Early Adverse Reactions to Snake Antivenom: Poison Center Data Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design
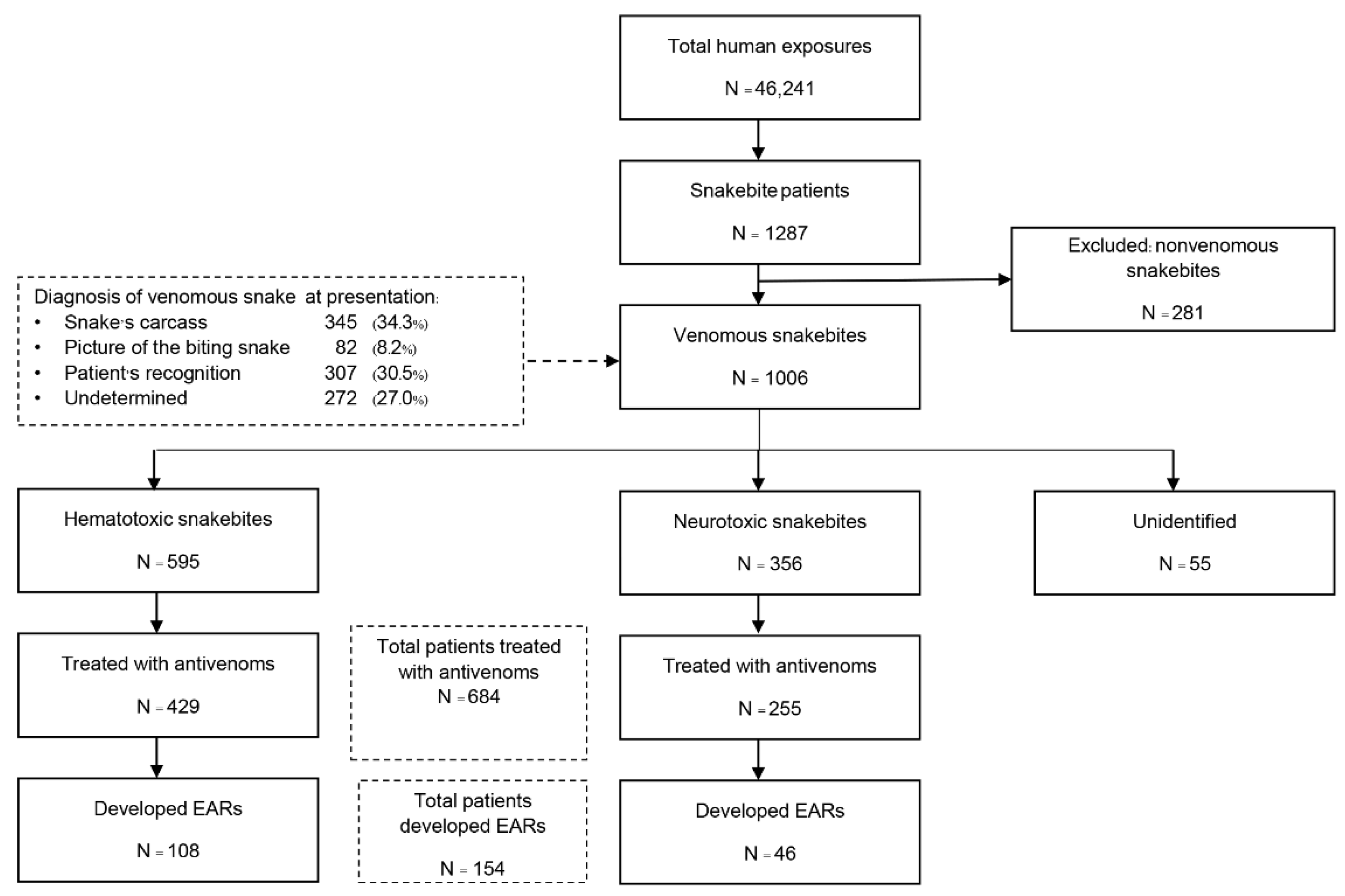
2.2. Study Site and Population
2.3. Study Protocol
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Snakebite-Envenomed Patients and Antivenom Therapy
3.2. Incidences of Early Adverse Reactions to Antivenom Per Patient
3.3. Early Adverse Reaction Rate Per Dose of Each Antivenom
4. Clinical Features, Severity Grading, and Treatment of Patients with EARs
Factors Associated with EARs
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Disclosure Statement
References
- World Health Organization. Snakebite Envenoming: A Strategy for Prevention and Control; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151564-1.
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; De Silva, H.J. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Thumtecho, S.; Tangtrongchitr, T.; Srisuma, S.; Kaewrueang, T.; Rittilert, P.; Pradoo, A.; Tongpoo, A.; Wananukul, W. Hematotoxic Manifestations and Management of Green Pit Viper Bites in Thailand. Ther. Clin. Risk Manag. 2020, 16, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Tongpoo, A.; Sriapha, C.; Pradoo, A.; Udomsubpayakul, U.; Srisuma, S.; Wananukul, W.; Trakulsrichai, S. Krait envenomation in Thailand. Ther. Clin. Risk Manag. 2018, 14, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Tangtrongchitr, T.; Thumtecho, S.; Janprasert, J.; Sanprasert, K.; Tongpoo, A.; Tanpudsa, Y.; Trakulsrichai, S.; Wananukul, W.; Srisuma, S. Malayan Pit Viper Envenomation and Treatment in Thailand. Ther. Clin. Risk Manag. 2021, 17, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Bureau of Epidemiology Department of Disease Control, Ministry of Public Health. Annual Epidemiological Surveillance Report 2018. Available online: https://apps-doe.moph.go.th/boeeng/download/AW_Annual_Mix%206212_14_r1.pdf (accessed on 19 May 2022).
- WHO Regional Office for South-East Asia. Guidelines for the Management of Snakebites, 2nd ed.; WHO Regional Office for South-East Asia: New Delhi, India, 2016; ISBN 978-929-022-530-0. [Google Scholar]
- Leon, G.; Herrera, M.; Segura, A.; Villalta, M.; Vargas, M.; Gutierrez, J.M. Pathogenic Mechanisms Underlying Adverse Reactions Induced by Intravenous Administration of Snake Antivenoms. Toxicon 2013, 76, 63–76. [Google Scholar] [CrossRef]
- Clark, R.F.; McKinney, P.E.; Chase, P.B.; Walter, F.G. Immediate and delayed allergic reactions to Crotalidae polyvalent immune Fab (ovine) antivenom. Ann. Emerg. Med. 2002, 39, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Dart, R.C.; McNally, J. Efficacy, safety, and use of snake antivenoms in the United States. Ann. Emerg. Med. 2001, 37, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Thiansookon, A.; Rojnuckarin, P. Low incidence of early reactions to horse-derived F(ab′)2 antivenom for snakebites in Thailand. Acta Trop. 2008, 105, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.R.; Mamun, S.M.H.; Rashid, R.; Rahman, M.; Ghose, A.; Sharmin, S.; Rahman, M.R.; Faiz, M.A. An-ti-snake Venom: Use and Adverse Reaction in a Snake Bite Study Clinic in Bangladesh. J. Venom. Anim. Toxins Incl. Trop. Dis. 2008, 14, 660–672. [Google Scholar] [CrossRef]
- Gutiérrez, J.M. Global Availability of Antivenoms: The Relevance of Public Manufacturing Laboratories. Toxins 2018, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Wangpradit, O.; Chokdeesrijun, C. UHC Investment and Management to Access Orphan Antidotes. In The Companion Book for Field Trips in PMAC 2020, UHC Forum 2020; Thammatacharee, J., Turner, K., Jamniandamrongkarn, S., Eds.; Sahamitr Printing & Publishing Co., Ltd.: Nonthaburi, Thailand, 2020; pp. 117–133. ISBN 978-616-490-022-6. [Google Scholar]
- Needleman, R.K.; Neylan, I.P.; Erickson, T. Potential Environmental and Ecological Effects of Global Climate Change on Venomous Terrestrial Species in the Wilderness. Wilderness Environ. Med. 2018, 29, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G. Snakebite: When the Human Touch Becomes a Bad Touch. Toxins 2018, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Vongphoumy, I.; Chanthilat, P.; Vilayvong, P.; Blessmann, J. Prospective, consecutive case series of 158 snakebite patients treated at Savannakhet provincial hospital, Lao People’s Democratic Republic with high incidence of anaphylactic shock to horse derived F(ab’)2 antivenom. Toxicon 2016, 117, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Mong, R.; Ng, V.C.H.; Tse, M.L. Safety Profile of Snake Antivenom (use) in Hong Kong-A Review of 191 Cases from 2008 to 2015. Clin. Toxicol. 2017, 55, 1066–1071. [Google Scholar] [CrossRef]
- Veto, T.; Price, R.; Silsby, J.F.; Carter, J.A. Treatment of the first known case of king cobra envenomation in the United Kingdom, complicated by severe anaphylaxis. Anaesthesia 2007, 62, 75–78. [Google Scholar] [CrossRef]
- Jagpal, P.S.; Williams, H.A.; Eddleston, M.; Lalloo, D.; Warrell, D.; Sandilands, E.A.; Thanacoody, R.; Gray, L.; Bradberry, S.M. Bites by Exotic Snakes Reported to the UK National Poisons Information Service 2009–2020. Clin. Toxicol. 2022, 19, 1–7. [Google Scholar] [CrossRef]
- Queen Saovabha Memorial Institute. The Thai Red Cross Society. Development and Progress in Biological Pro-duction, Queen Saovabha Memorial Institute 1922–2014. In Commemoration on the Auspicious Occasion of Her Royal Highness Princess Maha Chaki Sirindhorn 5th Cycle Birthday Anniversary; ID Print Co., Ltd.: Bangkok, Thailand, 2015; ISBN 978-616-782-940-1. [Google Scholar]
- Fung, H.T.; Lam, K.K.; Kam, C.W. Efficacy and Safety of Snake Antivenom Therapy: Experience of a Regional Hospital. Hong Kong J. Emerg. Med. 2006, 13, 70–78. [Google Scholar] [CrossRef]
- Chanhome, L.; Cox, M.J.; Vasaruchapong, T.; Chaiyabutra, N.; Sitprija, V. Characterization of Venomous Snakes of Thailand. Asian Biomed 2011, 5, 311–328. [Google Scholar] [CrossRef]
- Thai National Parks Snake Species in Thailand. Available online: https://www.thainationalparks.com/list-of-snakes-in-thailand (accessed on 19 May 2022).
- Klinpaj, O. Ultrafiltration for Purification and Concentration of Snake Antivenom. Thai. Pharm. Health Sci. J. 2020, 15, 49–55. [Google Scholar]
- Queen Saovabha Memorial Institute, The Thai Red Cross Society. Available online: http://nvi.ddc.moph.go.th/e-books/qsmi.pdf (accessed on 19 September 2022).
- Sibunruang, S.; Suteparuk, S.; Sitprija, V. Manual of Practical Management of Snakebites and Animal Toxin Injury; Pentagon Advertising: Bangkok, Thailand, 2013; ISBN 978-616-782-789-8. [Google Scholar]
- Suteparuk, S. Snake Antivenoms. In Antidote Book 3; Sriapha, C., Ed.; Scan and Print Co., Ltd.: Samut Prakan, Thailand, 2013; pp. 29–34. ISBN 978-616-348-099-6. [Google Scholar]
- Suchonwanich, N.; Wananukul, W. Improving access to antidotes and antivenoms, Thailand. Bull. World Heal. Organ. 2018, 96, 853–857. [Google Scholar] [CrossRef]
- Rojnuckarin, P.; Suteparak, S.; Sibunruang, S. Diagnosis and Management of Venomous Snakebites in Southeast Asia. Asian Biomed 2012, 6, 795–805. [Google Scholar] [CrossRef]
- Brown, S.G. Clinical features and severity grading of anaphylaxis. J. Allergy Clin. Immunol. 2004, 114, 371–376. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Ardusso, L.R.F.; Bilò, M.B.; El-Gamal, Y.M.; Ledford, D.K.; Ring, J.; Sanchez-Borges, M.; Senna, G.E.; Sheikh, A.; Thong, B.Y.; et al. World Allergy Organization Guidelines for the Assessment and Management of Anaphylaxis. World Allergy Organ. J. 2011, 4, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, C.; Jürgensen, J.A.; Føns, S.; Haack, A.M.; Friis, R.U.W.; Dam, S.H.; Bush, S.P.; White, J.; Laustsen, A.H. Snakebite Envenoming Diagnosis and Diagnostics. Front. Immunol. 2021, 12, 661457. [Google Scholar] [CrossRef]
- De Silva, H.A.; Ryan, N.M.; de Silva, H.J. Adverse reactions to snake antivenom, and their prevention and treatment. Br. J. Clin. Pharmacol. 2016, 81, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Leon, G.; Lomonte, B.; Angulo, Y. Antivenoms for snakebite envenomings. Inflamm. Allergy Drug Targets 2011, 10, 369–380. [Google Scholar] [CrossRef]
- Stone, S.F.; Isbister, G.K.; Shahmy, S.; Mohamed, F.; Abeysinghe, C.; Karunathilake, H.; Ariaratnam, A.; Jacoby-Alner, T.E.; Cotterell, C.L.; Brown, S.G. Immune Response to Snake Envenoming and Treatment with Antivenom; Complement Activation, Cytokine Production and Mast Cell Degranulation. PLoS Neglected Trop. Dis. 2013, 7, e2326. [Google Scholar] [CrossRef]
- Morais, V.; Berasain, P.; Ifrán, S.; Carreira, S.; Tortorella, M.N.; Negrín, A.; Massaldi, H. Humoral immune responses to venom and antivenom of patients bitten by Bothrops snakes. Toxicon 2012, 59, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P.; Stock, R.P.; Massougbodji, A. Antivenom Safety and Tolerance for the Strategy of Snake Enven-omation Management. In Snake Venoms; Gopalakrishnakone, P., Inagaki, H., Vogel, C.W., Mukherjee, A.K., Rahmy, T.R., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 475–495. ISBN 978-940-076-409-5. [Google Scholar]
- De Silva, H.A.; Pathmeswaran, A.; Ranasinha, C.D.; Jayamanne, S.; Samarakoon, S.B.; Hittharage, A.; Kalupahana, R.; Ratnatilaka, G.A.; Uluwatthage, W.; Aronson, J.K.; et al. Low-Dose Adrenaline, Promethazine, and Hydrocortisone in the Prevention of Acute Adverse Reactions to Antivenom following Snakebite: A Randomised, Double-Blind, Placebo-Controlled Trial. PLoS Med. 2011, 8, e1000435. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.P.; Motghare, V.M.; Padwal, S.L.; Pore, R.R.; Bhamare, C.G.; Deshmukh, V.S.; Pise, H.N. Adverse drug reaction profile of anti-snake venom in a rural tertiary care teaching hospital. J. Young Pharm. 2013, 5, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Otero-Patino, R.; Segura, A.; Herrera, M.; Angulo, Y.; Leon, G.; Gutierrez, J.M.; Barona, J.; Estrada, S.; Pereanez, A.; Quintana, J.C.; et al. Comparative Study of the Efficacy and Safety of Two Polyvalent, Caprylic Acid Frac-tionated [IgG and F(ab’)2] Antivenoms in Bothrops Asper Bites in Colombia. Toxicon 2012, 59, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.; Čačala, S.R.; Wood, D.; Klopper, J.; Oosthuizen, G.V. A retrospective study of antivenom-associated adverse reaction and anaphylaxis at Ngwelezana Hospital, South Africa. Toxicon 2022, 217, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kraisawat, K.; Promwang, N. Duration after Malayan Pit Viper Bite to Detect Coagulopathy in Songklanagarind Hospital. J. Heal. Sci. Med Res. 2020, 38, 93–101. [Google Scholar] [CrossRef]
- Blessmann, J.; Khonesavanh, C.; Outhaithit, P.; Manichanh, S.; Somphanthabansouk, K.; Siboualipha, P. Ven-omous Snake Bites in Lao PDR: A Retrospective Study of 21 Snakebite Victims in a Provincial Hospital. South East Asian J. Trop. Med. Public Health 2010, 41, 195–202. [Google Scholar]
- Holstege, C.P.; Wu, J.; Baer, A.B. Immediate hypersensitivity reaction associated with the rapid infusion of Crotalidae polyvalent immune Fab (ovine). Ann. Emerg. Med. 2002, 39, 677–679. [Google Scholar] [CrossRef]
- Isbister, G.K.; Shahmy, S.; Mohamed, F.; Abeysinghe, C.; Karunathilake, H.; Ariaratnam, A. A Randomised Controlled Trial of Two Infusion Rates to Decrease Reactions to Antivenom. PLoS ONE 2012, 7, e38739. [Google Scholar] [CrossRef]


| Characteristics | Total Venomous Snakebite Patients (N = 1006) |
|---|---|
| Age in years: Median (IQR, range) | 38 (22–55, 0.6–97) |
| Gender ratio, Female:Male: N (%) | 402:604 (40:60) |
| VENOMOUS SNAKES CATEGORIZED BY SYSTEMIC ENVENOMING: N (%) | |
| Hematotoxic snakes, N = 595 (59.1%) | |
| Green pit viper (Trimeresurus species) | 336 (33.4%) |
| Malayan pit viper (Calloselasma rhodostoma) | 168 (16.7%) |
| Russell’s viper (Daboia siamenis) | 30 (3.0%) |
| Red-necked keelback (Rhabdophis subminiatus) | 10 (1.0%) |
| Mountain pit viper (Ovophis monticola) | 1 (0.1%) |
| Brown-spotted pit viper (Protobothrops mucrosquamatus) | 1 (0.1%) |
| Unknown hematotoxic snakes | 49 (4.9%) |
| Neurotoxic snakes, N = 356 (35.4%) | |
| Cobra (Naja species) | 271 (26.9%) |
| Malayan krait (Bungarus candidus) | 25 (2.5%) |
| King cobra (Ophiophagus Hannah) | 10 (1.0%) |
| Coral snakes (Calliophis species) | 9 (0.9%) |
| Banded krait (Bungarus fasciatus) | 2 (0.2%) |
| Unknown neurotoxic snakes | 39 (3.9%) |
| Unidentified venomous snakes, N = 55 (5.5%) | |
| Characteristics | No. of Patients Receiving AV | No. of Patients Receiving AV with EARs | Incidence of EARs (%) |
|---|---|---|---|
| Age in years: Median (IQR, range) | 38 (21–55, 1–97) | 35 (19–50, 1–87) | |
| Gender ratio, Female:Male: N (%) | 266:418 (38.9:61.1) | 34.4:65.6 (53:101) | |
| FREQUENCY OF AV USED PER PATIENT, Median (IQR, range): | |||
| Hematotoxic envenomation Total doses/patient Total vials/patient | 1 (1–2, 1–15) 5 (3–9, 1–75) | 2 (1–2.8, 1–6) 5.5 (3–9, 1–18) | |
| Neurotoxic envenomation Total doses/patient Total vials/patient | 1 (1–1, 1–4) 10 (10–10, 1–30) | 1 (1–1, 1–4) 10 (10–10,1–24) | |
| VENOMOUS SNAKES CATEGORIZED BY SYSTEMIC ENVENOMING, N (%): | |||
| Hematotoxic snakes: | a 429 (62.7%) | a 108 (70.1%) | 25.2 |
| Green pit viper | b 213 (31.1%) | b 44 (28.6%) | 20.7 |
| Malayan pit viper | b 143 (20.9%) | b 54 (35.1%) | 37.8 |
| Russell’s viper | b 28 (4.1%) | b 2 (1.3%) | 7.1 |
| Red-necked keelback | 1 (0.1%) | 0 | 0 |
| Mountain pit viper | 1 (0.1%) | 0 | 0 |
| Unknown hematotoxic snakes | 43 (6.3%) | 8 (5.2%) | 18.6 |
| Neurotoxic snakes: | 255 (37.3%) | 46 (29.9%) | 18.0 |
| Cobra | 188 (27.5%) | 32 (20.8%) | 17.0 |
| Malayan krait | 20 (2.9%) | 4 (2.6%) | 20.0 |
| King cobra | 7 (1.0%) | 3 (1.9%) | 42.9 |
| Coral snakes | 0 | 0 | 0 |
| Banded krait | 2 (0.3%) | 1 (0.6%) | 50.0 |
| Unknown neurotoxic snakes | 38 (5.6%) | 6 (3.9%) | 15.8 |
| Total | 684 (100%) | 154 (100%) | 22.5 |
| NUMBER OF TYPES OF ANTIVENOM THERAPY IN EACH PATIENT, N (%): | |||
| * One | 651 (95.2%) | 145 (94.2%) | 22.3 |
| ** Two | 31 (4.5%) | 9 (5.8%) | 29.0 |
| *** Three | 2 (0.3%) | 0 | 0 |
| Types of AV Used (Quantity Given) | Frequency of AV Administration | EAR Rate (%) | Grading of EARs, N (%) | |||
|---|---|---|---|---|---|---|
| Total Doses Given N (%) | No. of Doses That Incurred EARs (N) | Mild | Moderate | Severe | ||
| Hematotoxic AV: | ||||||
| GPV (3860 vials) | 341 (29.5) | 39 | 11.4 | 14 (4.1) | 24 (7.0) | 1 (0.3) |
| MPV (3071 vials) | 283 (24.5) | 63 | 22.3 | 24 (8.5) | 24 (8.5) | 15 (5.3) |
| RV (1019 vials) | 68 (5.9) | 2 | 2.9 | 0 (0) | 0 (0) | 2 (2.9) |
| HPAV (1516 vials) | 146 (12.6) | 22 | 15.1 | 7 (4.8) | 10 (6.8) | 5 (3.4) |
| Total (9466 vials) | 838 (72.5) | 126 | 15.0 | 45 (5.4) | 58 (6.9) | 23 (2.7) |
| Neurotoxic AV: | ||||||
| Cobra (2754 vials) | 213 (18.4) | 31 | 14.6 | 17 (8.0) | 11 (5.2) | 3 (1.4) |
| MK (400 vials) | 26 (2.2) | 3 | 11.5 | 2 (7.7) | 1 (3.8) | 0 (0) |
| KC (164 vials) | 7 (0.6) | 1 | 14.3 | 0 (0) | 0 (0) | 1 (14.3) |
| NPAV (888 vials) | 73 (6.3) | 12 | 16.4 | 6 (8.2) | 3 (4.1) | 3 (4.1) |
| Total (4206 vials) | 319 (27.5) | 47 | 14.7 | 25 (7.8) | 15 (4.7) | 7 (2.2) |
| Overall Total (13,672 vials) | 1157 (100) | 173 | 15.0 | 70 (6.1) | 73 (6.3) | 30 (2.6) |
| Characteristics (Total 1157 Doses) | No. of Doses with EARs (N = 173) | No. of Doses without EARs (N = 984) | p-Value |
|---|---|---|---|
| Type of antivenom: N (%) | 0.897 | ||
| Received antivenom for hematotoxic snakes | 126 (72.8) | 721 (72.4) | |
| Received antivenom for neurotoxic snakes | 47 (27.2) | 272 (27.6) | |
| Antivenom given dose:N (%): | 0.585 | ||
| Appropriate dose | 165 (95.4) | 931 (94.6) | |
| Lower than appropriate dose | 8 (4.6) | 47 (4.8) | |
| Higher than appropriate dose | 0 (0) | 6 (0.6) | |
| Duration of IV antivenom administration: | 0.002 * | ||
| 30–60 min (recommended method) | 158 (91.3) | 954 (97.0) | |
| <30 min | 10 (5.8) | 16 (1.6) | |
| >60 min | 5 (2.9) | 14 (1.4) |
| Reference (Study Period) | Total Snakebite Patients | Type of Antivenom Used | Incidence of EARs % | Severe EARs % |
|---|---|---|---|---|
| Fung et al. [43] (2000–2005) | 192 | Cobra, GPV, KC | 0 | 0 |
| Thiansookon et al. [11] (1997–2006) | 382 | Cobra, GPV, MPV, RV | 3.5 | 1.6 |
| Blessmann et al. [44] (2007–2008) | 21 | MPV | 0 | 0 |
| Vongphoumy et al. [17] (2007–2008) | 158 | Cobra, GPV, MK, MPV, HPAV, NPAV | 53.0 | 30.0 |
| Mong et al. [18] (2008–2015) | 191 | GPV | 4.7 | 1.6 |
| Tongpoo et al. [4] (2008–2016) | 78 | BK, Cobra, MK, NPAV | 6.0 | 0 |
| Kraisawat and Promwang [42] (2006–2017) | 153 | MPV, HPAV | 8.0 | 0 |
| Characteristics | Previous [11] Thai Study (2008) | Laos Study [17] (2016) | Our Study |
|---|---|---|---|
| Study period (duration in years) | 1997–2006 (10) | 2013–2015 (3) | 2016–2017 (2) |
| Study design | Retrospective | Prospective | Retrospective |
| Age in years: Median (range) | 39.3 ± 16.3 (mean ± SD) | 32 (1.5–80) | 38 (0.6–97) |
| Gender: Male (%) | 60 | 68 | 60 |
| No. of patients receiving AV | 254 | 43 | 684 |
| No. of patients with EARs | 9 | 23 | 154 |
| Incidence of EARs/Severe reactions (%) | 3.5/1.6 | 53/30 | 22.5/2.6 |
| Type of AV given in each study (%) | GPV = 83.5% Cobra = 12.6% RV = 2.4% MPV = 1.6% | MPV = 53.5% GPV = 16.3% Cobra = 7.0% MK = 4.7% HPAV = 11.6% NPAV = 7.0% | GPV = 29.5% MPV = 24.5% Cobra = 18.4% HPAV = 12.6% NPAV = 6.3% RV = 5.9% MK = 2.2% KC = 0.6% |
| Method of administration | diluted 100 mL/dose IV drip in 1 h | Undiluted (diluent 10 mL/vial IV drip 50 mL/h | diluted 100 mL/dose IV drip in 1 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriapha, C.; Rittilert, P.; Vasaruchapong, T.; Srisuma, S.; Wananukul, W.; Trakulsrichai, S. Early Adverse Reactions to Snake Antivenom: Poison Center Data Analysis. Toxins 2022, 14, 694. https://doi.org/10.3390/toxins14100694
Sriapha C, Rittilert P, Vasaruchapong T, Srisuma S, Wananukul W, Trakulsrichai S. Early Adverse Reactions to Snake Antivenom: Poison Center Data Analysis. Toxins. 2022; 14(10):694. https://doi.org/10.3390/toxins14100694
Chicago/Turabian StyleSriapha, Charuwan, Panee Rittilert, Taksa Vasaruchapong, Sahaphume Srisuma, Winai Wananukul, and Satariya Trakulsrichai. 2022. "Early Adverse Reactions to Snake Antivenom: Poison Center Data Analysis" Toxins 14, no. 10: 694. https://doi.org/10.3390/toxins14100694
APA StyleSriapha, C., Rittilert, P., Vasaruchapong, T., Srisuma, S., Wananukul, W., & Trakulsrichai, S. (2022). Early Adverse Reactions to Snake Antivenom: Poison Center Data Analysis. Toxins, 14(10), 694. https://doi.org/10.3390/toxins14100694





