Nanobody-Nanoluciferase Fusion Protein-Enabled Immunoassay for Ochratoxin A in Coffee with Enhanced Specificity and Sensitivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
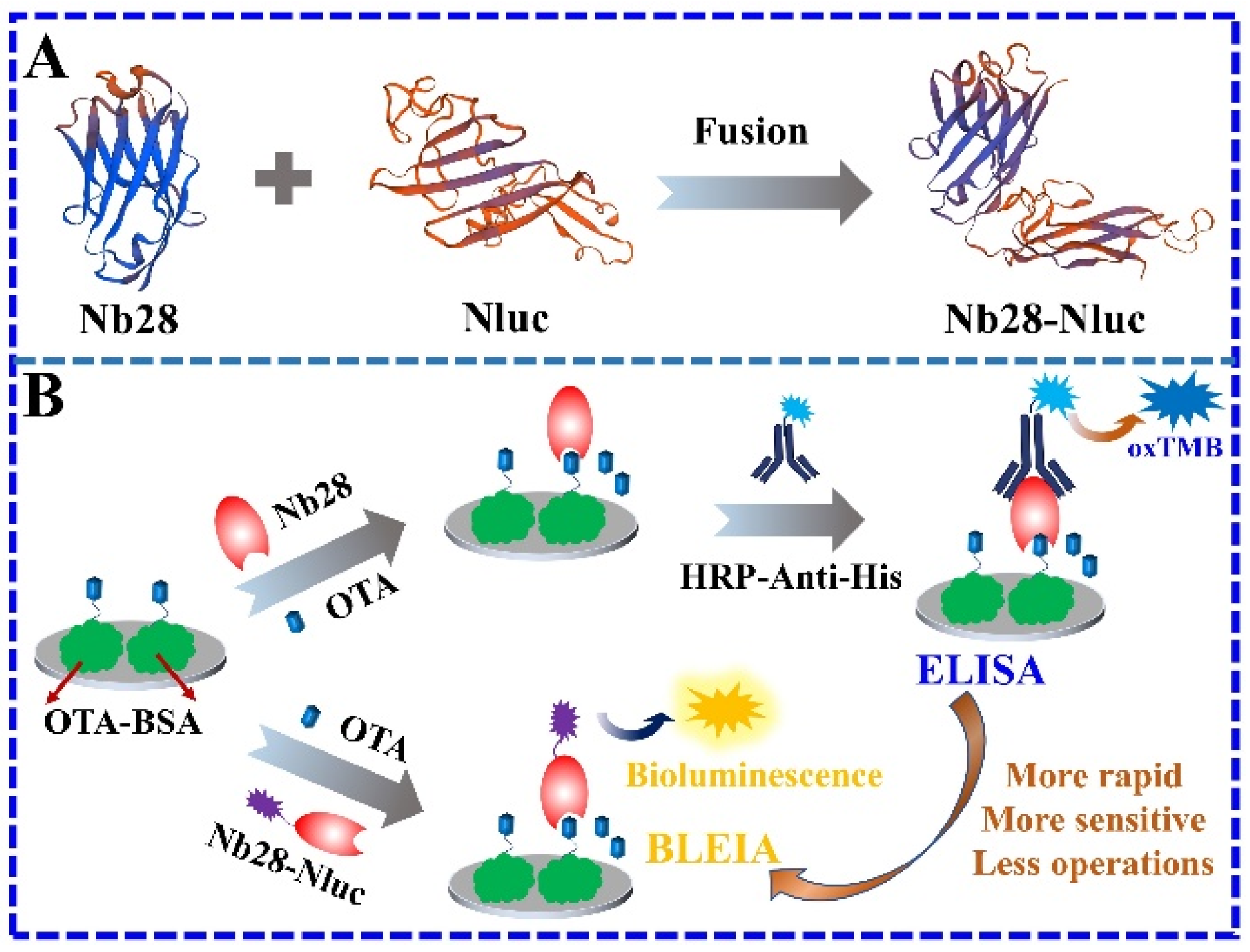
2.2. Construction of the pET25b-Nb28-Nluc Expression Vector
2.3. Expression and Identification of the Nb28-Nluc Fusion Protein
2.4. Construction of BLEIA Based on Nb28-Nluc Bifunctional Tracer
2.5. Specificity Evaluation of the Developed BLEIA
2.6. Practicability of the Assay
3. Results and Discussion
3.1. Expression, Characterisation and Purification of the Nb28-Nluc Fusion Protein
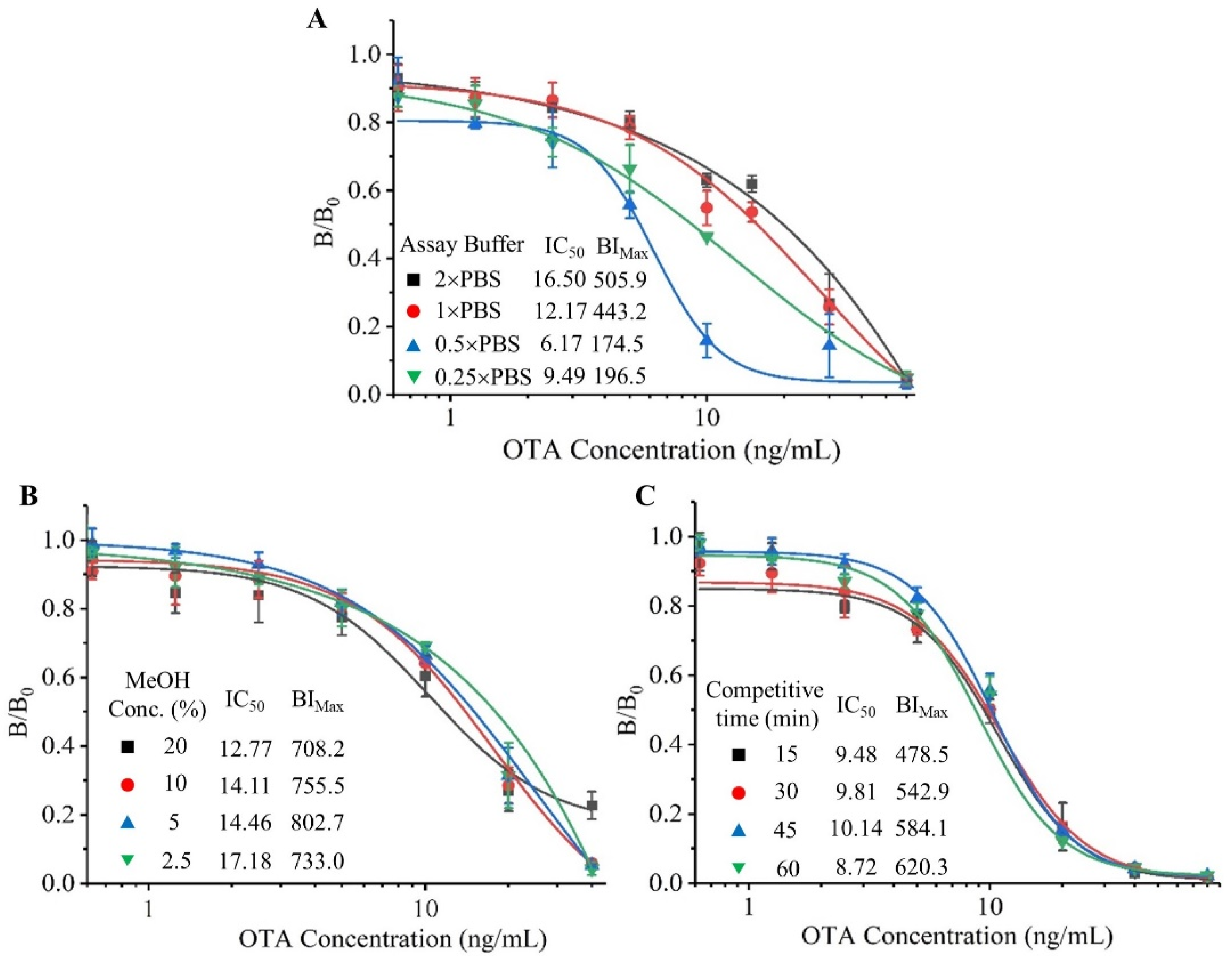
3.2. Optimization of BLEIA for OTA Detection
3.3. Cross-Reactivity of the Developed BLEIA
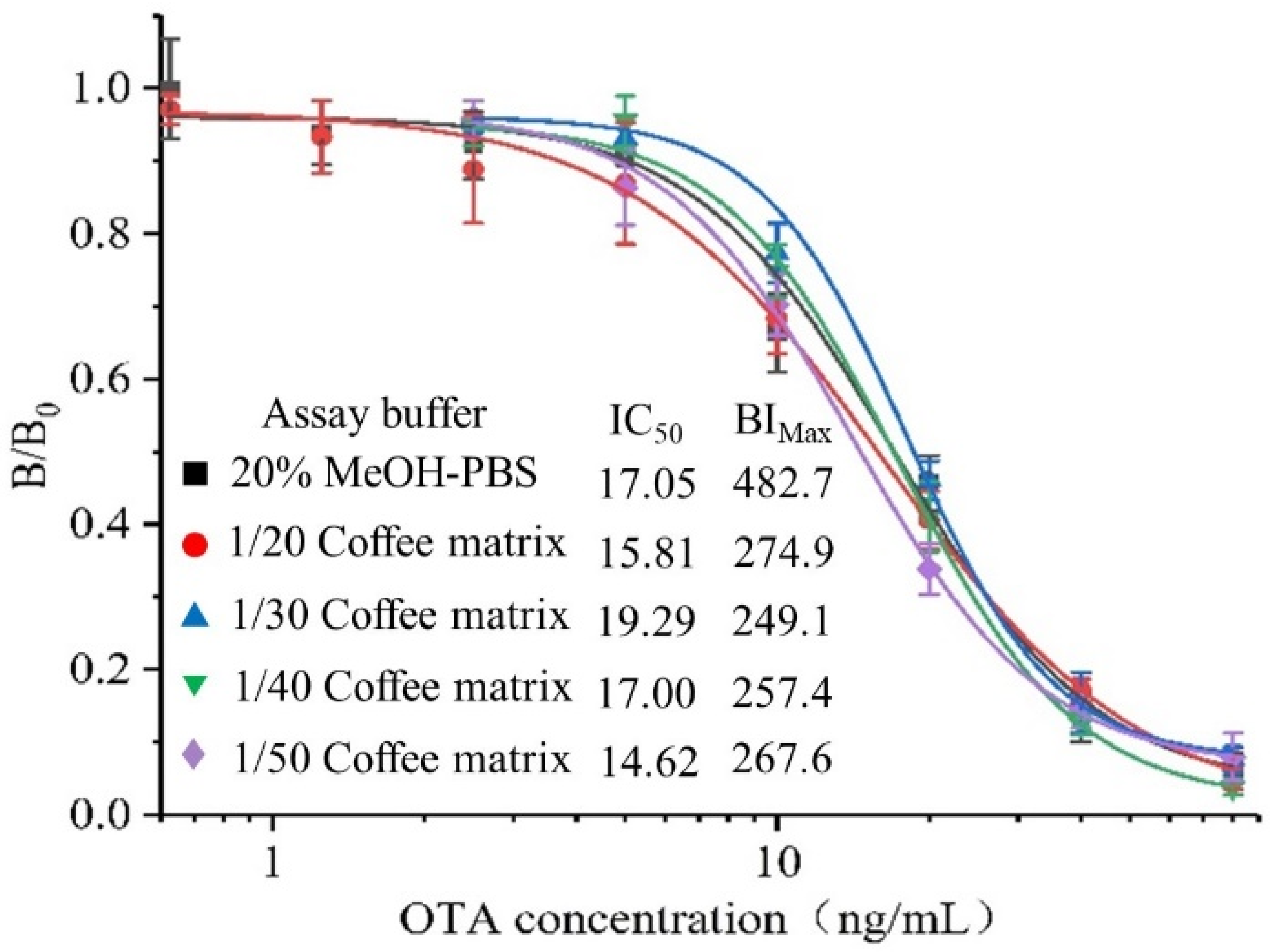
3.4. Matrix Effect
3.5. Practicability of the Developed BLEIA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sousa, T.M.A.; Batista, L.R.; Passamani, F.R.F.; Lira, N.A.; Cardoso, M.G.; Santiago, W.D.; Chalfoun, S.M. Evaluation of the effects of temperature on processed coffee beans in the presence of fungi and ochratoxin A. J. Food Saf. 2019, 39, e12584. [Google Scholar] [CrossRef]
- Wu, J.W.; Tan, Y.F.; Wang, Y.Q.; Xu, R. Occurrence of Ochratoxin A in Grain and Manufactured Food Products in China Detected by HPLC with Fluorescence Detection and Confirmed by LC-ESI-MS/MS. Mycopathologia 2012, 173, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kolakowski, B.; O’Rourke, S.M.; Bietlot, H.P.; Kurz, K.; Aweryn, B. Ochratoxin A Concentrations in a Variety of Grain-Based and Non-Grain-Based Foods on the Canadian Retail Market from 2009 to 2014. J. Food Prot. 2016, 79, 2143–2159. [Google Scholar] [CrossRef] [PubMed]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2010, 51, 61–99. [Google Scholar] [CrossRef] [PubMed]
- Taradolsirithitikul, P.; Sirisomboon, P.; Sirisomboon, C.D. Qualitative and quantitative analysis of ochratoxin A contamination in green coffee beans using Fourier transform near infrared spectroscopy. J. Sci. Food Agric. 2017, 97, 1260–1266. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- Costa, J.G.; Saraiva, N.; Guerreiro, P.S.; Louro, H.; Oliveira, N.G. Ochratoxin A-induced cytotoxicity, genotoxicity and reactive oxygen species in kidney cells: An integrative approach of complementary endpoints. Food Chem. Toxicol. 2016, 87, 65–76. [Google Scholar] [CrossRef]
- Lemming, E.W.; Montes, A.M.; Schmidt, J.; Cramer, B.; Humpf, H.U.; Moraeus, L.; Olsen, M. Mycotoxins in blood and urine of Swedish adolescents-possible associations to food intake and other background characteristics. Mycotoxin Res. 2020, 36, 193–206. [Google Scholar] [CrossRef]
- Guimares, E.R.; Santos, A.C.D.; Leme, P.H.M.V.; Azevedo, A.D.S. Direct Trade in the Specialty Coffee Market: Contributions, Limitations and New Lines of Research. Internext 2020, 15, 34. [Google Scholar] [CrossRef]
- Gebre, E. Factors Affecting Coffee Market Supply of Smallholder Farm Household: The Case of Gewata District Kaffa Zone, Southwest Ethiopia. Int. J. Econ. Financ. Res. 2020, 6, 14–21. [Google Scholar] [CrossRef]
- Commission, E. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 365–388. [Google Scholar]
- Quintela, S.; Ma, C.V.; Armentia, I.; Elejalde, E. Ochratoxin A in Spanish exportation wine market. Food Control 2012, 25, 501–504. [Google Scholar] [CrossRef]
- Mari?O-Repizo, L.; Gargantini, R.; Manzano, H.; Raba, J.; Cerutti, S. Assessment of ochratoxin A occurrence in Argentine red wines using a novel sensitive quechers-solid phase extraction approach prior to ultra high performance liquid chromatography-tandem mass spectrometry methodology. J. Sci. Food Agric. 2016, 97, 1260–1266. [Google Scholar]
- Zou, X.; Chen, C.; Huang, X.; Chen, X.; Xiong, Y. Phage-free peptide ELISA for ochratoxin A detection based on biotinylated mimotope as A competing antigen. Talanta 2016, 146, 394–400. [Google Scholar] [CrossRef] [PubMed]
- López-Puertollano, D.; Agulló, C.; Mercader, J.V.; Abad-Somovilla, A.; Abad-Fuentes, A. Immunoanalytical methods for ochratoxin A monitoring in wine and must based on innovative immunoreagents. Food Chem. 2021, 345, 128828. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Y.; Chen, Y.; Weng, B.; Xu, L.; Li, C. A highly sensitive aptasensor for OTA detection based on hybridization chain reaction and fluorescent perylene probe. Biosens. Bioelectron. 2016, 81, 125–130. [Google Scholar] [CrossRef]
- Xiong, Y.; Leng, Y.; Li, X.; Huang, X.; Xiong, Y. Emerging strategies to enhance the sensitivity of competitive ELISA for detection of chemical contaminants in food samples. Trends Anal. Chem. 2020, 126, 115861. [Google Scholar] [CrossRef]
- Wu, L.; Li, G.; Xu, X.; Zhu, L.; Huang, R.; Chen, X. Application of nano-ELISA in food analysis: Recent advances and challenges. Trends Anal. Chem. 2019, 113, 140–156. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Eyer, L.; Hruska, K. Single-domain antibody fragments derived from heavy-chain antibodies: A review. Veterinární Med. 2012, 57, 439–513. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Wang, Y.; Chen, Q.; Liu, X. Nanobody-alkaline phosphatase fusion-mediated phosphate-triggered fluorescence immunoassay for ochratoxin a detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117617. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhu, Y.; Wu, T.; Cheng, J.; Liu, Y. Nanobody-Ferritin Conjugate for Targeted Photodynamic Therapy. Chemistry 2020, 26, 7442–7450. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.Z.; Li, X.X.; Zhao, J.K.; Zhu, J.H.; Luo, Y.H.; Duan, H.; Ji, P.P.; Wang, K.; Liu, B.Y.; Wang, X.T.; et al. Nanobody-horseradish peroxidase and -EGFP fusions as reagents to detect porcine parvovirus in the immunoassays. J. Nanobiotechnol. 2020, 18, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.W.; Liu, X.; Su, B.C.; Chen, Q.; Cao, H.M.; Yun, Y.H.; Xu, Y.; Hammock, B.D. Ultrasensitive and rapid detection of ochratoxin A in agro-products by a nanobody-mediated FRET-based immunosensor. J. Hazard. Mater. 2020, 387, 121678. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Vasylieva, N.; Wan, D.; Yin, Z.; Dong, J.; Hammock, B.D. An Ultra-Sensitive Bioluminscent Enzyme Immunoassay Based on Nanobody/Nanoluciferase Heptamer Fusion for the Detection of Tetrabromobisphenol A in Sediment. Anal. Chem. 2020, 92, 10083–10090. [Google Scholar] [CrossRef]
- Boutel, N.; Lowe, P.; Berger, S.; Malissard, M.; Robert, A.; Tesar, M. NanoLuc Luciferase—A Multifunctional Tool for High Throughput Antibody Screening. Front. Pharmacol. 2016, 7, 00027. [Google Scholar]
- England, C.G.; Ehlerding, E.B.; Cai, W. NanoLuc: A Small Luciferase Is Brightening Up the Field of Bioluminescence. Bioconjugate Chem. 2016, 27, 1175–1187. [Google Scholar] [CrossRef]
- Sun, S.; Yang, X.; Wang, Y.; Shen, X. In Vivo Analysis of Protein-Protein Interactions with Bioluminescence Resonance Energy Transfer (BRET): Progress and Prospects. Int. J. Mol. Sci. 2016, 17, 1704. [Google Scholar] [CrossRef]
- Duong, H.D.; Appiah-Kwarteng, C.; Takashima, Y.; Aye, K.M.; Nagayasu, E.; Yoshida, A. A novel luciferase-linked antibody capture assay (LACA) for the diagnosis of Toxoplasma gondii infection in chickens. Parasitol. Int. 2020, 77, 102125. [Google Scholar] [CrossRef]
- Kamkaew, A.; Sun, H.; England, C.G.; Cheng, L.; Liu, Z.; Cai, W. Quantum dot-NanoLuc bioluminescence resonance energy transfer enables tumor imaging and lymph node mapping in vivo. Chem. Commun. 2016, 52, 6997–7000. [Google Scholar] [CrossRef]
- Ren, W.; Li, Z.; Xu, Y.; Wan, D.; Barnych, B.; Li, Y.; Tu, Z.; He, Q.; Fu, J.; Hammock, B.D. One-Step Ultrasensitive Bioluminescent Enzyme Immunoassay Based on Nanobody/Nanoluciferase Fusion for Detection of Aflatoxin B1 in Cereal. J. Agric. Food Chem. 2019, 67, 5221–5229. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Z.; Yang, Y.Y.; Wan, D.; Vasylieva, N.; Zhang, Y.Q.; Cai, J.; Wang, H.; Shen, Y.D.; Xu, Z.L.; et al. Chemiluminescent Enzyme Immunoassay and Bioluminescent Enzyme Immunoassay for Tenuazonic Acid Mycotoxin by Exploitation of Nanobody and Nanobody-Nanoluciferase Fusion. Anal. Chem. 2020, 92, 11935–11942. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, D.Y.; Pei, H.; Su, B.C.; Bao, K.L.; Cao, H.M.; Zhang, C.H.; Hammockc, B.D.; Liu, X. Development of a nanobody tagged with streptavidin-binding peptide and its application in a Luminex fluoroimmunoassay for alpha fetal protein in serum. RSC Adv. 2020, 10, 23767–23774. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.L.; Liu, X.; Xu, Q.; Su, B.C.; Liu, Z.L.; Cao, H.M.; Chen, Q. Nanobody multimerization strategy to enhance the sensitivity of competitive ELISA for detection of ochratoxin A in coffee samples. Food Control 2021, 126, 108167. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Xiong, Y.H.; Tu, Z.; Li, Y.P.; He, Z.Y.; Qiu, Y.L.; Fu, J.H.; Gee, S.J.; Hammock, B.D. VHH Phage-Based Competitive Real-Time Immuno-Polymerase Chain Reaction for Ultrasensitive Detection of Ochratoxin A in Cereal. Anal. Chem. 2014, 86, 7471–7477. [Google Scholar] [CrossRef] [PubMed]

| Spiked OTA (mg/kg) | Nb28-Nluc-Based BLEIA | HPLC-FLD | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Recovery (%) | RSD (%) | Mean ± SD | Recovery (%) | RSD (%) | ||
| Intra-assay (n = 3) | 1 | 1.10 ± 0.17 | 110.08% | 15.9% | 1.01 ± 0.09 | 101.27% | 9.19% |
| 2 | 1.98 ± 0.22 | 98.92% | 11.3% | 2.03 ± 0.13 | 101.33% | 6.28% | |
| 3 | 2.65 ± 0.14 | 88.41% | 5.2% | 2.68 ± 0.24 | 89.22% | 9.13% | |
| Inter-assay (n = 3) | 1 | 1.20 ± 0.30 | 120.23% | 24.7% | 1.00 ± 0.10 | 99.93% | 10.47% |
| 2 | 1.85 ± 0.17 | 92.63% | 9.3% | 2.03 ± 0.14 | 101.56% | 6.68% | |
| 3 | 2.52 ± 0.17 | 83.88% | 6.9% | 2.82 ± 0.18 | 94.11% | 6.42% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, K.; Liu, X.; Liao, Y.; Liu, Z.; Cao, H.; Wu, L.; Chen, Q. Nanobody-Nanoluciferase Fusion Protein-Enabled Immunoassay for Ochratoxin A in Coffee with Enhanced Specificity and Sensitivity. Toxins 2022, 14, 713. https://doi.org/10.3390/toxins14100713
Bao K, Liu X, Liao Y, Liu Z, Cao H, Wu L, Chen Q. Nanobody-Nanoluciferase Fusion Protein-Enabled Immunoassay for Ochratoxin A in Coffee with Enhanced Specificity and Sensitivity. Toxins. 2022; 14(10):713. https://doi.org/10.3390/toxins14100713
Chicago/Turabian StyleBao, Kunlu, Xing Liu, Yujing Liao, Zilong Liu, Hongmei Cao, Long Wu, and Qi Chen. 2022. "Nanobody-Nanoluciferase Fusion Protein-Enabled Immunoassay for Ochratoxin A in Coffee with Enhanced Specificity and Sensitivity" Toxins 14, no. 10: 713. https://doi.org/10.3390/toxins14100713
APA StyleBao, K., Liu, X., Liao, Y., Liu, Z., Cao, H., Wu, L., & Chen, Q. (2022). Nanobody-Nanoluciferase Fusion Protein-Enabled Immunoassay for Ochratoxin A in Coffee with Enhanced Specificity and Sensitivity. Toxins, 14(10), 713. https://doi.org/10.3390/toxins14100713






