Zearalenone Exposure Affects the Keap1–Nrf2 Signaling Pathway and Glucose Nutrient Absorption Related Genes of Porcine Jejunal Epithelial Cells
Abstract
1. Introduction
2. Results
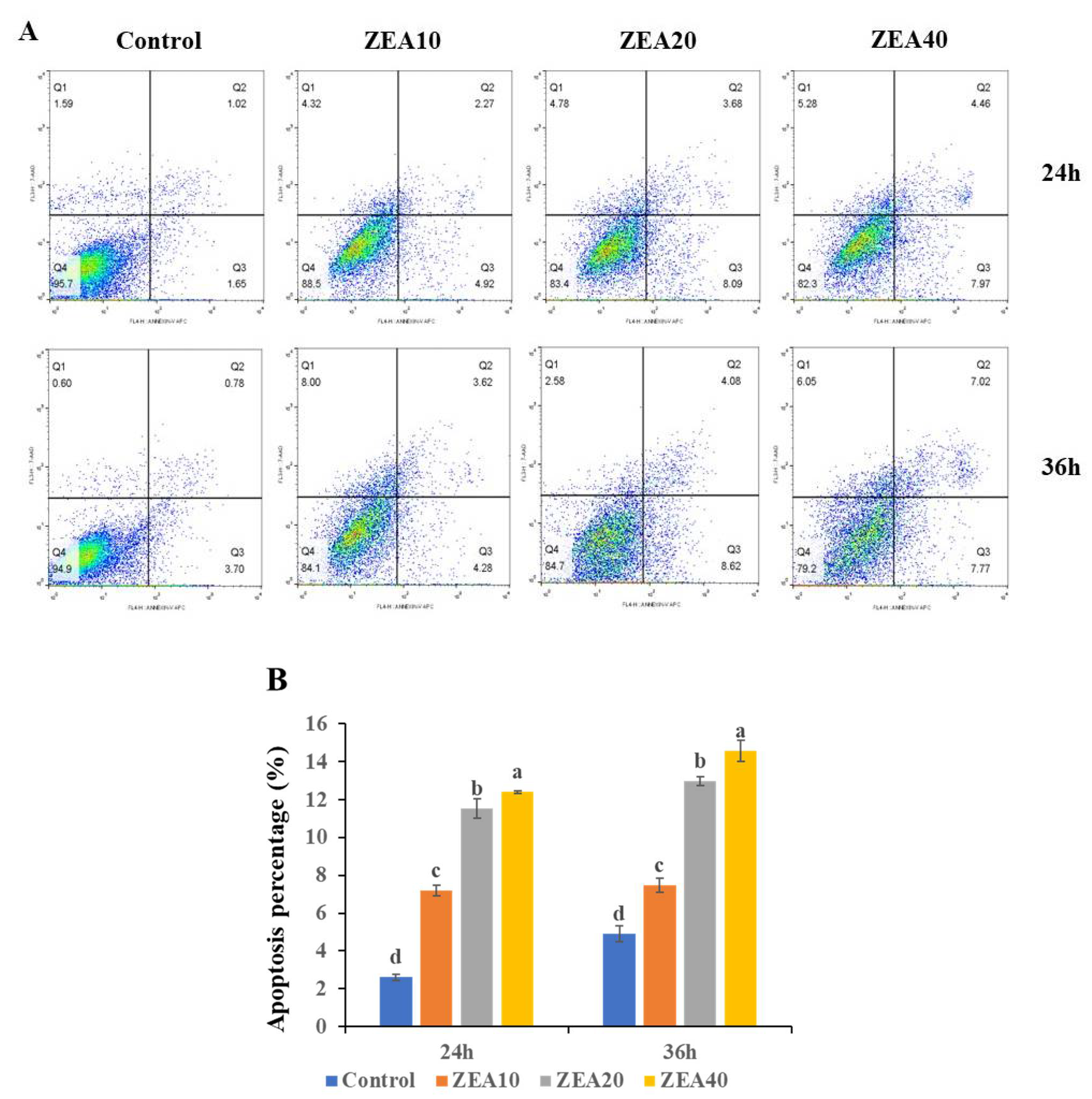
2.1. IPEC-J2 Cells’ Morphology and Apoptosis
2.2. The TEER of IPEC-J2 Cells
2.3. The Relative mRNA and Proteins Expression of Sglt1 and Glut2
2.4. The Immunofluorescence Localization of Sglt1 in IPEC-J2 Cells
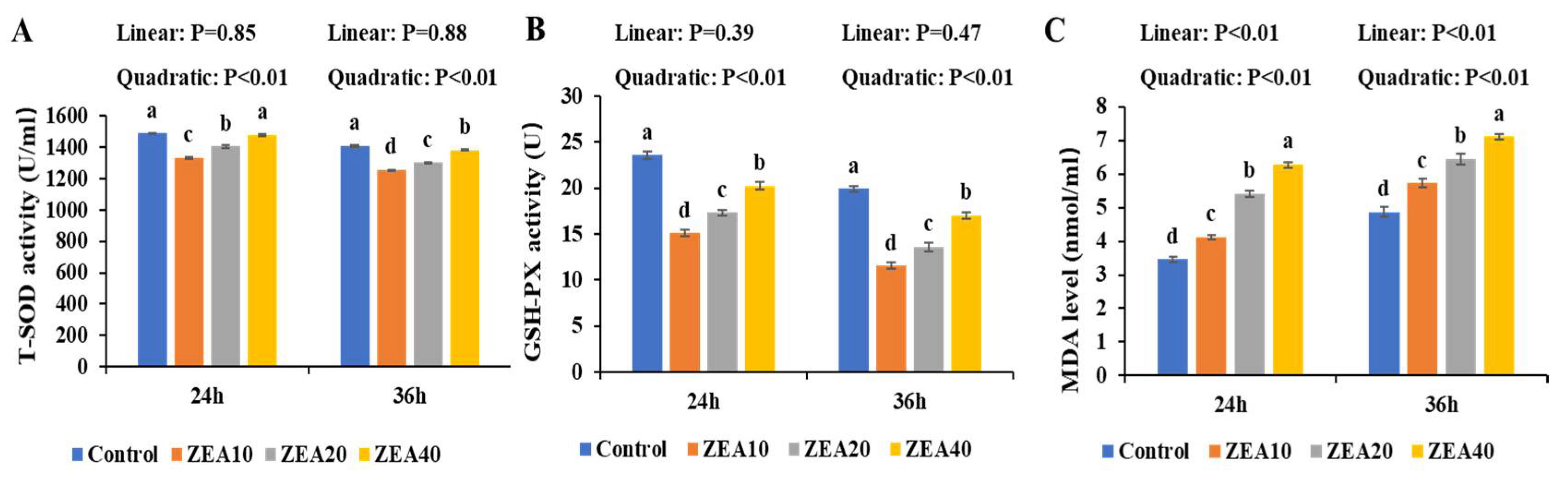
2.5. The Antioxidant Enzyme Activity of IPEC-J2 Cells
2.6. The Level ROS of IPEC-J2 Cells
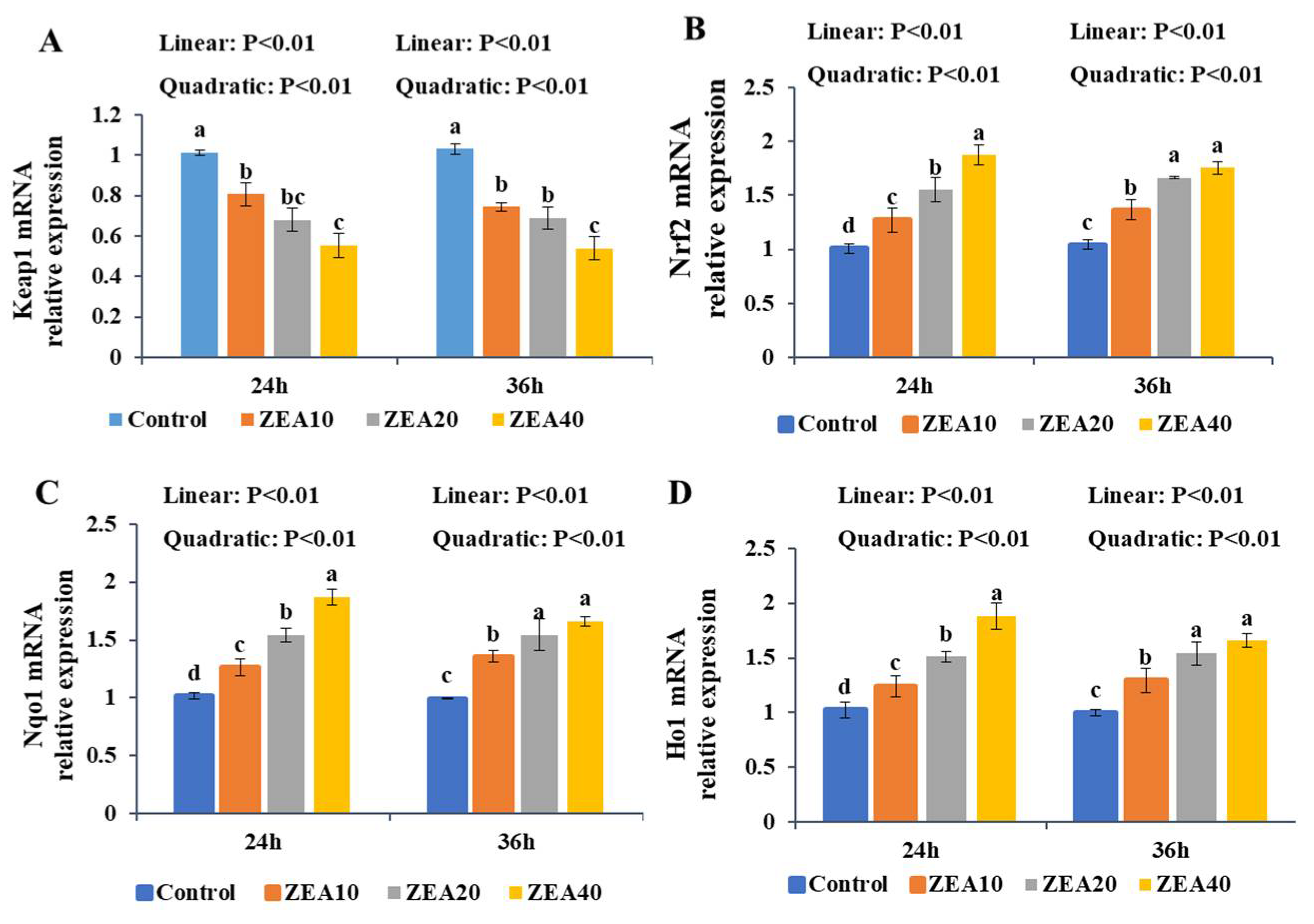
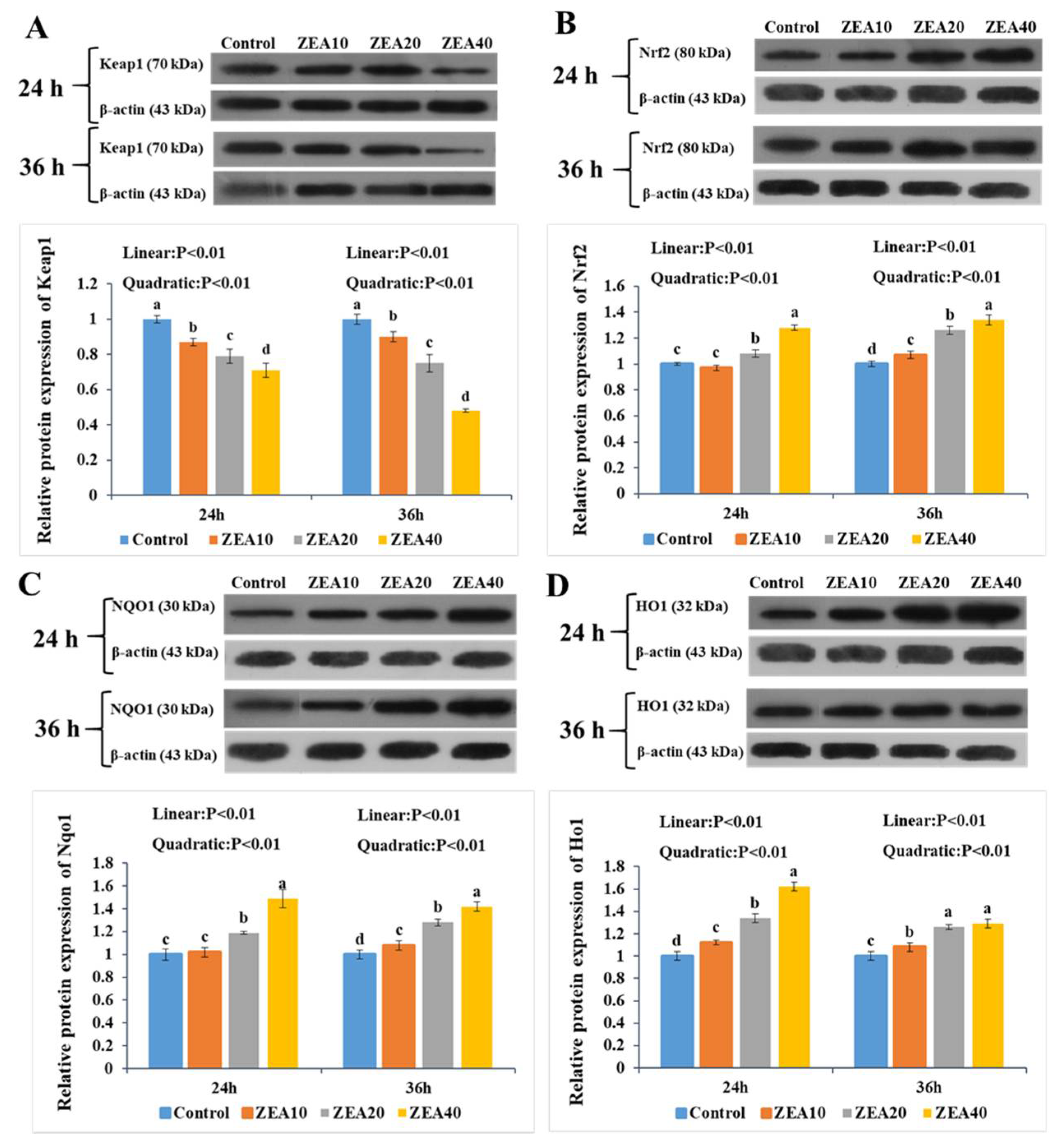
2.7. The Relative Expression of Keap1, Nrf2, Nqo1, and Ho1 mRNA and Protein
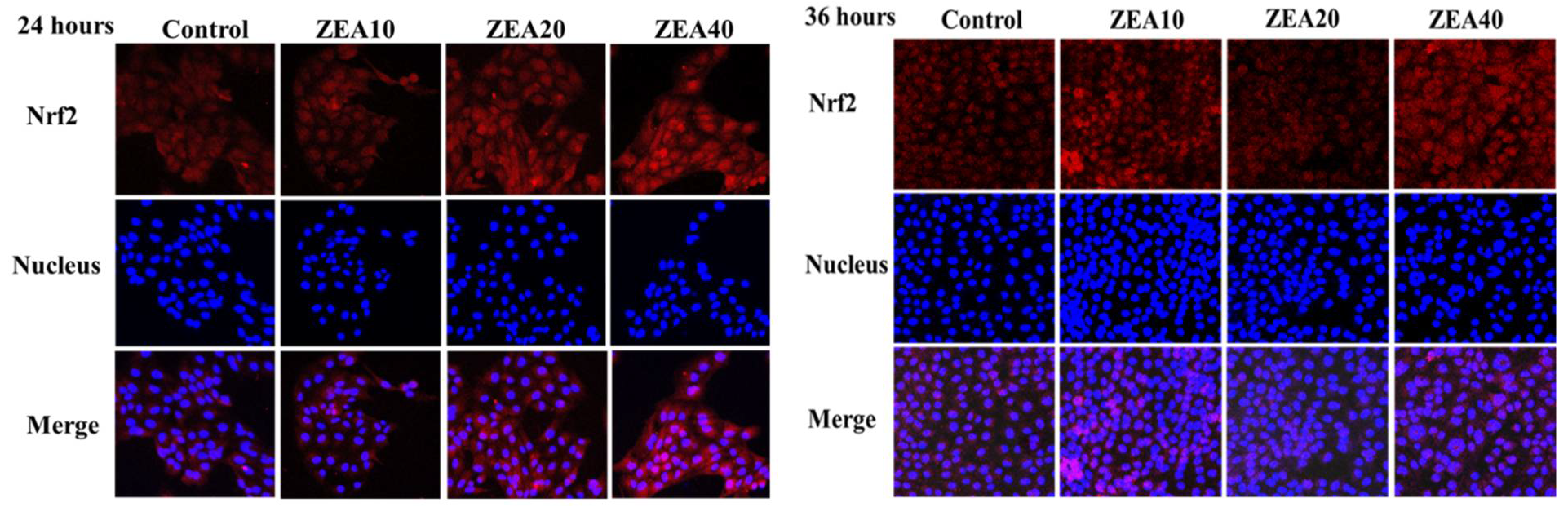
2.8. The Immunofluorescence localization of ROS and Nrf2 in the IPEC-J2 Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of IPEC-J2 Cells Culture
5.2. Preparation of ZEA Treatment of IPEC-J2 Cells
5.3. Determination of Apoptosis in IPEC-J2 Cells
5.4. Determination of Cell Transepithelial Electrical Resistance (TEER) of IPEC-J2 Cells
5.5. Determination of Antioxidant Enzyme Activity
5.6. Determinations of Relative mRNA Expressions
5.7. Determination of Sglt1, ROS, and Nrf2 Distribution in IPEC-J2 Cells
5.8. Determination of Protein Expression
5.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frizzell, C.; Ndossi, D.; Verhaegen, S.; Dahl, E.; Eriksen, G.; Sřrlie, M.; Ropstad, E.; Muller, M.; Elliott, C.T.; Connolly, L. Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol. Lett. 2011, 206, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Agahi, F.; Juan, C.; Font, G.; Juan-García, A. In silico methods for metabolomic and toxicity prediction of zearalenone, α-zearalenone and β-zearalenone. Food Chem. Toxicol. 2020, 146, 111818. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, S.; Berrada, H.; Juan-García, A.; Mañes, J.; Juan, C. Multiple mycotoxin determination on Tunisian cereals-based food and evaluation of the population exposure. Food Anal. Method. 2020, 13, 1271–1281. [Google Scholar] [CrossRef]
- Kawano, N.; Koji, T.; Hishikawa, Y.; Murase, K.; Murata, I.; Kohno, S. Identification and localization of estrogen receptor alpha- and beta-positive cells in adult male and female mouse intestine at various estrogen levels. Histochem. Cell Biol. 2004, 121, 399–405. [Google Scholar] [CrossRef]
- Wada-Hiraike, O.; Imamov, O.; Hiraike, H.; Hultenby, K.; Schwend, T.; Omoto, Y.; Warner, M.; Gustafsson, J.A. Role of estrogen receptor beta in colonic epithelium. Proc. Natl. Acad. Sci. USA 2006, 103, 2959–2964. [Google Scholar] [CrossRef]
- Long, M.; Chen, X.; Wang, N.; Wang, M.; Pan, J.; Tong, J.; Li, P.; Yang, S.; He, J. Proanthocyanidins Protect Epithelial Cells from Zearalenone-Induced Apoptosis via Inhibition of Endoplasmic Reticulum Stress-Induced Apoptosis Pathways in Mouse Small Intestines. Molecules 2018, 23, 1508. [Google Scholar] [CrossRef]
- Gerez, J.R.; Pinton, P.; Callu, P.; Grosjean, F.; Oswald, I.P.; Bracarense, A.P. Deoxynivalenol alone or incombination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2015, 67, 89–98. [Google Scholar] [CrossRef]
- Liu, M.; Gao, R.; Meng, Q.; Zhang, Y.; Bi, C.; Shan, A. Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats. PLoS ONE 2014, 9, e106412. [Google Scholar] [CrossRef]
- Marin, D.E.; Pistol, G.C.; Neagoe, I.V.; Calin, L.; Taranu, I. Effects of zearalenone on oxidative stress and inflammation in weanling piglets. Food Chem. Toxicol. 2015, 58, 408–415. [Google Scholar] [CrossRef]
- Taranu, I.; Braicu, C.; Marin, D.E.; Pistol, G.C.; Motiu, M.; Balacescu, L.; Neagoe, I.B.; Burlacu, R. Exposure to zearalenonemycotoxin alters in vitro porcine intestinal epithelial cells by differential gene expression. Toxicol. Lett. 2015, 232, 310–325. [Google Scholar] [CrossRef]
- Cheng, Q.; Jiang, S.Z.; Huang, L.B.; Ge, J.S.; Wang, Y.X.; Yang, W.R. Zearalenone induced oxidative stress in the jejunum in postweaning gilts through modulation of the Keap1–Nrf2 signaling pathway and relevant genes. J. Anim. Sci. 2019, 97, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Jiang, S.Z.; Huang, L.B.; Wang, Y.X.; Yang, W.R.; Yang, Z.B.; Ge, J.S. Effects of zearalenone-induced oxidative stress and Keap1–Nrf2 signaling pathway-related gene expression in the ileum and mesenteric lymph nodes of post-weaning gilts. Toxicology 2020, 429, 152337. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Jiang, S.Z.; Huang, L.B.; Yang, W.R.; Yang, Z.B. Zearalenone regulates key factors of the Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1-nuclear factor erythroid 2-related factor 2 signaling pathway in duodenum of post-weaning gilts. Anim. Biosci. 2021, 34, 1403–1414. [Google Scholar] [CrossRef]
- Klinger, S.; Lange, P.; Brandt, E.; Hustedt, K.; Schröder, B.; Breves, G.; Herrmann, J. Degree of SGLT1 phosphorylation is associated with but does not determine segment-specific glucose transport features in the porcine small intestines. Physiol. Rep. 2018, 6, e13562. [Google Scholar] [CrossRef]
- Röder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The Role of SGLT1 and GLUT2 in Intestinal Glucose Transport and Sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef]
- Farrell, T.L.; Ellam, S.L.; Forrelli, T.; Williamson, G. Attenuation of glucose transport across Caco-2 cell monolayers by a polyphenol-rich herbal extract: Interactions with SGLT1 and GLUT2 transporters. BioFactors 2013, 39, 448–456. [Google Scholar] [CrossRef]
- Grefner, N.M.; Gromova, L.V.; Gruzdkov, A.A.; Komissarchik, Y.Y. Interaction of glucose transporters SGLT1 and GLUT2 with cytoskeleton in enterocytes and Caco2 cells during hexose absorption. Cell Tissue Biol. 2015, 9, 45–52. [Google Scholar] [CrossRef]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2014, 58, 221–232. [Google Scholar] [CrossRef]
- Ana, F.; Monteiro, R.; Pestana, D.; Freitas, V.D.; Mateus, N.; Azevedo, I.; Calhau, C. Intestinal oxidative state can alter nutrient and drug bioavailability. Oxidative Med. Cell. Longev. 2009, 2, 322–327. [Google Scholar] [CrossRef]
- Nelson, A.; Cláudia, S.; Fátima, M. The effect of oxidative stress upon intestinal sugar transport: An in vitro study using human intestinal epithelial (Caco-2) cells. Toxicol. Res. 2018, 7, 1236–1246. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, L.; Yuan, Q.; Chen, H.; Lei, H.; Su, J. Effect of fumonisin B 1 on oxidative stress and gene expression alteration of nutrient transporters in porcine intestinal cells. J. Biochem. Mol. Toxicol. 2021, 35, e22706. [Google Scholar] [CrossRef] [PubMed]
- Nasu, K.; Nishida, M.; Kawano, Y.; Tsuno, A.; Abe, W.; Yuge, A. Aberrant expression of apoptosis-related molecules in endometriosis: A possible mechanism underlying the pathogenesis of endometriosis. Reprod. Sci. 2011, 18, 206–218. [Google Scholar] [CrossRef]
- Fadeel, B.; Orrenius, S.; Fadeel, B.; Orrenius, S. Apoptosis: A basic biological phenomenon with wide-ranging implications in human disease. J. Intern. Med. 2006, 258, 479–517. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, B.; Huang, K.; He, X.; Luo, Y.; Liang, R.; Luo, H.; Shen, X.L.; Xu, W. Mitochondrial proteomic analysis reveals the molecular mechanisms underlying reproductive toxicity of zearalenone in MLTC-1 cells. Toxicology 2014, 324, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Wang, J.Q.; Zheng, B.Q.; Li, S.L.; Zhang, Y.D.; Li, F.D.; Zheng, N. Cytotoxicity induced by ochratoxin A, zearalenone, and α-zearalenol: Effects of individual and combined treatment. Food Chem. Toxicol. 2014, 71, 17–24. [Google Scholar] [CrossRef]
- Kim, I.H.; Son, H.Y.; Cho, S.W.; Ha, C.S.; Kang, B.H. Zearalenone induces male germ cell apoptosis in rats. Toxicol. Lett. 2003, 138, 185–192. [Google Scholar] [CrossRef]
- Jee, Y.; Noh, E.-M.; Cho, E.-S.; Son, H.-Y. Involvement of the Fas and Fas ligand in testicular germ cell apoptosis by zearalenone in rat. J. Vet. Sci. 2010, 11, 115. [Google Scholar] [CrossRef]
- Karaman, E.F.; Zeybel, M.; Ozden, S. Evaluation of the epigenetic alterations and gene expression levels of HepG2 cells exposed to zearalenone and α-zearalenol. Toxicol. Lett. 2020, 326, 52–60. [Google Scholar] [CrossRef]
- Huang, W.; Chang, J.; Wang, P.; Liu, C.; Yin, Q.; Song, A.D.; Gao, T.Z.; Dang, X.W.; Lu, F.S. Effect of Compound Probiotics and Mycotoxin Degradation Enzymes on Alleviating Cytotoxicity of Swine Jejunal Epithelial Cells Induced by Aflatoxin B1 and Zearalenone. Toxins 2019, 11, 12. [Google Scholar] [CrossRef]
- Lei, M.Y.; Zhang, N.Y.; Qi, D.S. In vitro investigation of individual and combined cytotoxic effects of aflatoxin B1 and other selected mycotoxins on the cell line porcine kidney 15. Exp. Toxicol. Pathol. 2013, 65, 1149–1157. [Google Scholar] [CrossRef]
- Tatay, E.; Meca, G.; Font, G.; Ruiz, M.J. Interactive effects of zearalenone and its metabolites on cytotoxicity and metabolization in ovarian CHO-K1 cells. Toxicol. Vitr. 2014, 28, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi. Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Pinton, P.; Nougayrède, J.P.; Del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Ares, I.; Ramos, E.; Castellano, V.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology 2016, 353, 21–33. [Google Scholar] [CrossRef]
- Mclaughlin, J.; Padfield, P.J.; Burt, J.P.H.; O’Neill, C.A. Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms. J. Physiol.-Cell Physiol. 2004, 287, C1412–C1417. [Google Scholar] [CrossRef]
- Diesing, A.; Nossol, C.; Dänicke, S.; Walk, N.; Post, A.; Kahlert, S.; Rothkötter, H. Vulnerability of polarized intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application. PLoS ONE 2011, 6, e17472. [Google Scholar] [CrossRef]
- Kishida, K.; Iida, T.; Yamada, T.; Toyoda, Y. d-Allose is absorbed via sodium-dependent glucose cotransporter 1 (SGLT1) in the rat small intestine. Metab. Open 2021, 11, 100112. [Google Scholar] [CrossRef]
- Arya, A.; Al-Obaidi, M.M.; Karim, R.B.; Taha, H.; Khan, A.K.; Shahid, N.; Sayem, A.S.; Looi, C.Y.; Mustafa, M.R.; Mohd, M.A.; et al. Extract of Woodfordia fruticose flowers ameliorates hyperglycemia, oxidative stress and improves beta-cell function in streptozotocin-nicotinamide induced diabetic rats. J. Ethnopharmacol. 2015, 175, 229–240. [Google Scholar] [CrossRef]
- Debray-Garcia, Y.; Sanchez, E.I.; Rodriguez-Munoz, R.; Venegas, M.A.; Velazquez, J.; Reyes, J.L. Diabetes and exposure to peritoneal dialysis solutions alter tight junction proteins and glucose transporters of rat peritoneal mesothelial cells. Life Sci. 2016, 161, 78–89. [Google Scholar] [CrossRef]
- Yeong, J.; Tan, T.; Chow, Z.L.; Cheng, Q.; Lee, B.; Seet, A.; Lim, J.X.; Lim, J.C.T.; Ong, C.C.H.; Thike, A.A.; et al. Multiplex immunohistochemistry/immunofluorescence (mIHC/IF) for PD-L1 testing in triple-negative breast cancer: A translational assay compared with conventional IHC. J. Clin. Pathol. 2020, 73, 557–562. [Google Scholar] [CrossRef]
- Maresca, M.; Mahfoud, R.; Garmy, N.; Fantini, J. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J. Nutr. 2002, 9, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Mallebrera, B.; Juan-García, A.; Font, G.; Ruiz, M.J. Mechanisms of beauvericin toxicity and antioxidant cellular defense. Toxicol. Lett. 2016, 246, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Liu, M.; Qu, Z.; Zhang, Y.; Yin, S.; Shan, A. Toxic effects of zearalenone on oxidative stress, inflammatory cytokines, biochemical and pathological changes induced by this toxin in the kidney of pregnant rats. Environ. Toxicol. Pharmacol. 2014, 37, 580–591. [Google Scholar] [CrossRef]
- Ben Salem, I.; Prola, A.; Boussabbeh, M.; Guilbert, A.; Bacha, H.; Abid-Essefi, S.; Lemaire, C. Crocin and Quercetin protect HCT116 and HEK293 cells from Zearalenone-induced apoptosis by reducing endoplasmic reticulum stress. Cell Stress Chaperones 2015, 20, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Taranu, I.; Pistol, G.; Stancu, M. Effects of zearalenone and its metabolites on the swine epithelial intestinal cell line: IPEC 1. Proc. Nutr. Soc. 2013, 72, 85–89. [Google Scholar] [CrossRef]
- Qin, X.; Cao, M.; Lai, F.; Yang, F.G.W.; Zhang, X.; Cheng, S.; Sun, X.; Qin, G.; Shen, W.; Li, L. Oxidative stress induced by zearalenone in porcine granulosa cells and its rescue by curcumin in vitro. PLoS ONE 2015, 10, e0127551. [Google Scholar] [CrossRef]
- Mallebrera, B.; Font, G.; Ruiz, M.J. Disturbance of antioxidant capacity produced by beauvericin in CHO-K1 cells. Toxicol. Lett. 2014, 226, 337–342. [Google Scholar] [CrossRef]
- Zhou, J.; Ao, X.; Lei, Y.; Ji, C.; Ma, Q. Bacillus subtilis ansb01g culture alleviates oxidative stress and cell apoptosis induced by dietary zearalenone in first-parity gestation sows. Anim. Nutr. 2020, 6, 372–378. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.J. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis, and involves in Nrf2 signal pathway in primary broiler hepatocytes. Anim. Sci. J. 2016, 87, 1490–1500. [Google Scholar] [CrossRef]
- Gao, F.; Jiang, L.P.; Chen, M.; Geng, C.Y.; Yang, G.; Ji, F.; Zhong, L.F.; Liu, X.F. Genotoxic effects induced by zearalenone in a human embryonic kidney cell line. Mutat. Res.-Gen. Tox. En. 2013, 755, 6–10. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D.L. Activation of the p62-keap1-nrf2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, J.; Nie, S.; Ding, L.; Wang, L.; Liu, J.; Liu, W.; Zhang, T. Direct inhibition of keap1–nrf2 interaction by egg-derived peptides dkk and ddw revealed by molecular docking and fluorescence polarization. RSC Adv. 2017, 7, 34963–34971. [Google Scholar] [CrossRef]
- Jin, J.; Xiong, T.; Hou, X.; Sun, X.; Liao, J.; Huang, Z.; Huang, M.; Zhao, Z. Role of Nrf2 activation and NF-κB inhibition in valproic acid induced hepatotoxicity and in diammonium glycyrrhizinate induced protection in mice. Food Chem. Toxicol. 2014, 73, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Yang, Z.B.; Yang, W.R.; Wang, S.J.; Liu, F.X.; Johnston, L.A.; Chi, F.; Wang, Y. Effect of purified zearalenone with or without modified montmorillonite on nutrient availability, genital organs and serum hormones in post-weaning piglets. Livest. Sci. 2012, 144, 110–118. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]







| Target Gene | Primer Sequences (5’ to 3’) | Accession No. |
|---|---|---|
| Nrf2 | F: GAGTTAGATAGTGCCCCTGGAA R: ACTGGAGCACTATTACCCTGAG | XM_005671981.3 |
| Keap1 | F: GTGTGTGCTCCATGTCATGAAT R: CTCCCCAAAGTGCATGTAGATG | NM_001114671.1 |
| Ho1 | F: AGGTCCTCAAGAAGATTGCTCA R: CATCTCCAGAGTGTTCATTCGG | NM_001004027.1 |
| Nqo1 | F: AAAAGCACTGATCATACTGGCC R: TTCTGGAGATGACGGGATTGAA | NM_ 001159613.1 |
| Sglt1 | F: CGTCCATCTTTAACAGCAGCAG R: GCATGTAGATGAAGAGCTGCC | NM_001012297.1 |
| Glut2 | F: ACCGACAGCCTATTCTAGTAGC R: AGGAAAACAGAGAGAGCAGTGA | NM_001097417.1 |
| β-actin | F: AGATCACTCCCCCAATGACAG R: AGAGCAAGAGAGGCATCCTG | XM_003124280.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Q.; Jiang, S.; Huang, L.; Wang, Y.; Yang, W. Zearalenone Exposure Affects the Keap1–Nrf2 Signaling Pathway and Glucose Nutrient Absorption Related Genes of Porcine Jejunal Epithelial Cells. Toxins 2022, 14, 793. https://doi.org/10.3390/toxins14110793
Cheng Q, Jiang S, Huang L, Wang Y, Yang W. Zearalenone Exposure Affects the Keap1–Nrf2 Signaling Pathway and Glucose Nutrient Absorption Related Genes of Porcine Jejunal Epithelial Cells. Toxins. 2022; 14(11):793. https://doi.org/10.3390/toxins14110793
Chicago/Turabian StyleCheng, Qun, Shuzhen Jiang, Libo Huang, Yuxi Wang, and Weiren Yang. 2022. "Zearalenone Exposure Affects the Keap1–Nrf2 Signaling Pathway and Glucose Nutrient Absorption Related Genes of Porcine Jejunal Epithelial Cells" Toxins 14, no. 11: 793. https://doi.org/10.3390/toxins14110793
APA StyleCheng, Q., Jiang, S., Huang, L., Wang, Y., & Yang, W. (2022). Zearalenone Exposure Affects the Keap1–Nrf2 Signaling Pathway and Glucose Nutrient Absorption Related Genes of Porcine Jejunal Epithelial Cells. Toxins, 14(11), 793. https://doi.org/10.3390/toxins14110793




