Ochratoxin A in Dry-Cured Ham: OTA-Producing Fungi, Prevalence, Detection Methods, and Biocontrol Strategies—A Review
Abstract
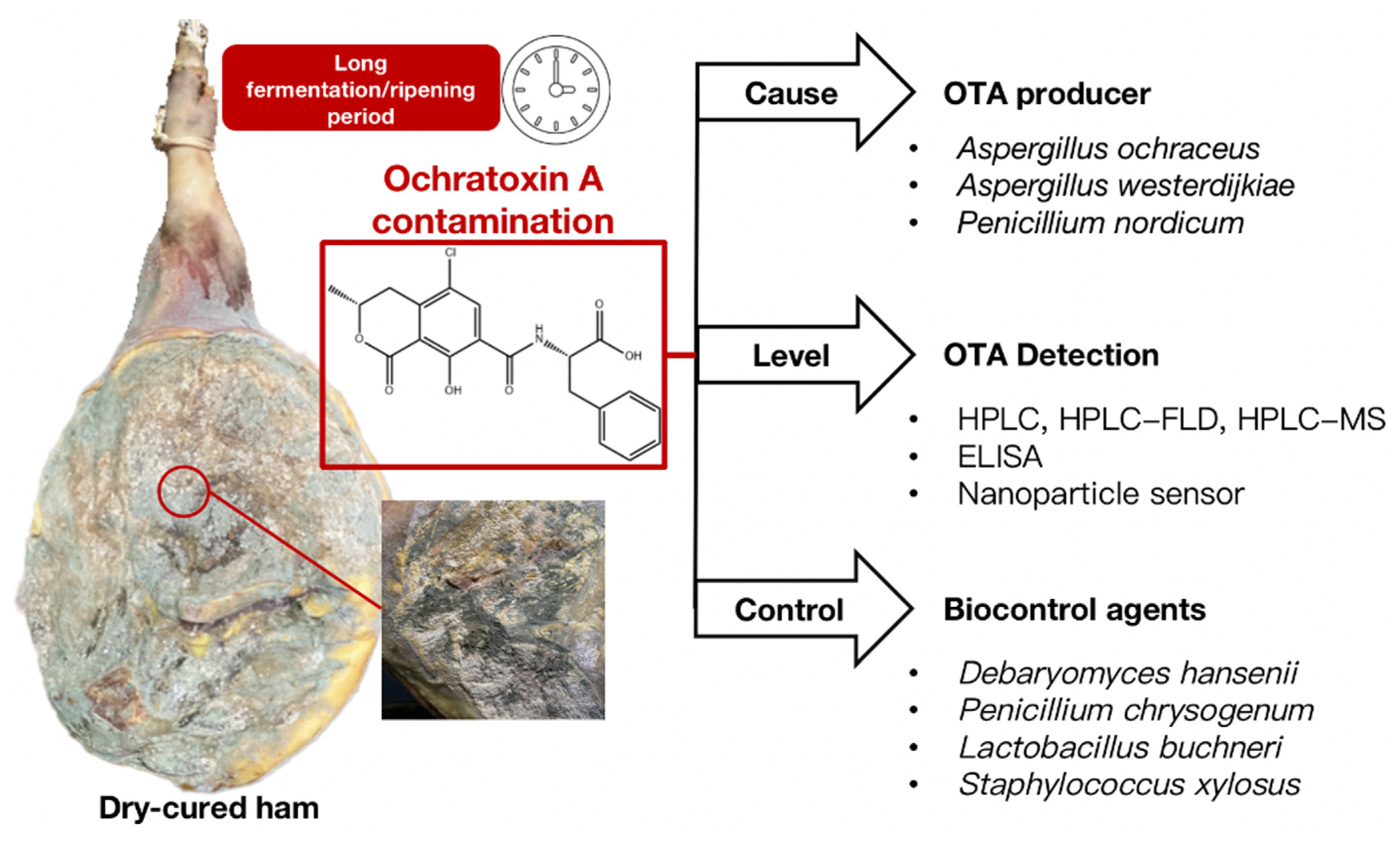
:1. Introduction
2. OTA-Producing Fungi in Dry-Cured Hams
3. The Prevalence of OTA Contamination in Dry-Cured Ham
4. OTA Detection Methods for Dry-Cured Hams
5. Biocontrol Strategies
| Biocontrol Strains | OTA Producer | Food Product | Inhibition Rate (%) | Reference |
|---|---|---|---|---|
| D. hansenii | P. nordicum | Dry-cured pork | 96.27 | [65] |
| D. hansenii | P. nordicum | Dry-cured ham slice | 65 | [37] |
| Saccharomycopsis fibuligera | A. ochraceus | Speck | 100 | [78] |
| D. hansenii | A. ochraceus | Speck | 100 | [78] |
| D. hansenii | P. verrucosum | Dry-cured ham | 80 | [64] |
| P. chrysogenum and D. hansenii | P. nordicum | Dry-cured ham | 98.51 | [69] |
| D. hansenii | P. nordicum | Dry-cured ham | 68.24 | [68] |
| P. chrysogenum | P. nordicum | Dry-cured ham | 80 | [68] |
| D. hansenii and L.buchneri | A. westerdijkiae | Dry-cured ham | 100 | [75] |
| S. xylosus | P. nordicum | Dry-cured ham-based media | 86.59 | [70] |
| D. hansenii | P. nordicum | Cured meat | 100 | [63] |
| D. hansenii | P. nordicum | Dry-cured fermented sausage | 54.97 | [79] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laureati, M.; Buratti, S.; Giovanelli, G.; Corazzin, M.; Lo Fiego, D.P.; Pagliarini, E. Characterization and differentiation of Italian Parma, San Daniele and Toscano dry-cured hams: A multi-disciplinary approach. Meat Sci. 2014, 96, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, P.; Diaz-Caro, C.; del Puerto, I.; Ortiz, A.; Escribano, M.; Tejerina, D. What effect does the presence of sustainability and traceability certifications have on consumers of traditional meat products? The case of Iberian cured products in Spain. Meat Sci. 2022, 187, 108752. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, F.; Chen, J.; Sun, Z.; Wang, F.; Wang, C.; Fu, L. High-throughput sequencing-based characterization of the predominant microbial community associated with characteristic flavor formation in Jinhua Ham. Food Microbiol. 2021, 94, 103643. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef] [PubMed]
- Petzinger, E.; Ziegler, K. Ochratoxin A from a toxicological perspective. J. Vet. Pharmacol. Ther. 2000, 23, 91–98. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Rodriguez, A.; Magista, D.; Magan, N. Insights into existing and future fungal and mycotoxin contamination of cured meats. Curr. Opin. Food Sci. 2019, 29, 20–27. [Google Scholar] [CrossRef]
- Escher, F.E.; Koehler, P.E.; Ayres, J.C. Production of ochratoxins A and B on country cured ham. Appl. Microbiol. 1973, 26, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Gareis, M. Fate of ochratoxin A on processing of meat products. Food Addit. Contam. 1996, 13, 35–37. [Google Scholar] [PubMed]
- Spotti, E.; Busolli, C.; Palmia, F. Sviluppo di colture fungine di importanza rilevante nell’industria dei prodotti carnei. Ind Conserve 1999, 74, 23–33. [Google Scholar]
- Comi, G.; Orlic, S.; Redzepovic, S.; Urso, R.; Iacumin, L. Moulds isolated from Istrian dried ham at the pre-ripening and ripening level. Int. J. Food Microbiol. 2004, 96, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Chiavaro, E.; Lepiani, A.; Colla, F.; Bettoni, P.; Pari, E.; Spotti, E. Ochratoxin A determination in ham by immunoaffinity clean-up and a quick fluorometric method. Food Addit. Contam. 2002, 19, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Matrella, R.; Monaci, L.; Milillo, M.A.; Palmisano, F. Ochratoxin A determination in paired kidneys and muscle samples from swine slaughtered in southern Italy. Food Control 2006, 17, 114–117. [Google Scholar] [CrossRef]
- Pietri, A.; Bertuzzi, T.; Gualla, A.; Piva, G. Occurrence of ochratoxin A in raw ham muscles and in pork products from Northern Italy. Ital. J. Food Sci. 2006, 18, 99–106. [Google Scholar]
- Dall’Asta, C.; Galaverna, G.; Bertuzzi, T.; Moseriti, A.; Pietri, A.; Dossena, A.; Marchelli, R. Occurrence of ochratoxin A in raw ham muscle, salami and dry-cured ham from pigs fed with contaminated diet. Food Chem. 2010, 120, 978–983. [Google Scholar] [CrossRef]
- Rodriguez, A.; Rodriguez, M.; Martin, A.; Delgado, J.; Cordoba, J.J. Presence of ochratoxin A on the surface of dry-cured Iberian ham after initial fungal growth in the drying stage. Meat Sci. 2012, 92, 728–734. [Google Scholar] [CrossRef] [PubMed]
- della Sanità, M. Direttive in materia di controllo ufficiale sui prodotti alimentari: Valori massimi ammissibili di micotossine nelle derrate alimentari di origine nazionale, comunitaria e Paesi terzi. Gazz. Uff. della Repubb. Ital. 1999, 135, 52–57. [Google Scholar]
- Delgado, J.; da Cruz Cabral, L.; Rodriguez, M.; Rodriguez, A. Influence of ochratoxin A on adaptation of Penicillium nordicum on a NaCl-rich dry-cured ham-based medium. Int. J. Food Microbiol. 2018, 272, 22–28. [Google Scholar] [CrossRef]
- Ferrara, M.; Magista, D.; Lippolis, V.; Cervellieri, S.; Susca, A.; Perrone, G. Effect of Penicillium nordicum contamination rates on ochratoxin A accumulation in dry-cured salami. Food Control 2016, 67, 235–239. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Vazquez, C.; Sardinas, N.; Teresa Gonzalez-Jaen, M.; Patino, B. Revision of ochratoxin a production capacity by the main species of Aspergillus section Circumdati Aspergillus steynii revealed as the main risk of OTA contamination. Food Control 2011, 22, 343–345. [Google Scholar] [CrossRef]
- Vipotnik, Z.; Rodriguez, A.; Rodrigues, P. Aspergillus westerdijkiae as a major ochratoxin A risk in dry-cured ham based-media. Int. J. Food Microbiol. 2017, 241, 244–251. [Google Scholar] [CrossRef]
- Abarca, M.L.; Bragulat, M.R.; Castella, G.; Cabanes, F.J. Ochratoxin A production by strains of Aspergillus niger var. niger. Appl. Environ. Microbiol. 1994, 60, 2650–2652. [Google Scholar] [CrossRef]
- Abdel-Hadi, A.; Alshehri, B.; Waly, M.; Aboamer, M.; Banawas, S.; Alaidarous, M.; Palanisamy, M.; Awad, M.; Baazeem, A. Predictive Modeling and Validation on Growth, Production of Asexual Spores and Ochratoxin A of Aspergillus Ochraceus Group under Abiotic Climatic Variables. Microorganisms 2021, 9, 1321. [Google Scholar] [CrossRef]
- Castella, G.; Larsen, T.O.; Cabanes, J.; Schmidt, H.; Alboresi, A.; Niessen, L.; Farber, P.; Geisen, R. Molecular characterization of ochratoxin A producing strains of the genus Penicillium. Syst. Appl. Microbiol. 2002, 25, 74–83. [Google Scholar] [CrossRef]
- Vlachou, M.; Pexara, A.; Solomakos, N.; Govaris, A. Ochratoxin A in Slaughtered Pigs and Pork Products. Toxins 2022, 14, 67. [Google Scholar] [CrossRef]
- Alapont, C.; Lopez-Mendoza, M.C.; Gil, J.V.; Martinez-Culebras, P.V. Mycobiota and toxigenic Penicillium species on two Spanish dry-cured ham manufacturing plants. Food Addit. Contam. Part A-Chem. Anal. Control Expo. Risk Assess. 2014, 31, 93–104. [Google Scholar] [CrossRef]
- Meftah, S.; Abid, S.; Dias, T.; Rodrigues, P. Mechanisms underlying the effect of commercial starter cultures and a native yeast on ochratoxin A production in meat products. Lwt-Food Sci. Technol. 2020, 117, 108611. [Google Scholar] [CrossRef]
- Leggieri, M.C.; Pont, N.P.; Battilani, P.; Magan, N. Detection and discrimination between ochratoxin producer and non-producer strains of Penicillium nordicum on a ham-based medium using an electronic nose. Mycotoxin Res. 2011, 27, 29–35. [Google Scholar] [CrossRef]
- Rodriguez, A.; Rodriguez, M.; Martin, A.; Nunez, F.; Cordoba, J.J. Evaluation of hazard of aflatoxin B1, ochratoxin A and patulin production in dry-cured ham and early detection of producing moulds by qPCR. Food Control 2012, 27, 118–126. [Google Scholar] [CrossRef]
- Zadravec, M.; Vahcic, N.; Brnic, D.; Markov, K.; Frece, J.; Beck, R.; Lesic, T.; Pleadin, J. A study of surface moulds and mycotoxins in Croatian traditional dry-cured meat products. Int. J. Food Microbiol. 2020, 317, 108459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, H.; Neng, J.; Gao, J.; Yang, B.L.; Liu, Y. The Influence of NaCl and Glucose Content on Growth and Ochratoxin A Production byAspergillus ochraceus, Aspergillus carbonarius and Penicillium nordicum. Toxins 2020, 12, 515. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Montero, L.; Cordoba, J.J.; Peromingo, B.; Alvarez, M.; Nunez, F. Effects of environmental conditions and substrate on growth and ochratoxin A production by Penicillium verrucosum and Penicillium nordicum: Relative risk assessment of OTA in dry-cured meat products. Food Res. Int. 2019, 121, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Medina, A.; Cordoba, J.J.; Magan, N. The influence of salt (NaCl) on ochratoxin A biosynthetic genes, growth and ochratoxin A production by three strains of Penicillium nordicum on a dry-cured ham-based medium. Int. J. Food Microbiol. 2014, 178, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Capela, D.; Medina, A.; Cordoba, J.J.; Magan, N. Relationship between ecophysiological factors, growth and ochratoxin A contamination of dry-cured sausage based matrices. Int. J. Food Microbiol. 2015, 194, 71–77. [Google Scholar] [CrossRef]
- Meftah, S.; Abid, S.; Dias, T.; Rodrigues, P. Effect of dry-sausage starter culture and endogenous yeasts on Aspergillus westerdijkiae and Penicillium nordicum growth and OTA production. LWT-Food Sci. Technol. 2017, 87, 250–258. [Google Scholar] [CrossRef]
- Rodriguez, A.; Bernaldez, V.; Rodriguez, M.; Andrade, M.J.; Nunez, F.; Cordoba, J.J. Effect of selected protective cultures on ochratoxin A accumulation in dry-cured Iberian ham during its ripening process. Lwt-Food Sci. Technol. 2015, 60, 923–928. [Google Scholar] [CrossRef]
- Andrade, M.; Thorsen, L.; Rodríguez, A.; Córdoba, J.; Jespersen, L. Inhibition of ochratoxigenic moulds by Debaryomyces hansenii strains for biopreservation of dry-cured meat products. Int. J. Food Microbiol. 2013, 170C, 70–77. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- Vulic, A.; Vahcic, N.; Hengl, B.; Gross-Boskovic, A.; Jurkovic, M.; Kudumija, N.; Pleadin, J. Assessment of possible human exposure to ochratoxin A in Croatia due to the consumption of dry-cured and fermented meat products. Food Addit. Contam. Part A-Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1428–1434. [Google Scholar] [CrossRef]
- Toscani, T.; Moseriti, A.; Dossena, A.; Dall’Asta, C.; Simoncini, N.; Virgili, R. Determination of ochratoxin A in dry-cured meat products by a HPLC-FLD quantitative method. J. Chromatography. B Anal. Technol. Biomed. Life Sci. 2007, 855, 242–248. [Google Scholar] [CrossRef]
- Pietri, A.; Gualla, A.; Rastelli, S.; Bertuzzi, T. Enzyme-assisted extraction for the HPLC determination of ochratoxin A in pork and dry-cured ham. Food Addit. Contam. Part A-Chem. Anal. Control Expo. Risk Assess. 2011, 28, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, T.; Gualla, A.; Morlacchini, M.; Pietri, A. Direct and indirect contamination with ochratoxin A of ripened pork products. Food Control 2013, 34, 79–83. [Google Scholar] [CrossRef]
- Pleadin, J.; Stayer, M.M.; Vahcic, N.; Kovatcevic, D.; Milone, S.; Saftic, L.; Scortichini, G. Survey of aflatoxin B-1 and ochratoxin A occurrence in traditional meat products coming from Croatian households and markets. Food Control 2015, 52, 71–77. [Google Scholar] [CrossRef]
- Pleadin, J.; Vasilj, V.; Lešić, T.; Frece, J.; Markov, K.; Krešić, G.; Vulić, A.; Bogdanović, T.; Zadravec, M.; Vahčić, N. Chemical composition and occurrence of mycotoxins in traditional meat products from the households of Bosnia and Herzegovina. Microporous Mesoporoud Mater. 2017, XIX, 331–337. [Google Scholar] [CrossRef]
- Rodrigues, P.; Silva, D.; Costa, P.; Abrunhosa, L.; Venancio, A.; Teixeira, A. Mycobiota and mycotoxins in Portuguese pork, goat and sheep dry-cured hams. Mycotoxin Res. 2019, 35, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Peromingo, B.; Sulyok, M.; Lemmens, M.; Rodriguez, A.; Rodriguez, M. Diffusion of mycotoxins and secondary metabolites in dry-cured meat products. Food Control 2019, 101, 144–150. [Google Scholar] [CrossRef]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef]
- Adunphatcharaphon, S.; Elliott, C.T.; Sooksimuang, T.; Charlermroj, R.; Petchkongkaew, A.; Karoonuthaisiri, N. The evolution of multiplex detection of mycotoxins using immunoassay platform technologies. J. Hazard. Mater. 2022, 432, 128706. [Google Scholar] [CrossRef]
- Li, C.L.; Wen, K.; Mi, T.J.; Zhang, X.Y.; Zhang, H.Y.; Zhang, S.X.; Shen, J.Z.; Wang, Z.H. A universal multi-wavelength fluorescence polarization immunoassay for multiplexed detection of mycotoxins in maize. Biosens. Bioelectron. 2016, 79, 258–265. [Google Scholar] [CrossRef]
- Zhang, Z.; Lai, J.H.; Wu, K.S.; Huang, X.C.; Guo, S.; Zhang, L.L.; Liu, J. Peroxidase-catalyzed chemiluminescence system and its application in immunoassay. Talanta 2018, 180, 260–270. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Dolatabadi, J.E.N.; Torbati, M.; Tazehkand, A.P.; Homayouni-Rad, A.; de la Guardia, M. Nanomaterials and new biorecognition molecules based surface plasmon resonance biosensors for mycotoxin detection. Biosens. Bioelectron. 2019, 143, 111603. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.X.; Liao, X.F.; Fang, L.; Jia, B.Y.; Liu, M.; Li, D.H.; Zhou, L.D.; Kong, W.J. Recent advances on immunosensors for mycotoxins in foods and other commodities. Trac-Trends Anal. Chem. 2021, 136, 116193. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q.; Xu, X.F.; Jin, Y.P.; Wang, Y.; Zhang, L.Y.; Yang, W.J.; He, L.D.; Feng, X.Y.; Chen, Y.Q. Microarray surface enhanced Raman scattering based immunosensor for multiplexing detection of mycotoxin in foodstuff. Sens. Actuators B-Chem. 2018, 266, 115–123. [Google Scholar] [CrossRef]
- He, Y.; Tian, F.Y.; Zhou, J.; Zhao, Q.Y.; Fu, R.J.; Jiao, B.N. Colorimetric aptasensor for ochratoxin A detection based on enzyme-induced gold nanoparticle aggregation. J. Hazard. Mater. 2020, 388, 121758. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.-H.; Bhardwaj, N. Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.; Sohrabi, H.; Arbabzadeh, O.; Khaaki, P.; Majidi, M.R. Frontiers in conventional and nanomaterials based electrochemical sensing and biosensing approaches for Ochratoxin A analysis in foodstuffs: A review. Food Chem. Toxicol. 2021, 149, 112030. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Nie, D.; Sun, X.; Xu, Y.; He, J.; Yang, L.; Yang, L. A versatile Y shaped DNA nanostructure for simple, rapid and one-step detection of mycotoxins. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2022, 281, 121634. [Google Scholar] [CrossRef] [PubMed]
- Altunbas, O.; Ozdas, A.; Yilmaz, M.D. Luminescent detection of Ochratoxin A using terbium chelated mesoporous silica nanoparticles. J. Hazard. Mater. 2020, 382, 121049. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Dong, Y.; Hong, F.; Zhang, X.; Wang, X.; Wang, J.; Chen, Y. Polydopamine nanoparticle-mediated, click chemistry triggered, microparticle-counting immunosensor for the sensitive detection of ochratoxin A. J. Hazard. Mater. 2022, 428, 128206. [Google Scholar] [CrossRef] [PubMed]
- Montanha, F.P.; Anater, A.; Burchard, J.F.; Luciano, F.B.; Meca, G.; Manyes, L.; Pimpao, C.T. Mycotoxins in dry-cured meats: A review. Food Chem. Toxicol. 2018, 111, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, F.; Lara, M.; Arévalo, A.B.; Delgado, J.; Sánchez-Montero, L.; Andrade, M. Selection and evaluation of Debaryomyces hansenii isolates as potential bioprotective agents against toxigenic penicillia in dry-fermented sausages. Food Microbiol. 2015, 46, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; Cesare, A.; Hilbert, F.; Lindqvist, R.; Nauta, M.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 14: Suitability of taxonomic units notified to EFSA until March 2021. EFSA J. 2021, 19, e06689. [Google Scholar] [CrossRef]
- Álvarez, M.; Nuñez, F.; Delgado, J.; Andrade, M.; Rodríguez, M.; Rodríguez, A. Competitiveness of three biocontrol candidates against ochratoxigenic Penicillium nordicum under dry-cured meat environmental and nutritional conditions. Fungal Biol. 2020, 125, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Peromingo, B.; Nunez, F.; Rodriguez, A.; Alia, A.; Andrade, M.J. Potential of yeasts isolated from dry-cured ham to control ochratoxin A production in meat models. Int. J. Food Microbiol. 2018, 268, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Simoncini, N.; Spadola, G.; Battilani, P. Autochthonous yeasts as potential biocontrol agents in dry-cured meat products. Food Control 2014, 46, 160–167. [Google Scholar] [CrossRef]
- Acosta, R.; Rodriguez-Martin, A.; Martin, A.; Nunez, F.; Asensio, M.A. Selection of antifungal protein-producing molds from dry-cured meat products. Int. J. Food Microbiol. 2009, 135, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martin, A.; Acosta, R.; Liddell, S.; Nunez, F.; Jose Benito, M.; Asensio, M.A. Characterization of the novel antifungal protein PgAFP and the encoding gene of Penicillium chrysogenum. Peptides 2010, 31, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Nuñez, F.; Asensio, M.; Owens, R. Quantitative proteomic profiling of ochratoxin A repression in Penicillium nordicum by protective cultures. Int. J. Food Microbiol. 2019, 305, 108243. [Google Scholar] [CrossRef] [PubMed]
- Cebrian, E.; Rodriguez, M.; Peromingo, B.; Bermudez, E.; Nunez, F. Efficacy of the Combined Protective Cultures of Penicillium chrysogenum and Debaryomyces hansenii for the Control of Ochratoxin A Hazard in Dry-Cured Ham. Toxins 2019, 11, 710. [Google Scholar] [CrossRef]
- Cebrián, E.; Nuñez, F.; Galvez, F.; Delgado, J.; Bermúdez, E.; Rodríguez, M. Selection and Evaluation of Staphylococcus xylosus as a Biocontrol Agent against Toxigenic Moulds in a Dry-Cured Ham Model System. Microorganisms 2020, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Beux, M.; Pagnoncelli, M.; Thomaz-Soccol, V.; Rodrigues, C.; Soccol, C. Isolation, selection and evaluation of antagonistic yeasts and lactic acid bacteria against ochratoxigenic fungus Aspergillus westerdijkiae on coffee beans. Lett. Appl. Microbiol. 2015, 62, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.; Mahony, J.; Van Sinderen, D. Broad-spectrum antifungal-producing lactic acid bacteria and their application in fruit models. Folia Microbiol. 2012, 58, 291–299. [Google Scholar] [CrossRef]
- Sathe, S.; Nawani, N.; Dhakephalkar, P.; Kapadnis, B. Antifungal lactic acid bacteria with potential to prolong shelf-life of fresh vegetables. J. Appl. Microbiol. 2008, 103, 2622–2628. [Google Scholar] [CrossRef] [PubMed]
- Iacumin, L.; Arnoldi, M.; Comi, G. Effect of a Debaryomyces hansenii and Lactobacillus buchneri Starter Culture on Aspergillus westerdijkiae Ochratoxin A Production and Growth during the Manufacture of Short Seasoned Dry-Cured Ham. Microorganisms 2020, 8, 1623. [Google Scholar] [CrossRef]
- Caridi, A. New perspectives in safety and quality enhancement of wine through selection of yeasts based on the parietal adsorption activity. Int. J. Food Microbiol. 2007, 120, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Gil-Serna, J.; Patino, B.; Cortés, L.; González-Jaén, M.; Vázquez, C. Mechanisms involved in reduction of ochratoxin A produced by Aspergillus westerdijkiae using Debaryomyces hansenii CYC 1244. Int. J. Food Microbiol. 2011, 151, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Iacumin, L.; Manzano, M.; Andyanto, D.; Comi, G. Biocontrol of ochratoxigenic moulds (Aspergillus ochraceus and Penicillium nordicum) by Debaryomyces hansenii and Saccharomycopsis fibuligera during speck production. Food Microbiol. 2017, 62, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Cebrian, E.; Nunez, F.; Alvarez, M.; Roncero, E.; Rodriguez, M. Biocontrol of ochratoxigenic Penicillium nordicum in dry-cured fermented sausages by Debaryomyces hansenii and Staphylococcus xylosus. Int. J. Food Microbiol. 2022, 375, 109744. [Google Scholar] [CrossRef] [PubMed]
| Hams Species | OTA Producer | Growing Condition | Toxigenic Condition | Reference | ||
|---|---|---|---|---|---|---|
| Temperature (°C) | Water Activity (Aw) | Temperature (°C) | Water Activity (Aw) | |||
| American country ham | A.ochraceus | 25–30 | / | 25–30 | / | [8] |
| Italy dry-cured ham | P. nordicum | 25 | 0.95–0.98 | 25 | 0.98 | [28] |
| Spanish dry-cured ham | P. nordicum | 25 | 0.84 | 25 | 0.92 | [29] |
| A.ochraceus | 25 | 0.84 | 25 | 0.92 | ||
| Portugal dry-cured ham | A. westerdijkiae | 25–30 | 0.93–0.97 | 20–25 | 0.94–0.97 | [21] |
| P. nordicum | 19–25 | 0.93–0.97 | 18–22 | 0.95–0.97 | ||
| Country | Source | Sample Volume | Incidence of Positives (%) | Mean of Positives (μg/kg) | Concentration Range (μg/kg) | LOD (μg/kg) | Analytical Method | Reference |
|---|---|---|---|---|---|---|---|---|
| Italy | manufacturer | 21 | 80.95 | 1.22 | 0.20–2.20 | 0.04 | HPLC | [12] |
| manufacturer | 30 | 40 | 1.62 | 0.01–28.42 | 0.01 | HPLC-FLD | [14] | |
| market | 5 | 60 | 2.67 | <0.02–7.28 | 0.02 | HPLC-FLD | [40] | |
| market | 110 | 76.36 | 0.53 | 0.10–12.51 | 0.1 | HPLC-FLD | [15] | |
| manufacturer | 40 | 100 | 3.27 | 1.14–6.29 | 0.06 | HPLC | [41] | |
| household | 6 | 83.33 | 21.40 | <0.04–104 | 0.05 | HPLC | [42] | |
| Spanish | manufacturer | 20 | 50 | 27.1 | 2.00–160.90 | 1 | HPLC-MS | [16] |
| household | 15 | 33.33 | 3.20 | 2.00–6.30 | 1 | HPLC-MS | [36] | |
| Croatia | household and market | 105 | 17.14 | 0.77 | 0.97–9.95 | 0.15 | ELISA/HPLC-FLD | [43] |
| market | 54 | 22.22 | 3.16 | 1.56–9.95 | 0.32 | ELISA | [39] | |
| household | 67 | 22.38 | 4.3 | 2.16–6.86 | 0.91 | ELISA | [30] | |
| Bosnia | household | 8 | 25 | 3.29 | 2.11–4.47 | 1.5 | ELISA | [44] |
| Portugal | manufacturer | 47 | 42.55 | 14.90 | <0.90–99.10 | 0.3 | HPLC-FLD | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Chen, J.; Zhu, Q.; Wan, J. Ochratoxin A in Dry-Cured Ham: OTA-Producing Fungi, Prevalence, Detection Methods, and Biocontrol Strategies—A Review. Toxins 2022, 14, 693. https://doi.org/10.3390/toxins14100693
Chen Y, Chen J, Zhu Q, Wan J. Ochratoxin A in Dry-Cured Ham: OTA-Producing Fungi, Prevalence, Detection Methods, and Biocontrol Strategies—A Review. Toxins. 2022; 14(10):693. https://doi.org/10.3390/toxins14100693
Chicago/Turabian StyleChen, Yuanshan, Jiang Chen, Qiujin Zhu, and Jing Wan. 2022. "Ochratoxin A in Dry-Cured Ham: OTA-Producing Fungi, Prevalence, Detection Methods, and Biocontrol Strategies—A Review" Toxins 14, no. 10: 693. https://doi.org/10.3390/toxins14100693





