Current Insights in the Mechanisms of Cobra Venom Cytotoxins and Their Complexes in Inducing Toxicity: Implications in Antivenom Therapy
Abstract
:1. Introduction: Epidemiology of Cobra Bites in the World
2. Composition of Cobra Venom: A Summary
3. Discovery, Occurrence, and Classification of Cobra Venom CTXs
4. Complex Formation of Cobra Venom CTXs with Other Components of Venoms
5. Pharmacological Mechanism(s) of Cobra Venom CTXs Vis-à-Vis Their Complexes: Correlation to Cobra Venom-Induced Pathophysiology and Toxicity
5.1. Cytolytic Action by Disruption of Membrane Integrity
5.1.1. Interaction with the Lipid Bilayer
5.1.2. Interaction with Some “Receptors” on The Cell Membrane
5.1.3. Destruction of Lysosomes and Mitochondria
5.1.4. Disturbance of Intracellular Cascades
5.2. Membrane Depolarization and Contraction
5.3. Activation of Cell Cycle Arrest and Apoptotic Cell Death Pathways
5.4. Activation of Necrotic and Necroptotic Cell Death Pathways
6. A Comparative Study on Neutralization of Cobra Venom CTXs and Their Complexes with Commercial Antivenoms
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Gutierrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17079. [Google Scholar] [CrossRef] [PubMed]
- Feola, A.; Marella, G.L.; Carfora, A.; Della Pietra, B.; Zangani, P.; Campobasso, C.P. Snakebite envenoming a challenging diagnosis for the forensic pathologist: A systematic review. Toxins 2020, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasoulis, T.; Isbister, G.K. A review and database of snake venom proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [Green Version]
- WHO. Snakebite Envenoming: A Strategy for Prevention and Control; WHO: Geneva, Switzerland, 2019.
- Wallach, V.; Wuester, W.; Broadley, D.G. In praise of subgenera: Taxonomic status of cobras of the genus Naja Laurenti (Serpentes: Elapidae). Zootaxa 2009, 2236, 26–36. [Google Scholar] [CrossRef]
- Wuster, W. Taxonomic changes and toxinology: Systematic revisions of the Asiatic cobras (Naja naja species complex). Toxicon 1996, 34, 399–406. [Google Scholar] [CrossRef]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Suchithra, N.; Pappachan, J.M.; Sujathan, P. Snakebite envenoming in Kerala, South India: Clinical profile and factors involved in adverse outcomes. Emerg. Med. J. 2008, 25, 200–204. [Google Scholar] [CrossRef]
- Tan, N.H.; Fung, S.Y.; Tan, K.Y.; Yap, M.K.; Gnanathasan, C.A.; Tan, C.H. Functional venomics of the Sri Lankan Russell’s viper (Daboia russelii) and its toxinological correlations. J. Proteom. 2015, 128, 403–423. [Google Scholar] [CrossRef]
- Kalita, B.; Singh, S.; Patra, A.; Mukherjee, A.K. Quantitative proteomic analysis and antivenom study revealing that neurotoxic phospholipase A2 enzymes, the major toxin class of Russell’s viper venom from southern India, shows the least immuno-recognition and neutralization by commercial polyvalent antivenom. Int. J. Biol. Macromol. 2018, 118, 375–385. [Google Scholar] [CrossRef]
- Puzari, U.; Mukherjee, A.K. Recent developments in diagnostic tools and bioanalytical methods for analysis of snake venom: A critical review. Anal. Chim. Acta 2020, 1137, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Mackessy, S.P. Prevention and improvement of clinical management of snakebite in Southern Asian countries: A proposed road map. Toxicon 2021, 200, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.C.; Liu, P.Y.; Chiang, L.C.; Lai, C.S.; Lai, K.L.; Ho, C.H.; Wang, T.H.; Yang, C.C. Naja atra snakebite in Taiwan. Clin. Toxicol. 2018, 56, 273–280. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Budagoda, B.D.; Gawarammana, I.B.; Kularatne, W.K. Epidemiology, clinical profile and management issues of cobra (Naja naja) bites in Sri Lanka: First authenticated case series. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, Q.F.; Yin, R.X.; Zhu, J.J.; Li, Q.B.; Chang, H.H.; Wu, Y.B.; Michelson, E. Clinical features and treatment experience: A review of 292 Chinese cobra snakebites. Environ. Toxicol. Pharmacol. 2014, 37, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Bawaskar, H.S.; Bawaskar, P.H. Envenoming by the common krait (Bungarus caeruleus) and Asian cobra (Naja naja): Clinical manifestations and their management in a rural setting. Wilderness Environ. Med. 2004, 15, 257–266. [Google Scholar] [CrossRef]
- Faiz, M.A.; Ahsan, M.F.; Ghose, A.; Rahman, M.R.; Amin, R.; Hossain, M.; Tareq, M.N.U.; Jalil, M.A.; Kuch, U.; Theakston, R.D.G.; et al. Bites by the Monocled Cobra, Naja kaouthia, in Chittagong Division, Bangladesh: Epidemiology, clinical features of envenoming and management of 70 identified cases. Am. J. Trop. Med. Hyg. 2017, 96, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Utkin, Y.N. Last decade update for three-finger toxins: Newly emerging structures and biological activities. World J. Biol. Chem. 2019, 10, 17–27. [Google Scholar] [CrossRef]
- Utkin, Y.N. Three-finger toxins, a deadly weapon of elapid venom—Milestones of discovery. Toxicon 2013, 62, 50–55. [Google Scholar] [CrossRef]
- Dutta, S.; Chanda, A.; Kalita, B.; Islam, T.; Patra, A.; Mukherjee, A.K. Proteomic analysis to unravel the complex venom proteome of eastern India Naja naja: Correlation of venom composition with its biochemical and pharmacological properties. J. Proteom. 2017, 156, 29–39. [Google Scholar] [CrossRef]
- Chanda, A.; Kalita, B.; Patra, A.; Senevirathne, W.; Mukherjee, A.K. Proteomic analysis and antivenomics study of Western India Naja naja venom: Correlation between venom composition and clinical manifestations of cobra bite in this region. Expert Rev. Proteom. 2019, 16, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Patra, A.; Kalita, B.; Mukherjee, A.K. Proteomics analysis to compare the venom composition between Naja naja and Naja kaouthia from the same geographical location of eastern India: Correlation with pathophysiology of envenomation and immunological cross-reactivity towards commercial polyantivenom. Expert Rev. Proteom. 2018, 15, 949–961. [Google Scholar] [CrossRef]
- Chanda, A.; Mukherjee, A.K. Quantitative proteomics to reveal the composition of Southern India spectacled cobra (Naja naja) venom and its immunological cross-reactivity towards commercial antivenom. Int. J. Biol. Macromol. 2020, 160, 224–232. [Google Scholar] [CrossRef]
- Petras, D.; Sanz, L.; Segura, A.; Herrera, M.; Villalta, M.; Solano, D.; Vargas, M.; Leon, G.; Warrell, D.A.; Theakston, R.D.; et al. Snake venomics of African spitting cobras: Toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J. Proteome Res. 2011, 10, 1266–1280. [Google Scholar] [CrossRef]
- Kakati, H.; Patra, A.; Kalita, B.; Chanda, A.; Rapole, S.; Mukherjee, A.K. A comparison of two different analytical workflows to determine the venom proteome composition of Naja kaouthia from North-East India and immunological profiling of venom against commercial antivenoms. Int. J. Biol. Macromol. 2022, 208, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Wong, K.Y.; Chong, H.P.; Tan, N.H.; Tan, K.Y. Proteomic insights into short neurotoxin-driven, highly neurotoxic venom of Philippine cobra (Naja philippinensis) and toxicity correlation of cobra envenomation in Asia. J. Proteom. 2019, 206, 103418. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Maity, C.R. The composition of Naja naja venom samples from three districts of West Bengal, India. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 119, 621–627. [Google Scholar] [CrossRef]
- Shashidharamurthy, R.; Jagadeesha, D.K.; Girish, K.S.; Kemparaju, K. Variations in biochemical and pharmacological properties of Indian cobra (Naja naja naja) venom due to geographical distribution. Mol. Cell. Biochem. 2002, 229, 93–101. [Google Scholar] [CrossRef]
- Tasoulis, T.; Pukala, T.L.; Isbister, G.K. Investigating toxin diversity and abundance in snake venom proteomes. Front. Pharmacol. 2021, 12, 768015. [Google Scholar] [CrossRef]
- Chanda, A.; Mukherjee, A.K. Mass spectrometric analysis to unravel the venom proteome composition of Indian snakes: Opening new avenues in clinical research. Expert Rev. Proteom. 2020, 17, 411–423. [Google Scholar] [CrossRef]
- Panagides, N.; Jackson, T.N.; Ikonomopoulou, M.P.; Arbuckle, K.; Pretzler, R.; Yang, D.C.; Ali, S.A.; Koludarov, I.; Dobson, J.; Sanker, B.; et al. How the cobra got its flesh-eating venom: Cytotoxicity as a defensive innovation and its co-evolution with hooding, aposematic marking, and spitting. Toxins 2017, 9, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asad, M.H.; McCleary, R.J.; Salafutdinov, I.; Alam, F.; Shah, H.S.; Bibi, S.; Ali, A.; Khalid, S.; Hasan, S.M.; Sabatier, J.-M. Proteomics study of Southern Punjab Pakistani cobra (Naja naja: Formerly Naja naja karachiensis) venom. Toxicol. Environ. Chem. 2019, 101, 91–116. [Google Scholar] [CrossRef]
- Beraldo, E.; Coelho, G.R.; Sciani, J.M.; Pimenta, D.C. Proteomic characterization of Naja mandalayensis venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200125. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.K.; Fung, S.Y.; Tan, K.Y.; Tan, N.H. Proteomic characterization of venom of the medically important Southeast Asian Naja sumatrana (Equatorial spitting cobra). Acta Trop. 2014, 133, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhao, H.Y.; Yin, Y.; Shen, S.S.; Shan, L.L.; Chen, C.X.; Zhang, Y.X.; Gao, J.F.; Ji, X. Combined venomics, antivenomics and venom gland transcriptome analysis of the monocoled cobra (Naja kaouthia) from China. J. Proteom. 2017, 159, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Tan, C.H.; Tan, K.Y.; Quraishi, N.H.; Tan, N.H. Elucidating the biogeographical variation of the venom of Naja naja (spectacled cobra) from Pakistan through a venom-decomplexing proteomic study. J. Proteom. 2018, 175, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Tan, K.Y.; Tan, N.H.; Tan, C.H. A neurotoxic snake venom without Phospholipase A2: Proteomics and cross-neutralization of the venom from Senegalese cobra, Naja senegalensis (Subgenus: Uraeus). Toxins 2021, 13, 60. [Google Scholar] [CrossRef]
- Tan, N.H.; Wong, K.Y.; Tan, C.H. Venomics of Naja sputatrix, the Javan spitting cobra: A short neurotoxin-driven venom needing improved antivenom neutralization. J. Proteom. 2017, 157, 18–32. [Google Scholar] [CrossRef]
- Tan, K.Y.; Wong, K.Y.; Tan, N.H.; Tan, C.H. Quantitative proteomics of Naja annulifera (sub-Saharan snouted cobra) venom and neutralization activities of two antivenoms in Africa. Int. J. Biol. Macromol. 2020, 158, 605–606. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia. J. Proteom. 2015, 120, 105–125. [Google Scholar] [CrossRef]
- Sintiprungrat, K.; Watcharatanyatip, K.; Senevirathne, W.D.; Chaisuriya, P.; Chokchaichamnankit, D.; Srisomsap, C.; Ratanabanangkoon, K. A comparative study of venomics of Naja naja from India and Sri Lanka, clinical manifestations and antivenomics of an Indian polyspecific antivenom. J. Proteom. 2016, 132, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.L.; Gao, J.F.; Zhang, Y.X.; Shen, S.S.; He, Y.; Wang, J.; Ma, X.M.; Ji, X. Proteomic characterization and comparison of venoms from two elapid snakes (Bungarus multicinctus and Naja atra) from China. J. Proteom. 2016, 138, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Malih, I.; Ahmad rusmili, M.R.; Tee, T.Y.; Saile, R.; Ghalim, N.; Othman, I. Proteomic analysis of Moroccan cobra Naja haje legionis venom using tandem mass spectrometry. J. Proteom. 2014, 96, 240–252. [Google Scholar] [CrossRef]
- Liu, C.C.; You, C.H.; Wang, P.J.; Yu, J.S.; Huang, G.J.; Liu, C.H.; Hsieh, W.C.; Lin, C.C. Analysis of the efficacy of Taiwanese freeze-dried neurotoxic antivenom against Naja kaouthia, Naja siamensis and Ophiophagus hannah through proteomics and animal model approaches. PLoS Negl. Trop. Dis. 2017, 11, e0006138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutierrez, J.M. Exploring the venom of the forest cobra snake: Toxicovenomics and antivenom profiling of Naja melanoleuca. J. Proteom. 2017, 150, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.W.; Liu, B.S.; Chien, K.Y.; Chiang, L.C.; Huang, S.Y.; Sung, W.C.; Wu, W.G. Cobra venom proteome and glycome determined from individual snakes of Naja atra reveal medically important dynamic range and systematic geographic variation. J. Proteom. 2015, 128, 92–104. [Google Scholar] [CrossRef]
- Condrea, E. Membrane-active polypeptides from snake venom: Cardiotoxins and haemocytotoxins. Experientia 1974, 30, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.K. Existence of a cardiotoxic principle in cobra venom. Ann. Biochem. Exp. Med. 1948, 8, 11–22. [Google Scholar]
- Aloof-Hirsch, S.; de Vries, A.; Berger, A. The direct lytic factor of cobra venom: Purification and chemical characterization. Biochim. Biophys. Acta 1968, 154, 53–60. [Google Scholar] [CrossRef]
- Larsen, P.R.; Wolff, J. The basic proteins of cobra venom. I. Isolation and characterization of cobramines A and B. J. Biol. Chem. 1968, 243, 1283–1289. [Google Scholar] [CrossRef]
- Slotta, K.H.; Vick, J.A. Identification of the direct lytic factor from cobra venom as cardiotoxin. Toxicon 1969, 6, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Dufton, M.J.; Hider, R.C. Conformational properties of the neurotoxins and cytotoxins isolated from Elapid snake venoms. CRC Crit. Rev. Biochem. 1983, 14, 113–171. [Google Scholar] [CrossRef]
- Hiu, J.J.; Yap, M.K.K. The myth of cobra venom cytotoxin: More than just direct cytolytic actions. Toxicon X 2022, 14, 100123. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.C.; Hsu, J.H.; Sheu, Y.C.; Chiang, C.M.; Wu, W.; Fann, W.; Tsao, P.H. Effect of D57N mutation on membrane activity and molecular unfolding of cobra cardiotoxin. Biophys. J. 1998, 75, 2382–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konshina, A.G.; Dubovskii, P.V.; Efremov, R.G. Structure and dynamics of cardiotoxins. Curr. Protein Pept. Sci. 2012, 13, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Dubovskii, P.V.; Dubova, K.M.; Bourenkov, G.; Starkov, V.G.; Konshina, A.G.; Efremov, R.G.; Utkin, Y.N.; Samygina, V.R. Variability in the spatial structure of the central loop in cobra cytotoxins revealed by X-ray analysis and molecular modeling. Toxins 2022, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Fryklund, L.; Eaker, D. Complete amino acid sequence of a nonneurotoxic hemolytic protein from the venom of Haemachatus haemachates (African ringhals cobra). Biochemistry 1973, 12, 661–667. [Google Scholar] [CrossRef]
- Joubert, F.J. Snake venom toxins. The amino-acid sequences of three toxins (9B, 11 and 12A) from Hemachatus haemachatus (Ringhals) venom. Eur. J. Biochem. 1977, 74, 387–396. [Google Scholar] [CrossRef]
- Joubert, F.J. Snake venom toxins—II. The primary structures of cytotoxin homologues S3C2 and S4C8 from Aspidelaps scutatus (shield or shield-nose snake) venom. Int. J. Biochem. 1988, 20, 337–345. [Google Scholar] [CrossRef]
- Chang, L.S.; Chen, K.C.; Lin, S.R.; Huang, H.B. Purification and characterization of Ophiophagus hannah cytotoxin-like proteins. Toxicon 2006, 48, 429–436. [Google Scholar] [CrossRef]
- Thakur, R.; Chattopadhyay, P.; Mukherjee, A.K. Biochemical and pharmacological characterization of a toxic fraction and its cytotoxin-like component isolated from Russell’s viper (Daboia russelii russelii) venom. Comp. Biochem. Physiology. Toxicol. Pharmacol. CBP 2015, 168, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.Y.; Chiang, C.M.; Hseu, Y.C.; Vyas, A.A.; Rule, G.S.; Wu, W. Two distinct types of cardiotoxin as revealed by the structure and activity relationship of their interaction with zwitterionic phospholipid dispersions. J. Biol. Chem. 1994, 269, 14473–14483. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wuster, W.; Kini, R.M.; Brusic, V.; Khan, A.; Venkataraman, D.; Rooney, A.P. Molecular evolution and phylogeny of elapid snake venom three-finger toxins. J. Mol. Evol. 2003, 57, 110–129. [Google Scholar] [CrossRef] [Green Version]
- Dubovskii, P.V.; Lesovoy, D.M.; Dubinnyi, M.A.; Konshina, A.G.; Utkin, Y.N.; Efremov, R.G.; Arseniev, A.S. Interaction of three-finger toxins with phospholipid membranes: Comparison of S- and P-type cytotoxins. Biochem. J. 2005, 387, 807–815. [Google Scholar] [CrossRef]
- Averin, A.S.; Goltyaev, M.V.; Andreeva, T.V.; Starkov, V.G.; Tsetlin, V.I.; Utkin, Y.N. S- and P-type cobra venom cardiotoxins differ in their action on isolated rat heart. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210110. [Google Scholar] [CrossRef]
- Averin, A.S.; Nenov, M.N.; Starkov, V.G.; Tsetlin, V.I.; Utkin, Y.N. Effects of cardiotoxins from Naja oxiana cobra venom on rat heart muscle and aorta: A comparative study of toxin-induced contraction mechanisms. Toxins 2022, 14, 88. [Google Scholar] [CrossRef]
- Condrea, E.; Devries, A.; Mager, J. Hemolysis and splitting of human erythrocyte phospholipids by snake venoms. Biochim. Biophys. Acta 1964, 84, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Chaim-Matyas, A.; Borkow, G.; Ovadia, M. Synergism between cytotoxin P4 from the snake venom of Naja nigricollis nigricollis and various phospholipases. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 110, 83–89. [Google Scholar] [CrossRef]
- Mukherjee, A.K. Non-covalent interaction of phospholipase A2 (PLA2) and kaouthiotoxin (KTX) from venom of Naja kaouthia exhibits marked synergism to potentiate their cytotoxicity on target cells. J. Venom Res. 2010, 1, 37–42. [Google Scholar] [PubMed]
- Mukherjee, A.K. Phospholipase A2-interacting weak neurotoxins from venom of monocled cobra Naja kaouthia display cell-specific cytotoxicity. Toxicon 2008, 51, 1538–1543. [Google Scholar] [CrossRef]
- Dutta, S.; Sinha, A.; Dasgupta, S.; Mukherjee, A.K. Binding of a Naja naja venom acidic phospholipase A2 cognate complex to membrane-bound vimentin of rat L6 cells: Implications in cobra venom-induced cytotoxicity. Biochim. Biophys. Acta Biomembr. 2019, 1861, 958–977. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Ahmadi, S.; Cerni, F.A.; Ledsgaard, L.; Sorensen, C.V.; McGeoghan, F.T.S.; Stewart, T.; Schoof, E.; Lomonte, B.; Auf dem Keller, U.; et al. Unity makes strength: Exploring intraspecies and interspecies toxin synergism between phospholipases A2 and cytotoxins. Front. Pharmacol. 2020, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Dubovskii, P.V.; Konshina, A.G.; Efremov, R.G. Cobra cardiotoxins: Membrane interactions and pharmacological potential. Curr. Med. Chem. 2014, 21, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Gorai, B.; Sivaraman, T. Delineating residues for haemolytic activities of snake venom cardiotoxin 1 from Naja naja as probed by molecular dynamics simulations and in vitro validations. Int. J. Biol. Macromol. 2017, 95, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Dubovskii, P.V.; Dubinnyi, M.A.; Volynsky, P.E.; Pustovalova, Y.E.; Konshina, A.G.; Utkin, Y.N.; Arseniev, A.S.; Efremov, R.G. Impact of membrane partitioning on the spatial structure of an S-type cobra cytotoxin. J. Biomol. Struct. Dyn. 2018, 36, 3463–3478. [Google Scholar] [CrossRef] [PubMed]
- Averin, A.S.; Astashev, M.E.; Andreeva, T.V.; Tsetlin, V.I.; Utkin, Y.N. Cardiotoxins from Cobra Naja oxiana change the force of contraction and the character of rhythmoinotropic phenomena in the rat myocardium. Doklady. Biochem. Biophys. 2019, 487, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Gasanov, S.E.; Shrivastava, I.H.; Israilov, F.S.; Kim, A.A.; Rylova, K.A.; Zhang, B.; Dagda, R.K. Naja naja oxiana Cobra venom cytotoxins CTI and CTII disrupt mitochondrial membrane integrity: Implications for basic three-fingered cytotoxins. PLoS ONE 2015, 10, e0129248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahim, K.; Shirazi, F.H.; Mirakabadi, A.Z.; Vatanpour, H. Cobra venom cytotoxins; apoptotic or necrotic agents? Toxicon 2015, 108, 134–140. [Google Scholar] [CrossRef]
- Konshina, A.G.; Dubovskii, P.V.; Efremov, R.G. Stepwise insertion of cobra cardiotoxin CT2 into a lipid bilayer occurs as an interplay of protein and membrane “Dynamic Molecular Portraits”. J. Chem. Inf. Model. 2021, 61, 385–399. [Google Scholar] [CrossRef]
- Ebrahim, K.; Shirazi, F.H.; Vatanpour, H.; Zare, A.; Kobarfard, F.; Rabiei, H. Anticancer activity of cobra venom polypeptide, cytotoxin-II, against human breast adenocarcinoma cell line (MCF-7) via the induction of apoptosis. J. Breast Cancer 2014, 17, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Chiou, J.T.; Wang, L.J.; Lee, Y.C.; Chang, L.S. Naja atra cardiotoxin 1 induces the FasL/Fas death pathway in human leukemia cells. Cells 2021, 10, 2073. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ming, W.; Tang, Y.; Zhou, S.; Kong, T.; Dong, W. The anticancer effect of cytotoxin 1 from Naja atra Cantor venom is mediated by a lysosomal cell death pathway involving lysosomal membrane permeabilization and cathepsin B release. Am. J. Chin. Med. 2013, 41, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ming, W.; Wang, Y.; Liu, S.; Qiu, Y.; Xiang, Y.; Hu, L.; Fan, L.; Peng, X.; Wang, H.; et al. Cytotoxin 1 from Naja atra Cantor venom induced necroptosis of leukemia cells. Toxicon 2019, 165, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Lin, C.C.; Wang, C.H.; Wu, P.L.; Huang, H.W.; Chang, C.I.; Wu, W.G. Endocytotic routes of cobra cardiotoxins depend on spatial distribution of positively charged and hydrophobic domains to target distinct types of sulfated glycoconjugates on cell surface. J. Biol. Chem. 2014, 289, 20170–20181. [Google Scholar] [CrossRef] [Green Version]
- Kwan, C.Y.; Kwan, T.K.; Huang, S.J. Effect of calcium on the vascular contraction induced by cobra venom cardiotoxin. Clin. Exp. Pharmacol. Physiol. 2002, 29, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.C.; Chiou, Y.L.; Kao, P.H.; Lin, S.R.; Chang, L.S. Taiwan cobra cardiotoxins induce apoptotic death of human neuroblastoma SK-N-SH cells mediated by reactive oxygen species generation and mitochondrial depolarization. Toxicon 2008, 51, 624–634. [Google Scholar] [CrossRef]
- Chien, C.M.; Yang, S.H.; Yang, C.C.; Chang, L.S.; Lin, S.R. Cardiotoxin III induces c-jun N-terminal kinase-dependent apoptosis in HL-60 human leukaemia cells. Cell Biochem. Funct. 2008, 26, 111–118. [Google Scholar] [CrossRef]
- Yang, S.H.; Chien, C.M.; Chang, L.S.; Lin, S.R. Involvement of c-jun N-terminal kinase in G2/M arrest and caspase-mediated apoptosis induced by cardiotoxin III (Naja naja atra) in K562 leukemia cells. Toxicon 2007, 49, 966–974. [Google Scholar] [CrossRef]
- Chien, C.M.; Chang, S.Y.; Lin, K.L.; Chiu, C.C.; Chang, L.S.; Lin, S.R. Taiwan cobra cardiotoxin III inhibits Src kinase leading to apoptosis and cell cycle arrest of oral squamous cell carcinoma Ca9-22 cells. Toxicon 2010, 56, 508–520. [Google Scholar] [CrossRef]
- Lin, K.L.; Su, J.C.; Chien, C.M.; Chuang, P.W.; Chang, L.S.; Lin, S.R. Down-regulation of the JAK2/PI3K-mediated signaling activation is involved in Taiwan cobra cardiotoxin III-induced apoptosis of human breast MDA-MB-231 cancer cells. Toxicon 2010, 55, 1263–1273. [Google Scholar] [CrossRef]
- Su, J.C.; Lin, K.L.; Chien, C.M.; Chuang, P.W.; Chang, L.S.; Lin, S.R. Concomitant inactivation of the epidermal growth factor receptor, phosphatidylinositol 3-kinase/Akt and Janus tyrosine kinase 2/signal transducer and activator of transcription 3 signalling pathways in cardiotoxin III-treated A549 cells. Clin. Exp. Pharmacol. Physiol. 2010, 37, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Yang, S.H.; Chien, C.M.; Lu, M.C.; Lo, C.S.; Lin, Y.H.; Hu, X.W.; Lin, S.R. Mechanisms of cardiotoxin lll-induced apoptosis in human colorectal cancer colo205 cells. Clin. Exp. Pharmacol. Physiol. 2006, 33, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Chien, C.M.; Lu, M.C.; Lin, Y.H.; Hu, X.W.; Lin, S.R. Up-regulation of Bax and endonuclease G, and down-modulation of Bcl-XL involved in cardiotoxin III-induced apoptosis in K562 cells. Exp. Mol. Med. 2006, 38, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Chiou, J.T.; Shi, Y.J.; Wang, L.J.; Huang, C.H.; Lee, Y.C.; Chang, L.S. Naja atra cardiotoxin 3 elicits autophagy and apoptosis in U937 human leukemia cells through the Ca2+/PP2A/AMPK axis. Toxins 2019, 11, 527. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.C.; Chou, Y.S.; Chen, C.Y.; Liu, K.L.; Huang, G.J.; Yu, J.S.; Wu, C.J.; Liaw, G.W.; Hsieh, C.H.; Chen, C.K. Pathogenesis of local necrosis induced by Naja atra venom: Assessment of the neutralization ability of Taiwanese freeze-dried neurotoxic antivenom in animal models. PLoS Negl. Trop. Dis. 2020, 14, e0008054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandopadhyay, P.; Halder, S.; Sarkar, M.; Kumar Bhunia, S.; Dey, S.; Gomes, A.; Giri, B. Molecular modeling of NK-CT1, from Indian monocellate cobra (Naja kaouthia) and its docking interaction with human DNA topoisomerase II alpha. Bioinformation 2016, 12, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debnath, A.; Saha, A.; Gomes, A.; Biswas, S.; Chakrabarti, P.; Giri, B.; Biswas, A.K.; Gupta, S.D.; Gomes, A. A lethal cardiotoxic-cytotoxic protein from the Indian monocellate cobra (Naja kaouthia) venom. Toxicon 2010, 56, 569–579. [Google Scholar] [CrossRef] [PubMed]
- El Hakim, A.E.; Gamal-Eldeen, A.M.; Shahein, Y.E.; Mansour, N.M.; Wahby, A.F.; Abouelella, A.M. Purification and characterization of a cytotoxic neurotoxin-like protein from Naja haje haje venom that induces mitochondrial apoptosis pathway. Arch. Toxicol. 2011, 85, 941–952. [Google Scholar] [CrossRef]
- Teoh, S.Q.; Yap, M.K.K. Naja sumatrana venom cytotoxin, suma CTX exhibits concentration-dependent cytotoxicity via caspase-activated mitochondrial-mediated apoptosis without transitioning to necrosis. Toxin Rev. 2021, 40, 886–900. [Google Scholar] [CrossRef]
- Hiu, J.J.; Yap, M.K.K. The effects of Naja sumatrana venom cytotoxin, sumaCTX on alteration of the secretome in MCF-7 breast cancer cells following membrane permeabilization. Int. J. Biol. Macromol. 2021, 184, 776–786. [Google Scholar] [CrossRef]
- Rivel, M.; Solano, D.; Herrera, M.; Vargas, M.; Villalta, M.; Segura, A.; Arias, A.S.; Leon, G.; Gutierrez, J.M. Pathogenesis of dermonecrosis induced by venom of the spitting cobra, Naja nigricollis: An experimental study in mice. Toxicon 2016, 119, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.L.; Shi, Y.J.; Huang, C.H.; Lee, Y.C.; Wang, L.J.; Chiou, J.T.; Lu, C.Y.; Chang, L.S. Status of Asp29 and Asp40 in the interaction of Naja atra cardiotoxins with lipid bilayers. Toxins 2020, 12, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.L.; Shi, Y.J.; Chiou, J.T.; Huang, C.H.; Lee, Y.C.; Wang, L.J.; Chang, L.S. Functional and structural properties of cardiotoxin isomers produced by blocking negatively charged groups. Arch. Biochem. Biophys. 2022, 722, 109209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, F.; Chen, Z.; Shrivastava, I.H.; Gasanoff, E.S.; Dagda, R.K. Naja mossambica mossambica cobra cardiotoxin targets mitochondria to disrupt mitochondrial membrane structure and function. Toxins 2019, 11, 152. [Google Scholar] [CrossRef] [Green Version]
- Abu-Khousa, M.; Fiegle, D.J.; Sommer, S.T.; Minabari, G.; Milting, H.; Heim, C.; Weyand, M.; Tomasi, R.; Dendorfer, A.; Volk, T.; et al. The degree of t-System remodeling predicts negative force-frequency relationship and prolonged relaxation time in failing human myocardium. Front. Physiol. 2020, 11, 182. [Google Scholar] [CrossRef]
- Averin, A.S.; Utkin, Y.N. Cardiovascular effects of snake toxins: Cardiotoxicity and cardioprotection. Acta Nat. 2021, 13, 4–14. [Google Scholar] [CrossRef]
- Marks, A.R. Calcium and the heart: A question of life and death. J. Clin. Investig. 2003, 111, 597–600. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.H.; Wu, W.G. Amphiphilic beta-sheet cobra cardiotoxin targets mitochondria and disrupts its network. FEBS Lett. 2005, 579, 3169–3174. [Google Scholar] [CrossRef]
- Portt, L.; Norman, G.; Clapp, C.; Greenwood, M.; Greenwood, M.T. Anti-apoptosis and cell survival: A review. Biochim. Biophys. Acta 2011, 1813, 238–259. [Google Scholar] [CrossRef] [Green Version]
- Ichim, G.; Tait, S.W. A fate worse than death: Apoptosis as an oncogenic process. Nat. Rev. Cancer 2016, 16, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L., Jr.; Loson, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shrivastava, I.H.; Hanlon, P.; Dagda, R.K.; Gasanoff, E.S. Molecular mechanism by which cobra venom cardiotoxins interact with the outer mitochondrial membrane. Toxins 2020, 12, 425. [Google Scholar] [CrossRef] [PubMed]
- Aripov, T.F.; Gasanov, S.E.; Salakhutdinov, B.A.; Rozenshtein, I.A.; Kamaev, F.G. Central Asian cobra venom cytotoxins-induced aggregation, permeability and fusion of liposomes. Gen. Physiol. Biophys. 1989, 8, 459–473. [Google Scholar] [PubMed]
- Syntichaki, P.; Tavernarakis, N. The biochemistry of neuronal necrosis: Rogue biology? Nat. Rev. Neurosci. 2003, 4, 672–684. [Google Scholar] [CrossRef]
- Yuan, J.; Kroemer, G. Alternative cell death mechanisms in development and beyond. Genes Dev. 2010, 24, 2592–2602. [Google Scholar] [CrossRef] [Green Version]
- Christofferson, D.E.; Yuan, J. Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 2010, 22, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Chan, F.K.; Luz, N.F.; Moriwaki, K. Programmed necrosis in the cross talk of cell death and inflammation. Annu. Rev. Immunol. 2015, 33, 79–106. [Google Scholar] [CrossRef]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef]
- Zhang, D.W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.J.; Lin, S.C.; Dong, M.Q.; Han, J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef]
- Feofanov, A.V.; Sharonov, G.V.; Astapova, M.V.; Rodionov, D.I.; Utkin, Y.N.; Arseniev, A.S. Cancer cell injury by cytotoxins from cobra venom is mediated through lysosomal damage. Biochem. J. 2005, 390, 11–18. [Google Scholar] [CrossRef]
- Turk, B.; Stoka, V. Protease signalling in cell death: Caspases versus cysteine cathepsins. FEBS Lett. 2007, 581, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Herrera, M.; Gutierrez, J.M.; Mukherjee, A.K. The application of laboratory-based analytical tools and techniques for the quality assessment and improvement of commercial antivenoms used in the treatment of snakebite envenomation. Drug Test. Anal. 2021, 13, 1471–1489. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Kalita, B.; Chanda, A.; Mukherjee, A.K. Proteomics and antivenomics of Echis carinatus carinatus venom: Correlation with pharmacological properties and pathophysiology of envenomation. Sci. Rep. 2017, 7, 17119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, A.; Kalita, B.; Mukherjee, A.K. Assessment of quality, safety, and pre-clinical toxicity of an equine polyvalent anti-snake venom (Pan Africa): Determination of immunological cross-reactivity of antivenom against venom samples of Elapidae and Viperidae snakes of Africa. Toxicon 2018, 153, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Chanda, A.; Mukherjee, A.K. Quantitative proteomic analysis of venom from Southern India common krait (Bungarus caeruleus) and identification of poorly immunogenic toxins by immune-profiling against commercial antivenom. Expert Rev. Proteom. 2019, 16, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Mukherjee, A.K. Proteomic analysis of Sri Lanka Echis carinatus venom: Immunological cross-reactivity and enzyme neutralization potency of indian polyantivenom. J. Proteome Res. 2020, 19, 3022–3032. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Patra, A.; Mukherjee, A.K. Unraveling the proteome composition and immuno-profiling of western India Russell’s viper venom for in-depth understanding of its pharmacological properties, clinical manifestations, and effective antivenom treatment. J. Proteome Res. 2017, 16, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Patra, A.; Das, A.; Mukherjee, A.K. Proteomic analysis and immuno-profiling of eastern India Russell’s viper (Daboia russelii) venom: Correlation between RVV composition and clinical manifestations post RV bite. J. Proteome Res. 2018, 17, 2819–2833. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, K.Y.; Wong, K.Y.; Tan, N.H.; Chong, H.P. Equatorial spitting cobra (Naja sumatrana) from Malaysia (Negeri Sembilan and Penang), southern Thailand, and Sumatra: Comparative venom proteomics, immunoreactivity and cross-neutralization by antivenom. Toxins 2022, 14, 522. [Google Scholar] [CrossRef]
- Mukherjee, A.K. Species-specific and geographical variation in venom composition of two major cobras in Indian subcontinent: Impact on polyvalent antivenom therapy. Toxicon 2020, 188, 150–158. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Gutierrez, J.M.; Lohse, B.; Rasmussen, A.R.; Fernandez, J.; Milbo, C.; Lomonte, B. Snake venomics of monocled cobra (Naja kaouthia) and investigation of human IgG response against venom toxins. Toxicon 2015, 99, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.P.; Tan, K.Y.; Liu, B.S.; Sung, W.C.; Tan, C.H. Cytotoxicity of venoms and cytotoxins from Asiatic cobras (Naja kaouthia, Naja sumatrana, Naja atra) and neutralization by antivenoms from Thailand, Vietnam, and Taiwan. Toxins 2022, 14, 334. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Kalita, B.; Dutta, S.; Patra, A.; Maiti, C.R.; Punde, D. Snake envenomation: Therapy and challenges in India. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2021; pp. 581–592. [Google Scholar]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Neutralization of the principal toxins from the venoms of Thai Naja kaouthia and Malaysian Hydrophis schistosus: Insights into toxin-specific neutralization by two different antivenoms. Toxins 2016, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.H.; Wong, K.Y.; Tan, N.H.; Ng, T.S.; Tan, K.Y. Distinctive distribution of secretory phospholipases A2 in the venoms of Afro-Asian cobras (Subgenus: Naja, Afronaja, Boulengerina and Uraeus). Toxins 2019, 11, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.H.; Sung, W.C.; Mu, H.W.; Hung, D.Z. Local cytotoxic effects in cobra envenoming: A pilot study. Toxins 2022, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chaou, C.H.; Tseng, C.Y. An investigation of snakebite antivenom usage in Taiwan. J. Formos. Med. Assoc. 2016, 115, 672–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, H.Y.; Wang, M.J.; Li, Y.H.; Tang, C.N.; Tsai, M.J. Can surgical need in patients with Naja atra (Taiwan or Chinese cobra) envenomation be predicted in the emergency department? Hong Kong Med. J. 2016, 22, 435–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-K.; Lin, C.-C.; Shih, F.-Y.; Chaou, C.-H.; Lin, J.C.-C.; Lai, T.-I.; Tseng, C.-Y.; Fang, C.-C. Population-based study of venomous snakebite in Taiwan. J. Acute Med. 2015, 5, 38–42. [Google Scholar] [CrossRef]
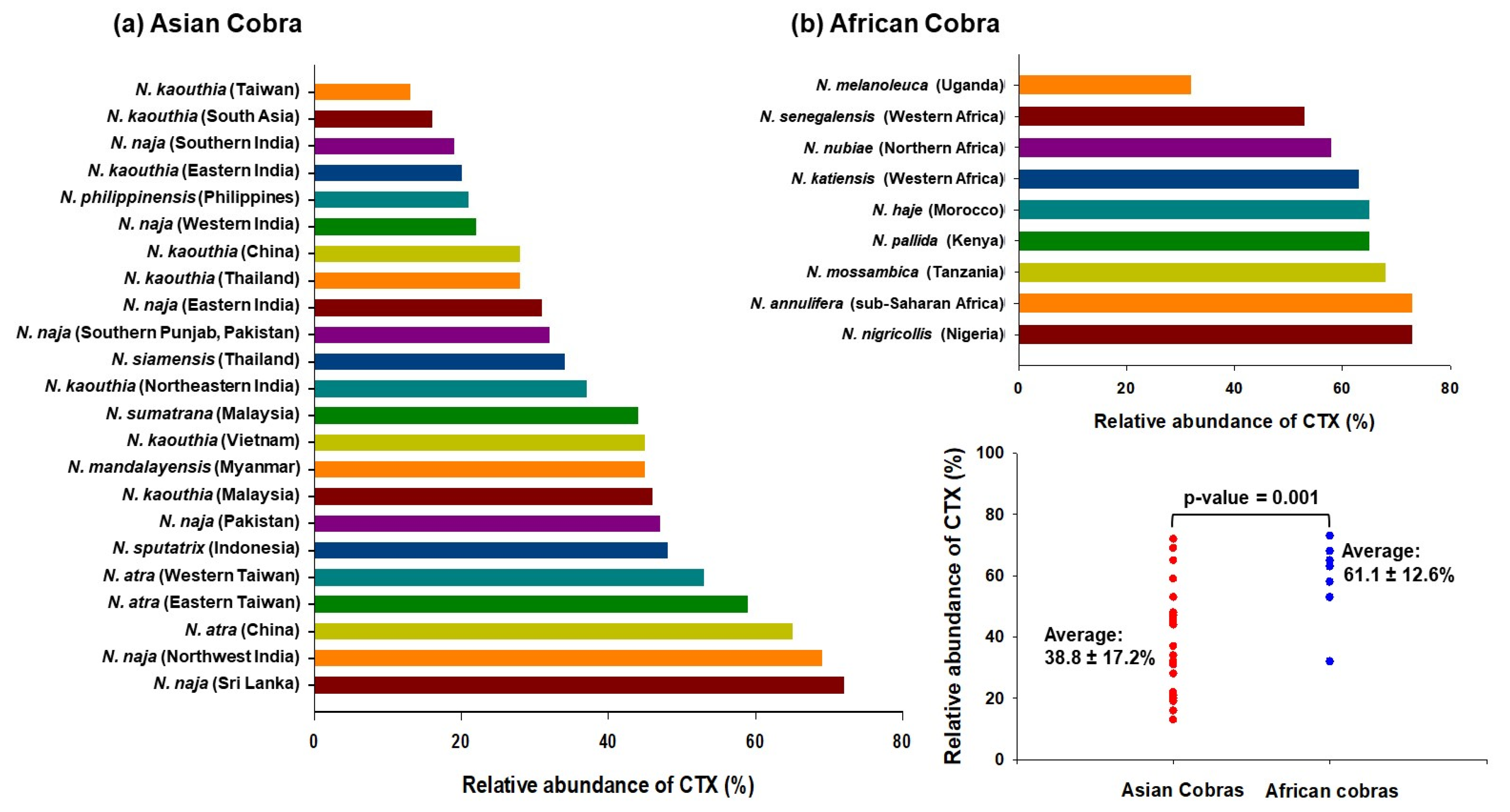
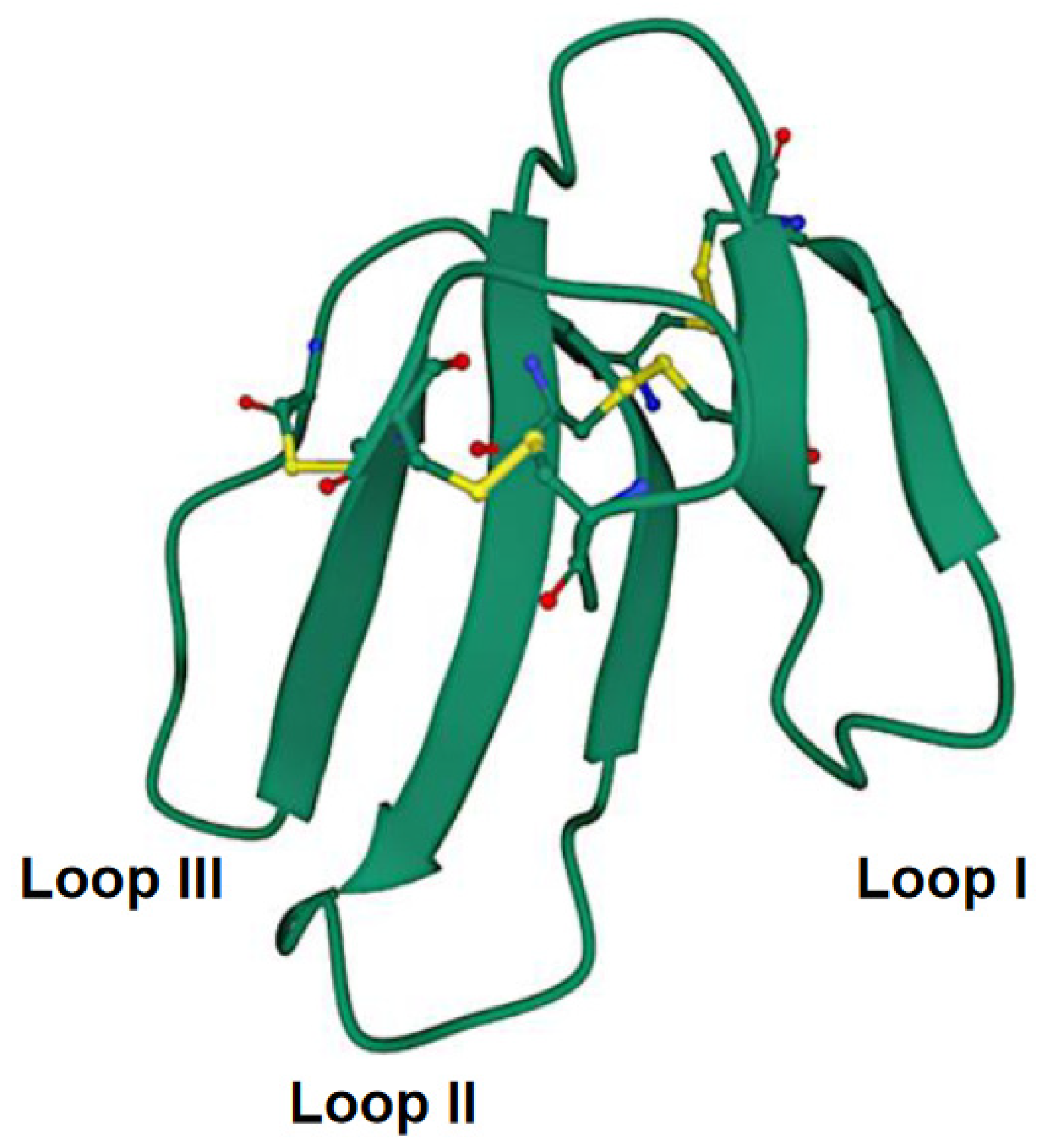

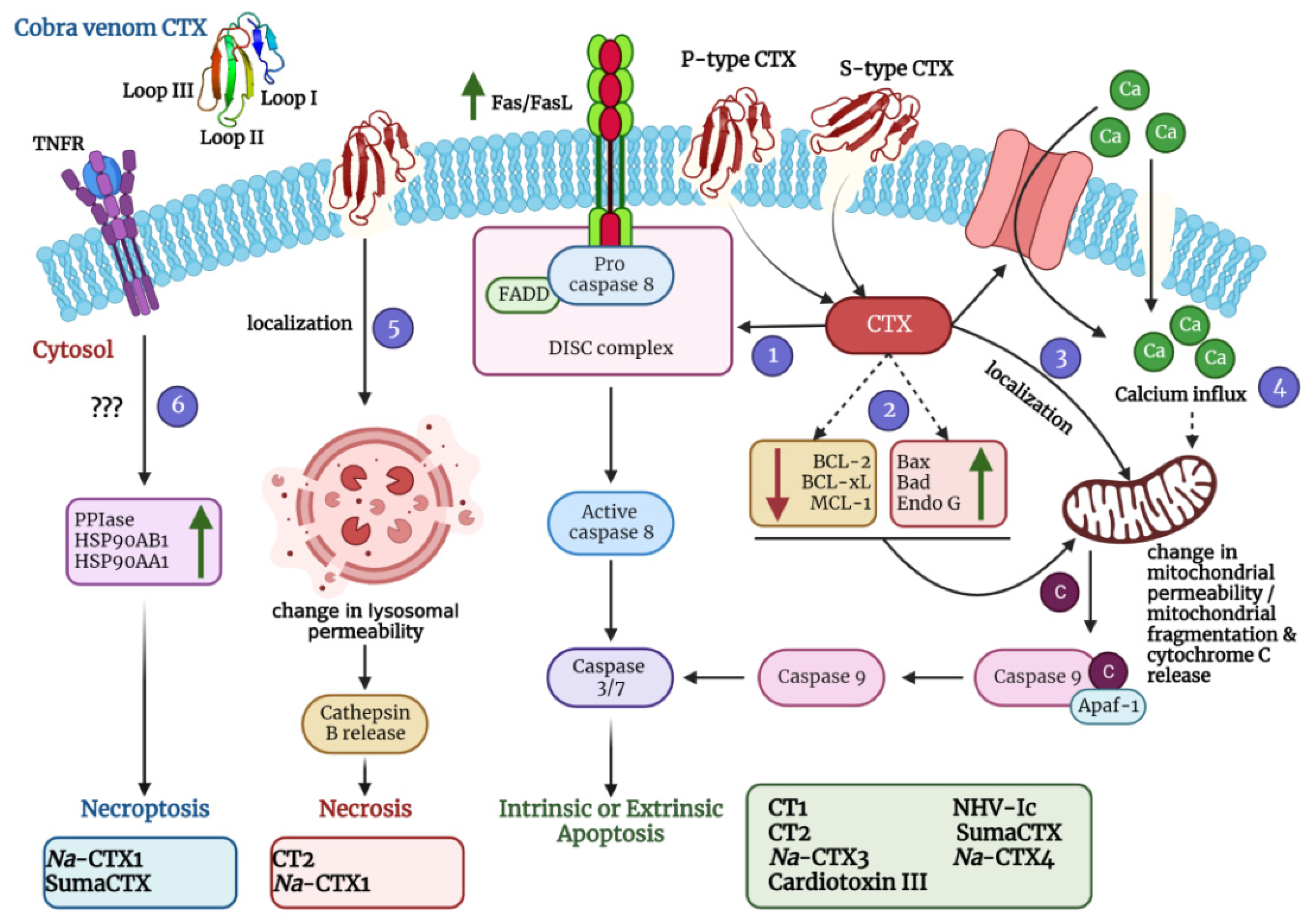
| Snake Species | Common Name | Medical Importance/ Category | Region of Distribution | Number of Countries | Human Population (2020) in This Species’ Range |
|---|---|---|---|---|---|
| N. anchietae | Anchieta’s cobra | Highest, Secondary | Africa | 6 | 19,008,230 |
| N. annulata | Banded water cobra | Highest, Secondary | Africa | 10 | 114,642,902 |
| N. annulifera | Snouted cobra | Highest | Africa | 8 | 70,731,878 |
| N. ashei | Ashe’s spitting cobra | Highest | Africa | 6 | 32,513,269 |
| N. atra | Chinese cobra | Highest | Asia and Australasia | 5 | 570,266,425 |
| N. christyi | Christy’s water cobra | Secondary | Africa | 3 | 15,111,896 |
| N. guineensis | Black forest cobra | Highest, Secondary | Africa | 7 | 55,106,930 |
| N. haje | Egyptian cobra | Highest, Secondary | Africa | 21 | 443,884,351 |
| N. kaouthia | Monocled cobra, Thai cobra | Highest, Secondary | Asia | 11 | 976,884,863 |
| N. katiensis | Mali cobra, West Africa brown spitting cobra | Highest, Secondary | Africa | 12 | 123,542,818 |
| N. mandalayensis | Mandalay spitting cobra | Highest | Asia and Australasia | 1 | 14,774,047 |
| N. melanoleuca | Black and white cobra, Forest cobra | Highest, Secondary | Africa | 11 | 244,375,176 |
| N. mossambica | Mozambique spitting cobra | Highest, Secondary | Africa | 10 | 130,049,980 |
| N. naja | Indian cobra, Spectacled cobra | Highest, Secondary | Asia and Australasia | 5 | 1,656,817,409 |
| N. nigricincta | Western barred spitting cobra, Zebra cobra | Highest, Secondary | Africa | 4 | 11,381,021 |
| N. nigricollis | Black-necked spitting cobra | Highest, Secondary | Africa | 33 | 727,256,279 |
| N. nivea | Cape cobra | Highest | Africa | 4 | 17,651,152 |
| N. nubiae | Nubian spitting cobra | Secondary | Africa | 5 | 39,843,095 |
| N. oxiana | Central Asian cobra, Transcaspian cobra | Highest, Secondary | Asia and Australasia, Middle East | 8 | 242,127,307 |
| N. pallida | Red spitting cobra | Secondary | Africa | 6 | 59,847,176 |
| N. peroescobari | Sao Tome cobra | Highest | Africa | 1 | 189,185 |
| N. philippinesis | Northern Philippine cobra | Highest | Asia and Australasia | 1 | 592,982,107 |
| N. sagittifera | Andaman cobra | Secondary | Asia and Australasia | 1 | 373,959 |
| N. samarensis | Southern Philippine cobra, Visayan cobra | Highest | Asia and Australasia | 1 | 30,350,207 |
| N. savannula | West African banded cobra | Highest, Secondary | Africa | 16 | 151,894,138 |
| N. senegalensis | Senegalese cobra | Highest, Secondary | Africa | 13 | 84,781,768 |
| N. siamensis | Indochinese spitting cobra, Siamese spitting cobra | Highest, Secondary | Asia and Australasia | 5 | 119,240,121 |
| N. sputatrix | Southern Indonesian spitting cobra | Highest, Secondary | Asia and Australasia | 2 | 167,089,984 |
| N. subfulva | Brown forest cobra | Highest, Secondary | Africa | 22 | 377,545,129 |
| N. sumatrana | Equatorial spitting cobra | Highest, Secondary | Asia and Australasia | 6 | 124,654,470 |
| Cobra Species | Cytotoxin | UniProt ID | Mechanism | Methodology/Tested Model | References |
|---|---|---|---|---|---|
| N. naja | CTX1 | P01447 | Interaction with erythrocyte membrane via ‘head groove’ and ‘loop groove’ of loop II | Molecular dynamics simulation | [75] |
| CT13Nn | A0A0U4N5W4 | Transformation from the “water” conformation to the “membrane” conformation in loop II during insertion into lipid membranes | X-ray crystallography and molecular dynamics simulation | [57] | |
| CTX2a | P86538 | Complex formation with PLA2 and NTX and entry into cells via specific binding of the PLA2 to Vimentin | L6 rat myogenic cells | [72] | |
| N. oxiana | CT1 | P01451 | Insertion into lipid membranes primarily with either the tip of loop I or both ends of loops I and II | NMR spectroscopy and molecular dynamics simulation | [76] |
| Contractions of papillary muscles | Cardiomyocytes from right ventricles of rat hearts | [77] | |||
| Formation of non-selective pores in the cell membrane that facilitates the influx of Ca2+ and stimulation of cardiomyocyte contracture | Isolated rat heart | [66] | |||
| Alteration of mitochondrial permeability and signaling, ultimately leading to the mitochondrial fragmentation and stimulation of intrinsic apoptosis | Bovine cardiomyocytes, MCF-7 breast cancer cells, Hep-G2 hepatocellular carcinoma cells | [78,79] | |||
| CT2 | P01441 | Insertion into lipid membranes via immersion of loop I | Molecular dynamics simulation | [80] | |
| Contractions of papillary muscles | Cardiomyocytes from right ventricles of rat hearts | [77] | |||
| Formation of non-selective pores in the cell membrane that facilitates the influx of Ca2+ and stimulation of cardiomyocyte contracture | Isolated rat heart | [66] | |||
| Alteration of mitochondrial permeability and signaling, ultimately leading to mitochondrial fragmentation and stimulation of intrinsic apoptosis | Bovine cardiomyocytes, MCF-7 breast cancer cells | [78,81] | |||
| Increase in lysosomal membrane permeability and cathepsin B protease activity, and necrosis | MCF-7 breast cancer cells, HepG2 liver cancer cells, DU-145 prostate cancer cells, HL-60 leukemia cells, MDCK Madin–Darby canine kidney cells | [79] | |||
| N. atra | Cardiotoxin 1/CTX1 | P60304 | Upregulation of FasL and Fas expression leading to extrinsic apoptosis | HL-60 and U937 leukemia cells | [82] |
| Increase in lysosomal membrane permeability and cathepsin B protease activity, and necrosis | 16HBE human bronchial epithelial cells, MCF-7 breast cancer cells, K562 and P388 leukemia cells, H22 liver cancer cells | [83] | |||
| Increase in lysosomal membrane permeability and release of cathepsin B, and necroptosis | KG1a and HL-60 leukemia cells | [84] | |||
| CTX A2 | P01442 | Interaction with low sulfated heparin domains of cell membrane for internalization | H9C2 rat cardiomyocytes and Chinese hamster ovary (CHO) cells | [85] | |
| CTX A4/CTX4 | P01443 | Interaction with fully sulfated heparin domains of cell membrane for internalization | H9C2 rat cardiomyocytes and Chinese hamster ovary (CHO) cells | [85] | |
| Activation of L-type calcium channels for the influx of Ca2+ and subsequent activation of calcium-dependent cardiomyocyte contraction | Rat aortic ring preparation | [86] | |||
| ROS generation followed by alteration in mitochondrial permeability, cytochrome c release and activation of intrinsic apoptosis | SK-N-SH human neuroblastoma cells | [87] | |||
| Cardiotoxin III/CTX3 | P60301 | Cell cycle arrest at sub-G1 stage | HL-60 leukemia cells | [88] | |
| Downregulation of cyclin B1, cyclin A, Cdc25C, and Cdk 1 expression | K562 leukemia cells and Ca9–22, SAS, and CAL27 oral squamous carcinoma cells | [89,90] | |||
| Upregulation of pro-apoptotic proteins (Bad, Bax, endonuclease G) and downregulation of anti-apoptotic proteins (Mcl-1, Bcl-2, survivin, Bcl-XL and XIAP) leading to intrinsic apoptosis | Ca9–22 oral squamous cell carcinoma cells, MDA-MB-231 breast cancer cells, A549 lung cancer cells, colo 205 colorectal cancer cells, and K562 leukemia cells | [90,91,92,93,94] | |||
| ROS generation followed by alteration in mitochondrial permeability, cytochrome c release and activation of intrinsic apoptosis | SK-N-SH human neuroblastoma cells | [87] | |||
| Ca2+ influx, phosphorylation of AMPK, mitochondrial fragmentation, cytochrome c release, and intrinsic apoptosis | U937 leukemia cells | [95] | |||
| RP-HPLC fraction containing CTX isoforms | Unavailable | Dermonecrosis | Littermate ICR (CD-1) mice | [96] | |
| N. kaouthia | NK-CT1 | P0CH80 | Interaction with oligonucleotide–human DNA topoisomerase II alpha complex for arresting cell growth | Molecular modelling and docking | [97] |
| Cell cycle arrest at sub-G1 stage | U937 and K562 leukemia cells | [98] | |||
| N. haje | NHV-Ic | P01389 | Alteration of mitochondrial permeability and signaling, ultimately leading to mitochondrial fragmentation and stimulation of intrinsic apoptosis | 1301 leukemia cells | [99] |
| N. sumatrana | SumaCTX | A0A7T7DMY7 | Alteration of mitochondrial permeability and signaling, ultimately leading to mitochondrial fragmentation and stimulation of intrinsic apoptosis | MCF-7 breast cancer cells | [100] |
| Upregulation of peptidyl–prolyl isomerase and heat shock proteins thereby leading to necroptosis | MCF-7 breast cancer cells | [101] | |||
| N. nigricollis | RP-HPLC fraction containing CTX and PLA2 | Unavailable | Dermonecrosis | CD-1 mice | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalita, B.; Utkin, Y.N.; Mukherjee, A.K. Current Insights in the Mechanisms of Cobra Venom Cytotoxins and Their Complexes in Inducing Toxicity: Implications in Antivenom Therapy. Toxins 2022, 14, 839. https://doi.org/10.3390/toxins14120839
Kalita B, Utkin YN, Mukherjee AK. Current Insights in the Mechanisms of Cobra Venom Cytotoxins and Their Complexes in Inducing Toxicity: Implications in Antivenom Therapy. Toxins. 2022; 14(12):839. https://doi.org/10.3390/toxins14120839
Chicago/Turabian StyleKalita, Bhargab, Yuri N. Utkin, and Ashis K. Mukherjee. 2022. "Current Insights in the Mechanisms of Cobra Venom Cytotoxins and Their Complexes in Inducing Toxicity: Implications in Antivenom Therapy" Toxins 14, no. 12: 839. https://doi.org/10.3390/toxins14120839
APA StyleKalita, B., Utkin, Y. N., & Mukherjee, A. K. (2022). Current Insights in the Mechanisms of Cobra Venom Cytotoxins and Their Complexes in Inducing Toxicity: Implications in Antivenom Therapy. Toxins, 14(12), 839. https://doi.org/10.3390/toxins14120839






