., and Roseburia spp. were concentrated in the control group (Figure 5L,M).
The top 30 most important genera were screened using random forest analysis, and the most important species at the genus level were BF311 spp., Roseburia spp., and Butyrivibrio spp. (Figure 6A). Thirty-three characteristic species were identified using a Venn diagram analysis of the LEfSe results (LDA score > 2; p < 0.05), and 10 genera were screened out from the top 30 genera based on significance scores (Figure 6B).
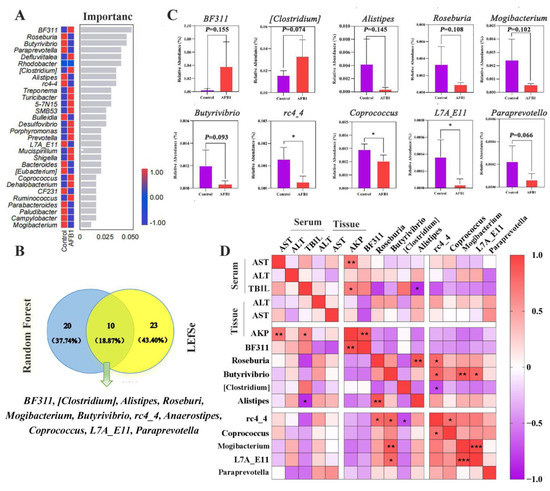
Figure 6.
Analysis of differential gut microbiota. (A) Gut microbiota random forest analysis at the genera level (Top 30). (B) Venn diagrams for the LEfSe and the random forest. (C) The analysis of relative abundances of the ten genera between groups. (D) Heat map showing the correlation between indicators of liver function and gut microbiota. * p < 0.05, ** p < 0.01, *** p < 0.001.
Furthermore, we noted that the abundances of BF311 spp. and (Clostridium) spp. were higher in the AFB1 group, whereas those of Alistipes spp., Roseburia spp., Mogibacterium spp., Butyrivibrio spp., rc4-4 spp., Coprococcus spp., L7A_E11 spp., and Paraprevotella spp. were lower in the AFB1 group than in the control group (p = 0.155, 0.074, 0.145, 0.108, 0.102, 0.093, 0.015, 0.037, 0.022, and 0.066 respectively; Figure 6C). Pearson correlation analysis revealed that the TBIL (serum) level was significantly negatively correlated with Alistipes spp. (r = 0.84, p = 0.036; Figure 6D), whereas AKP (tissue) levels were positively correlated with BF311 spp. (r = 0.897, p = 0.002; Figure 6D).
3. Discussion
Body temperature, breathing rate, and heart rate are important indicators of physical health [18]. In this study, the body temperatures of sheep increased and respiration and heart rates decreased after intake of AFB1. AFB1 exposure-induced gastrointestinal and neurological symptoms were also observed in this study. These results suggest that AFB1 exposure affects the health of sheep.
Jaundice is an important sign of liver dysfunction [19]. As AFB1 targets the liver, exposure to AFB1 may cause jaundice and change the color of the conjunctiva. Therefore, we measured conjunctival color and noted that AFB1 exposure significantly reduced the L values. However, no significant difference in b and a values was detected between the two groups, which could be attributed to the short-term exposure of the animals to AFB1 in this study.
The activities of serum enzymes such as ALT and AST, as well as TP, ALB, TBIL, and GLO concentration, have been defined as critical indicators of liver injury and function [20,21,22]. In this investigation, AFB1 exposure significantly increased the serum TBIL content of sheep, while the levels of serum TP, ALB, GLO, and A/G tended to decrease, and AST and ALT contents tended to increase. These phenomena suggest that exposure to AFB1 may harm the liver and impair liver function in sheep [23]. BUN and CRE are the main biochemical indexes of renal function, which increase with the aggravation of renal injury [24]. In this study, BUN and CRE levels tended to increase slightly, which may have been caused by the short acting time of AFB1 in this study. Ca2+ is an important signaling molecule involved in numerous cellular processes, and its content decreases with the aggravation of liver and kidney diseases [25]. AFB1 exposure significantly decreased the serum Ca content in sheep, indicating the possibility of liver damage. We next assessed liver tissue to explore whether AFB1 exposure would negatively affect sheep liver tissue.
AFB1 exposure resulted in hepatocyte degeneration and considerable inflammatory cell infiltration, suggesting liver tissue damage and corroborating the results of Tsiouris et al. (2021) [26]. In general, elevated ALT, AST, and AKP levels indicate liver dysfunction, which is critical for the differential diagnosis of liver diseases [27]. In the present study, AFB1 exposure increased ALT, AST, and AKP levels in the liver tissue of sheep compared with those of the control group, reflecting the negative impact of AFB1 exposure on liver function.
Numerous studies have demonstrated that AFB1 increases lipid peroxidation and induces the formation of high levels of reactive oxygen species and free radicals, damaging body organs through oxidative stress [28,29]. Since MDA is a byproduct of lipid peroxidation, the level of lipid peroxidation can be inferred from the MDA content in living things [30,31]. In this study, the MDA content increased slightly, indicating liver tissue injury. Antioxidant markers, including SOD, CAT, and T-AOC, are known to play key roles in attenuating oxidative stress by scavenging reactive oxygen species [32]. Although SOD and T-AOC levels were both reduced in the liver of sheep exposed to AFB1 compared to those of the control group, the difference was not significant. The activity of SOD, a particular antioxidant enzyme that neutralizes superoxide anions in ROS free radicals, indirectly reflects the capacity to neutralize oxygen free radicals and is crucial in liver injury [33]. Further examination of gene expression revealed that SOD-1 and SOD-2 levels were significantly decreased. However, an apparent increase in the CAT content was observed in the present study, which may have resulted from the defense mechanism of the body against AFB1 toxicity [8,34]. In this context, Cao et al. (2021) discovered that AFB1 intoxication significantly increases CAT activity in sheep exposed with AFB1 [8]. In addition, the correlation between liver function and antioxidant enzyme activity revealed that AFB1 exposure causes a decrease in liver function related to oxidative damage.
The inflammatory response is an important mechanism in AFB1 toxicity [35]. Oxidative stress can increase the production of different types of ROS in the body, and ROS can activate the nuclear factor-kappa B (NF-κB) pathway, leading to the production of inflammatory cytokines [36]. TNF-α is the most prominent pro-inflammatory cytokine involved in the activation of NF-κB, which induces the expression of IL-1β, IL-6, and other downstream inflammatory mediators [37]. AFB1 significantly increased the expression of the inflammatory factors IL-1β and IL-6, according to our findings. Furthermore, the anti-inflammatory IL-10 gene, which is linked to liver function, was drastically downregulated. The changes in the inflammatory factor expression levels in the liver tissue imply that exposure to AFB1 causes inflammatory injury to the liver.
It has been reported that AFB1 destroys the integrity of the cell membrane by stimulating phospholipids and inducing ROS formation [38]. When excessive ROS is produced and the scavenging capacity of the body decreases, it can lead to protein, DNA, and mitochondrial damage, thus inducing apoptosis [39]. Excessive apoptosis can lead to organ damage, which is considered one of the mechanisms of AFB1-induced toxicity [40]. Our findings showed that when compared with the control group, the AFB1 group had a significantly higher rate of hepatocyte apoptosis detected using the TUNEL method. These results are similar with the findings of a prior study by Xu et al. (2021) [41]. The release of mitochondrial cytochrome c is affected by changes in the ratio of Bcl-2 to Bax expression in cells, and a decrease in this ratio results in apoptosis, according to studies conducted on mammals [42,43]. At the same time, caspase-3 is a common effector of apoptosis [44]. In this study, we discovered that after AFB1 exposure, Bcl-2/Bax gene expression decreased while caspase-3 gene expression increased, indicating that AFB1 exposure can promote liver cell apoptosis. In addition, the correlation between liver function and apoptosis showed that the decline in liver function caused by AFB1 exposure is related to apoptosis.
Like most mycotoxins, AFB1 not only directly damages body organs but also interferes with the normal activities of animal intestinal flora via enterohepatic circulation [7,8]. For example, long-term feeding of AFB1 can significantly reduce most intestinal microbiota in mice [45], and microbiota decline was observed in the acute AFB1 poisoning experiment in this study. Moreover, intestinal flora can combine, transform, degrade, and transfer mycotoxins; promote the healthy growth of livestock and poultry; and participate in material metabolism [46,47,48]. Through amplicon sequencing, we discovered that Firmicutes and Bacteroidetes were the two largest phyla that made up the sheep intestinal flora, which is consistent with earlier research on mammalian intestinal flora [49]. Firmicutes/Bacteroidetes ratios are typically correlated with inflammatory marker levels and pathological conditions of intestinal metabolic homeostasis [49]. AFB1 exposure significantly raised the F/B ratio in this study, implying that AFB1 exposure may disrupt gut metabolic homeostasis and alter inflammatory metabolite levels. At the same time, by screening at the genus level, we identified two genera that deserve attention: BF311 spp. and Alistipes spp. Although there are few studies on the function of BF311 spp., some suggest that BF311 spp. may play a crucial role in the rumen ecosystem and even in rumen synchronization [50]. As a relatively newly identified bacterial genus, Alistipes spp. has been shown to be associated with liver fibrosis, cardiovascular disease, cancer immunotherapy, cardiovascular disease, colitis, and depression [51]. The decrease of Alistipes spp. causes a reduction in short-chain fatty acids (SCFA), which further leads to a decrease in the levels of anti-inflammatory cytokines and decreased inhibition of Th17 cells, resulting in liver fibrosis and hepatocellular carcinoma [51]. Our results suggest that exposure to AFB1 may lead to an increase in BF311 spp. and a decrease in Alistipes spp. populations, and the changes in these bacteria are related to liver dysfunction in sheep.
4. Conclusions
This study confirmed that oxidative stress, inflammatory injury, apoptosis, and gut microbiota are involved in the liver injury and liver dysfunction caused by AFB1 exposure in sheep. The study also offers a valuable reference for future research into the mechanism underlying the hepatotoxic effects of aflatoxin on sheep.
5. Materials and Methods
5.1. Toxin
AFB1 (>98% pure) was purchased from Pribolab Chemical Inc., Co. (Qingdao, China) and dissolved in 4% (v/v) ethanol.
5.2. Animals, Exposure Experiment
The study was mainly conducted at Henan Agricultural University’s Xuchang practical teaching base, China. Twelve Dorper RAMS with an average body weight of 22.34 ± 5.07 kg were individually identified by ear tag and randomly divided into two groups of three replicates and two sheep each. The sheep were immunized according to routine procedures. The control group received 4% ethanol (20 mL) through gavage, while the AFB1 group received AFB1 (1 mg/kg, dissolved in 20 mL 4% ethanol) (half of LD50) orally [8]. Following the gavage of AFB1, there was a 24 h fast from food and water, and surgical sampling was carried out 24 h later. The animal care and experimental procedures were approved by the Institutional Animal Welfare and Research Ethics Committee of Henan Agricultural University’s College of Veterinary Medicine (Zhengzhou, China) (Permit No: 17-0126, Year of approval: 2017). The experimental animals were kept under anesthesia during surgery and every effort was made to minimize their pain, suffering, and death.
5.3. The Color of Conjunctiva
A handheld colorimeter (#SR-62; Shenzhen 3nh Technology Co., Ltd., Shenzhen, China) was used to assess conjunctival color prior to before intragastric administration and surgical sampling. Reflectance spectrometry was used for the color determination using the CIELab method.
5.4. Sample Collection
Temperature, respiration, and heart rate were measured before and 24 h after intragastric administration. Rectal temperature and heart rate were measured using a mercury-in-glass thermometer (range 35–42 °C; accuracy ± 0.3) and stethoscope, respectively. By counting the movement of the abdominal muscles on both sides while breathing, the sheep’s breathing rate per minute was calculated. Blood samples were drawn from the jugular vein; serum was isolated through centrifugation at 4 °C for 15 min at 3000× g and stored at −20 °C. Fecal samples were collected from the sheep rectum before surgery and kept at −80 °C. The anterior abdominal midline of umbilicus was selected as the surgical incision. The liver was exposed by laparotomy, about 2 cm × 3 cm × 3 cm hepatic lobules were collected for liver evaluation, and clamp hemostasis or electrocautery hemostasis was used. The wound was strictly aseptic debridement; each layer of abdominal wall was closed and wrapped with elastic bandage. Part of the liver tissue was cut into 1 cm3 pieces and transferred in liquid nitrogen to the laboratory, where it was preserved at −80 °C for further analysis. The other portion was fixed for 24 h with 4% paraformaldehyde.
5.5. Serum Biochemistry
To determine the 16 biochemical indexes of the serum, a full-automatic biochemical analyzer (#SMT-120 V, Seamaty technology Co., Ltd., Chengdu, China) was used, and the reagent plate was obtained from Chengdu Polytech biological technology Co., Ltd., China. The following parameters were measured within 2 h: ALB, TP, CK, GLU, AMY, A/G, Ca, TBIL, AST, ALT, CRE, TG, P, BUN, and GLO.
5.6. Liver Histopathology
Paraformaldehyde-fixed liver tissue samples were washed and dehydrated in ethanol before being extracted with toluene and embedded in paraffin. For qualitative histological analysis, tissues were sectioned (5 μm) and stained with hematoxylin and eosin (H&E). Motic BA600-4 microscope was used to photograph the tissues (Motic, Xiamen, China).
5.7. Detection of Apoptosis
Paraffin slices of liver were dewaxed using xylene and anhydrous ethanol, then incubated in a 37 °C incubator for 22 min with protease K. TDT enzyme and dUTP were used to incubate in incubator at 37 °C for 2 h before dropping DAPI dye and incubating for 10 min at room temperature. A fluorescent microscope was used for photo imaging (Nikon Eclipse C1, Nikon, Japan). Apoptosis was indicated by green fluorescence.
5.8. Liver Function of Tissue
The liver tissue was homogenized to 10% by the dilution solution, and the activity of general marker enzymes like ALT, AST, and alkaline phosphatase (AKP) enzymes in liver tissue were assessed using kits (Nanjing Jiancheng Bioengineering Factory, Nanjing, China).
5.9. Liver Antioxidant Abilities
The activities of superoxide dismutase (SOD) and catalase (CAT), and the concentration of total antioxidant capacity (T-AOC) and malondialdehyde (MDA) in the liver tissue supernatant were detected by the kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
5.10. Extraction of Total RNA and Quantitative Real-Time PCR Analysis
The CFX96 real-time PCR detection system was used for real-time quantitative PCR (Bio-Rad, Munich, Germany). TRIZOL reagent (Takara Biotechnology Co., Ltd., Dalian, China) was used to extract total RNA from liver tissue, and Superscript II reverse transcriptase was used to prepare first strand cDNA (Roche, Basel, Switzerland). SYBR Green I PCR Master Mix (Vazyme Biotech Co.,Ltd, Nanjing, China) was used to determine mRNA levels, which were then calculated using the 2−ΔΔCT method. Sangon Biotech (Shanghai, China) designed specific primers (Table S1) for inflammatory genes (TNF-α, IL-1β, IL-6, and IL-10), antioxidant genes (SOD-1, SOD-2), and apoptosis genes (Bcl-2, Bax, and Caspase-3) based on the NCBI database sequence (Table S1).
5.11. Extraction of Faecal DNA, PCR Amplification, and Illumina Sequencing
Following the manufacturer’s instructions, total fecal DNA was extracted using the Fast DNA SPIN extraction kits (MP Biomedicals, Santa Ana, CA, USA). To amplify the V3-V4 region of the 16S rRNA gene, forward primer 338F and reverse primer 806R were used. QIIME2 dada2 and R package (v3.2.0) were primarily used for sequence data analysis. The National Center for Biotechnology Information Sequence Read Archive now contains the sequence information for the 16S rRNA gene obtained in our study (PRJNA844551).
5.12. Statistical Analysis
To ensure accuracy and reproducibility, every experiment was performed at least three times. Graphpad Prism (version 7.0) was used to analyze all of the data, which was expressed as means ± standard deviation (SD). The significance of any differences between the experimental groups was assessed using Student’s t-test. Statistics were considered significant at p < 0.05.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins14120840/s1, Table S1: Primer sequences for qPCR analysis; Table S2: Effects of AFB1 exposure on body temperature, breathing, and heart rate of sheep; Table S3: Effects of AFB1 exposure on serum biochemistry of sheep. Figure S1: Effects of AFB1 exposure on conjunctival color.
Author Contributions
Conceptualization, Y.L., H.W., X.B., H.D. (Hongyu Dai), X.B., F.L. and H.D. (Haiju Dong); methodology, Y.S., Y.L., S.Z., H.W., X.B., G.C., H.D. (Hongyu Dai), F.L. and H.D. (Haiju Dong); software, Y.S.; validation, S.Z.; data curation, Y.S. and Y.L.; writing—original draft preparation, Y.S.; writing—review and editing, S.H. and H.D. (Haiju Dong).; supervision, H.D. (Hongyu Dai), F.L. and H.D. (Haiju Dong); funding acquisition, H.D. (Haiju Dong). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Henan Province Project for Tackling Key Problems in Science and Technology (92102110079 and 222102110053), the Young Backbone Teachers Project of Colleges and Universities in Henan Province (2018GGJS031), and the National Natural Science Foundation of China (31402187).
Institutional Review Board Statement
All animals used in this experiment were cared for in accordance with the Institutional Animal Welfare and Research Ethics Committee of Henan Agricultural University’s College of Veterinary Medicine (Zhengzhou, China) (Permit No: 17-0126, date of approval: 26 January 2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
The National Center for Biotechnology Information Sequence Read Archive now contains the sequence information for the 16S rRNA gene obtained in our study (PRJNA844551).
Conflicts of Interest
The authors declare no conflict of interest.
References
- M Santhosh, N.; Shvalya, V.; Modic, M.; Hojnik, N.; Zavašnik, J.; Olenik, J.; Košiček, M.; Filipič, G.; Abdulhalim, I.; Cvelbar, U. Label-Free Mycotoxin Raman Identification by High-Performing Plasmonic Vertical Carbon Nanostructures. Small 2021, 17, e2103677. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.G.; Ross, P.F. Methods for Detection and Quantitation of Fumonisins in Corn, Cereal Products and Animal Excreta. J. Food Prot. 1994, 57, 536–540. [Google Scholar] [CrossRef]
- Crudo, F.; Varga, E.; Aichinger, G.; Galaverna, G.; Marko, D.; Dall’Asta, C.; Dellafiora, L. Co-Occurrence and Combinatory Effects of Alternaria Mycotoxins and other Xenobiotics of Food Origin: Current Scenario and Future Perspectives. Toxins 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Huang, C.; Yang, X.; Zhang, B.; He, X.; Xu, W.; Huang, K. Proteomics reveals the alleviation of zinc towards aflatoxin B1-induced cytotoxicity in human hepatocyes (HepG2 cells). Ecotoxicol. Environ. Saf. 2020, 198, 110596. [Google Scholar] [CrossRef] [PubMed]
- Solcan, C.; Gogu, M.; Floristean, V.; Oprisan, B.; Solcan, G. The hepatoprotective effect of sea buckthorn (Hippophae rhamnoides) berries on induced aflatoxin B1 poisoning in chickens 1. Poult. Sci. 2013, 92, 966–974. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Xu, F.; Huang, W.; Ji, Q.; Han, Y.; Shao, B.; Li, Y. Protective effects of lycopene against AFB(1)-induced erythrocyte dysfunction and oxidative stress in mice. Res. Vet. Sci. 2020, 129, 103–108. [Google Scholar] [CrossRef]
- Lin, L.; Fu, P.; Chen, N.; Gao, N.; Cao, Q.; Yue, K.; Xu, T.; Zhang, C.; Zhang, C.; Liu, F.; et al. Total flavonoids of Rhizoma Drynariae protect hepatocytes against aflatoxin B1-induced oxidative stress and apoptosis in broiler chickens. Ecotoxicol. Environ. Saf. 2022, 230, 113148. [Google Scholar] [CrossRef]
- Cao, Q.Q.; Lin, L.X.; Xu, T.T.; Lu, Y.; Zhang, C.D.; Yue, K.; Huang, S.C.; Dong, H.J.; Jian, F.C. Aflatoxin B1 alters meat quality associated with oxidative stress, inflammation, and gut-microbiota in sheep. Ecotoxicol. Environ. Saf. 2021, 225, 112754. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Li, S.; Muhammad, I.; Yu, H.; Sun, X.; Zhang, X. Detection of Aflatoxin adducts as potential markers and the role of curcumin in alleviating AFB1-induced liver damage in chickens. Ecotoxicol. Environ. Saf. 2019, 176, 137–145. [Google Scholar] [CrossRef]
- Budin, C.; Man, H.Y.; Al-Ayoubi, C.; Puel, S.; van Vugt-Lussenburg, B.M.A.; Brouwer, A.; Oswald, I.P.; van der Burg, B.; Soler, L. Versicolorin A enhances the genotoxicity of aflatoxin B1 in human liver cells by inducing the transactivation of the Ah-receptor. Food Chem. Toxicol. 2021, 153, 112258. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, Z.; Chen, J.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Bacillus amyloliquefaciens B10 can alleviate liver apoptosis and oxidative stress induced by aflatoxin B1. Food Chem. Toxicol. 2021, 151, 112124. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Yuan, Q.; Yan, H.; Tian, G.; Chen, D.; He, J.; Zheng, P.; Yu, J.; Mao, X.; Huang, Z.; et al. Effects of Chronic Exposure to Low Levels of Dietary Aflatoxin B(1) on Growth Performance, Apparent Total Tract Digestibility and Intestinal Health in Pigs. Animals 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, R.; Xia, S.; Wei, G.; Ishfaq, M.; Zhang, Y.; Zhang, X. Protective role of curcumin on aflatoxin B1-induced TLR4/RIPK pathway mediated-necroptosis and inflammation in chicken liver. Ecotoxicol. Environ. Saf. 2022, 233, 113319. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, M.R.; Hong, Y.C. Modification of the association of bisphenol A with abnormal liver function by polymorphisms of oxidative stress-related genes. Environ. Res. 2016, 147, 324–330. [Google Scholar] [CrossRef]
- Buccitelli, C.; Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020, 21, 630–644. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Tabas, I.; Pajvani, U.B. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology 2020, 158, 1913–1928. [Google Scholar] [CrossRef]
- Goergen, C.J.; Tweardy, M.J.; Steinhubl, S.R.; Wegerich, S.W.; Singh, K.; Mieloszyk, R.J.; Dunn, J. Detection and Monitoring of Viral Infections via Wearable Devices and Biometric Data. Annu. Rev. Biomed. Eng. 2021, 24, 1–27. [Google Scholar] [CrossRef]
- Polley, N.; Saha, S.; Singh, S.; Adhikari, A.; Das, S.; Choudhury, B.R.; Pal, S.K. Development and optimization of a noncontact optical device for online monitoring of jaundice in human subjects. J. Biomed. Opt. 2015, 20, 067001. [Google Scholar] [CrossRef]
- Bagherzadeh Kasmani, F.; Karimi Torshizi, M.A.; Allameh, A.; Shariatmadari, F. A novel aflatoxin-binding Bacillus probiotic: Performance, serum biochemistry, and immunological parameters in Japanese quail. Poult. Sci. 2012, 91, 1846–1853. [Google Scholar] [CrossRef]
- Lv, L.X.; Hu, X.J.; Qian, G.R.; Zhang, H.; Lu, H.F.; Zheng, B.W.; Jiang, L.; Li, L.J. Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 improves acute liver injury induced by D-galactosamine in rats. Appl. Microbiol. Biotechnol. 2014, 98, 5619–5632. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Bai, W.; Mao, D.; Chen, Y.; Wang, K.; Qiu, H.; Wu, J. Detoxification II Prescription Suppresses the Th-17/IL-17 Inflammatory Axis to Improve the Liver Function of ACLF-Rats via Inactivating the P38MAPK Pathway. J. Healthc. Eng. 2021, 2021, 7563383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, F.; Liu, M.; Zhou, X.; Wang, M.; Cao, K.; Jin, S.; Shan, A.; Feng, X. Curcumin mitigates aflatoxin B1-induced liver injury via regulating the NLRP3 inflammasome and Nrf2 signaling pathway. Food Chem. Toxicol. 2022, 161, 112823. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fu, W.; Huo, M.; He, B.; Liu, Y.; Tian, L.; Li, W.; Zhou, Z.; Wang, B.; Xia, J.; et al. Spatial-resolved metabolomics reveals tissue-specific metabolic reprogramming in diabetic nephropathy by using mass spectrometry imaging. Acta Pharm. Sin. B 2021, 11, 3665–3677. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Hsu, L.W.; Chen, K.D.; Chiu, K.W.; Chen, C.L.; Huang, K.T. Emerging Roles of Calcium Signaling in the Development of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2021, 23, 256. [Google Scholar] [CrossRef]
- Jin, S.; Yang, H.; Wang, Y.; Pang, Q.; Jiao, Y.; Shan, A.; Feng, X. Dietary Curcumin Alleviated Aflatoxin B1-Induced Acute Liver Damage in Ducks by Regulating NLRP3-Caspase-1 Signaling Pathways. Foods 2021, 10, 3086. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Fu, K.; Gong, L.H.; Zhang, Y.F.; Zhou, H.L.; Li, Y.X. Phillygenin Attenuates Carbon Tetrachloride-Induced Liver Fibrosis via Modulating Inflammation and Gut Microbiota. Front. Pharmacol. 2021, 12, 756924. [Google Scholar] [CrossRef]
- Bai, K.; Huang, Q.; Zhang, J.; He, J.; Zhang, L.; Wang, T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2017, 96, 74–82. [Google Scholar] [CrossRef]
- Fouad, A.M.; Ruan, D.; El-Senousey, H.K.; Chen, W.; Jiang, S.; Zheng, C. Harmful Effects and Control Strategies of Aflatoxin B1 Produced by Aspergillus flavus and Aspergillus parasiticus Strains on Poultry: Review. Toxins 2019, 11, 176. [Google Scholar] [CrossRef]
- Yang, R.L.; Li, W.; Shi, Y.H.; Le, G.W. Lipoic acid prevents high-fat diet–induced dyslipidemia and oxidative stress: A microarray analysis. Nutrition 2008, 24, 582–588. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, Y.; Ye, J.; Du, Z.; Kong, Y. An evaluation of replacing fish meal with fermented soybean meal in the diet of Macrobrachium nipponense: Growth, nonspecific immunity, and resistance to Aeromonas hydrophila. Fish Shellfish Immunol. 2015, 44, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yin, H.; Zuo, Z.; Yang, Z.; Yang, Y.; Wei, L.; Cui, H.; Deng, H.; Chen, X.; Zhu, Y.; et al. Oxidative stress-mediated apoptosis and autophagy involved in Ni-induced nephrotoxicity in the mice. Ecotoxicol. Environ. Saf. 2021, 228, 112954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, N.; Xu, Y.; Tan, H.Y.; Li, S.; Feng, Y. Molecular Mechanisms Involved in Oxidative Stress-Associated Liver Injury Induced by Chinese Herbal Medicine: An Experimental Evidence-Based Literature Review and Network Pharmacology Study. Int. J. Mol. Sci. 2018, 19, 2745. [Google Scholar] [CrossRef] [PubMed]
- Herrington, J.D.; Figueroa, J.A.; Kirstein, M.N.; Zamboni, W.C.; Stewart, C.F. Effect of hemodialysis on topotecan disposition in a patient with severe renal dysfunction. Cancer Chemother. Pharmacol. 2001, 47, 89–93. [Google Scholar] [CrossRef]
- Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Curcumin Mitigates AFB1-Induced Hepatic Toxicity by Triggering Cattle Antioxidant and Anti-inflammatory Pathways: A Whole Transcriptomic In Vitro Study. Antioxidants 2020, 9, 1059. [Google Scholar] [CrossRef]
- Li, H.; Huang, K.; Liu, X.; Liu, J.; Lu, X.; Tao, K.; Wang, G.; Wang, J. Lithium chloride suppresses colorectal cancer cell survival and proliferation through ROS/GSK-3β/NF-κB signaling pathway. Oxidative Med. Cell. Longev. 2014, 2014, 241864. [Google Scholar] [CrossRef]
- Rajendran, P.; Chen, Y.F.; Chen, Y.F.; Chung, L.C.; Tamilselvi, S.; Shen, C.Y.; Day, C.H.; Chen, R.J.; Viswanadha, V.P.; Kuo, W.W.; et al. The multifaceted link between inflammation and human diseases. J. Cell. Physiol. 2018, 233, 6458–6471. [Google Scholar] [CrossRef]
- Guo, Y.; Balasubramanian, B.; Zhao, Z.H.; Liu, W.C. Marine algal polysaccharides alleviate aflatoxin B1-induced bursa of Fabricius injury by regulating redox and apoptotic signaling pathway in broilers. Poult. Sci. 2021, 100, 844–857. [Google Scholar] [CrossRef]
- Yasin, M.; Mazdak, R.; Mino, I. Aflatoxin B1 impairs spermatogenesis: An experimental study for crosslink between oxidative stress and mitochondria-dependent apoptosis. Environ. Toxicol. 2018, 33, 1204–1213. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X.; Hu, X.; Sha, J.; Li, B.; Zhang, H.; Fan, H. Dexmedetomidine Ameliorates Acute Stress-Induced Kidney Injury by Attenuating Oxidative Stress and Apoptosis through Inhibition of the ROS/JNK Signaling Pathway. Oxidative Med. Cell. Longev. 2018, 2018, 4035310. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Cao, Z.; Zhang, J.; Huang, W. AFB(1)-induced mice liver injury involves mitochondrial dysfunction mediated by mitochondrial biogenesis inhibition. Ecotoxicol. Environ. Saf. 2021, 216, 112213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shao, D.; Wu, Y.; Cai, C.; Hu, C.; Shou, X.; Dai, B.; Ye, B.; Wang, M.; Jia, X. Apoptotic responses of Carassius auratus lymphocytes to nodularin exposure In Vitro. Fish Shellfish Immunol. 2012, 33, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Han, B.; Xing, X.; Li, Y.; Zhao, D.; Liu, M.; Wang, S. A Protein from Dioscorea polystachya (Chinese Yam) Improves Hydrocortisone-Induced Testicular Dysfunction by Alleviating Leydig Cell Injury via Upregulation of the Nrf2 Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 3575016. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, C.U.; Woad, K.J.; Barnes, N.; Gardner, D.S.; Robinson, R.S. Maternal protein restriction affects fetal ovary development in sheep. Reprod. Fertil. 2021, 2, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, L.; Chen, J.; Xiao, A. Response of Intestinal Bacterial Flora to the Long-term Feeding of Aflatoxin B1 (AFB1) in Mice. Toxins 2017, 9, 317. [Google Scholar] [CrossRef]
- Young, J.C.; Zhou, T.; Yu, H.; Zhu, H.; Gong, J. Degradation of trichothecene mycotoxins by chicken intestinal microbes. Food Chem. Toxicol. 2007, 45, 136–143. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Glenn, T.C.; Wang, J.S. Aflatoxin B1 Induced Compositional Changes in Gut Microbial Communities of Male F344 Rats. Toxicol. Sci. 2016, 150, 54–63. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, N.; Shen, W.; Zhao, S.; Wang, J. Synchrony Degree of Dietary Energy and Nitrogen Release Influences Microbial Community, Fermentation, and Protein Synthesis in a Rumen Simulation System. Microorganisms 2020, 8, 231. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).