Characterization of Potential Threats from Cyanobacterial Toxins in Lake Victoria Embayments and during Water Treatment
Abstract
1. Introduction
2. Results
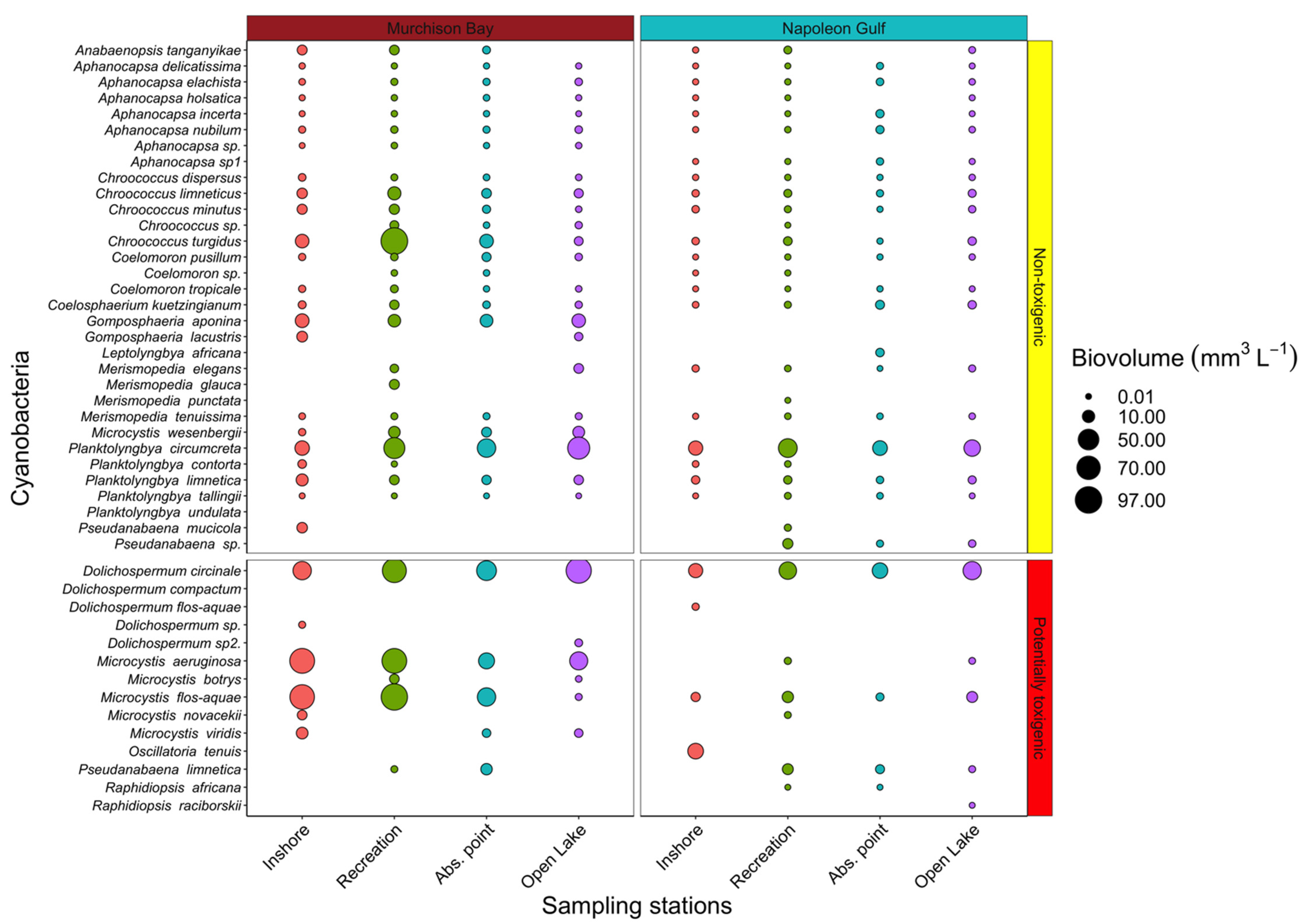
2.1. Toxigenic vs. Non-Toxigenic Cyanobacteria Diversity and Biovolume
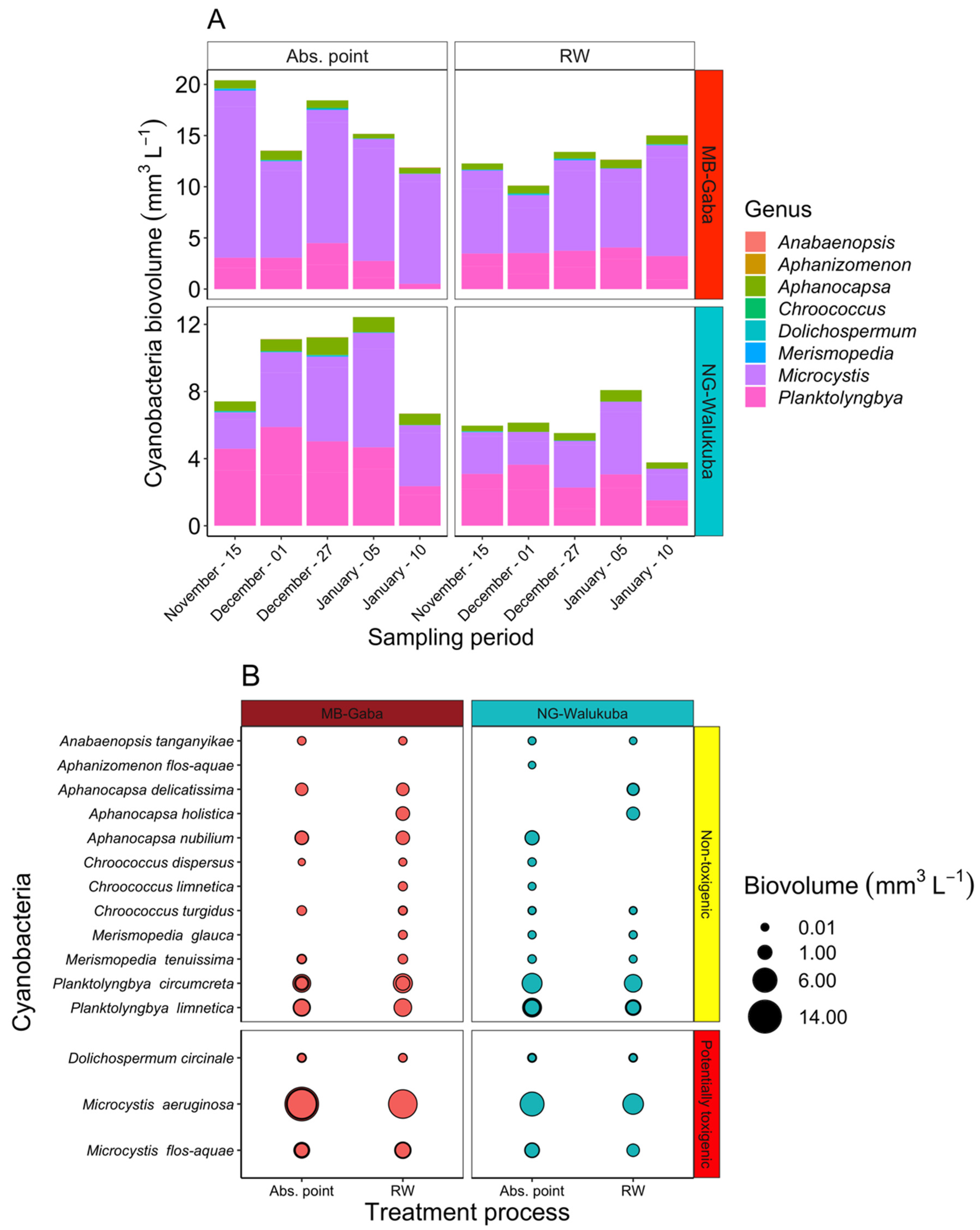
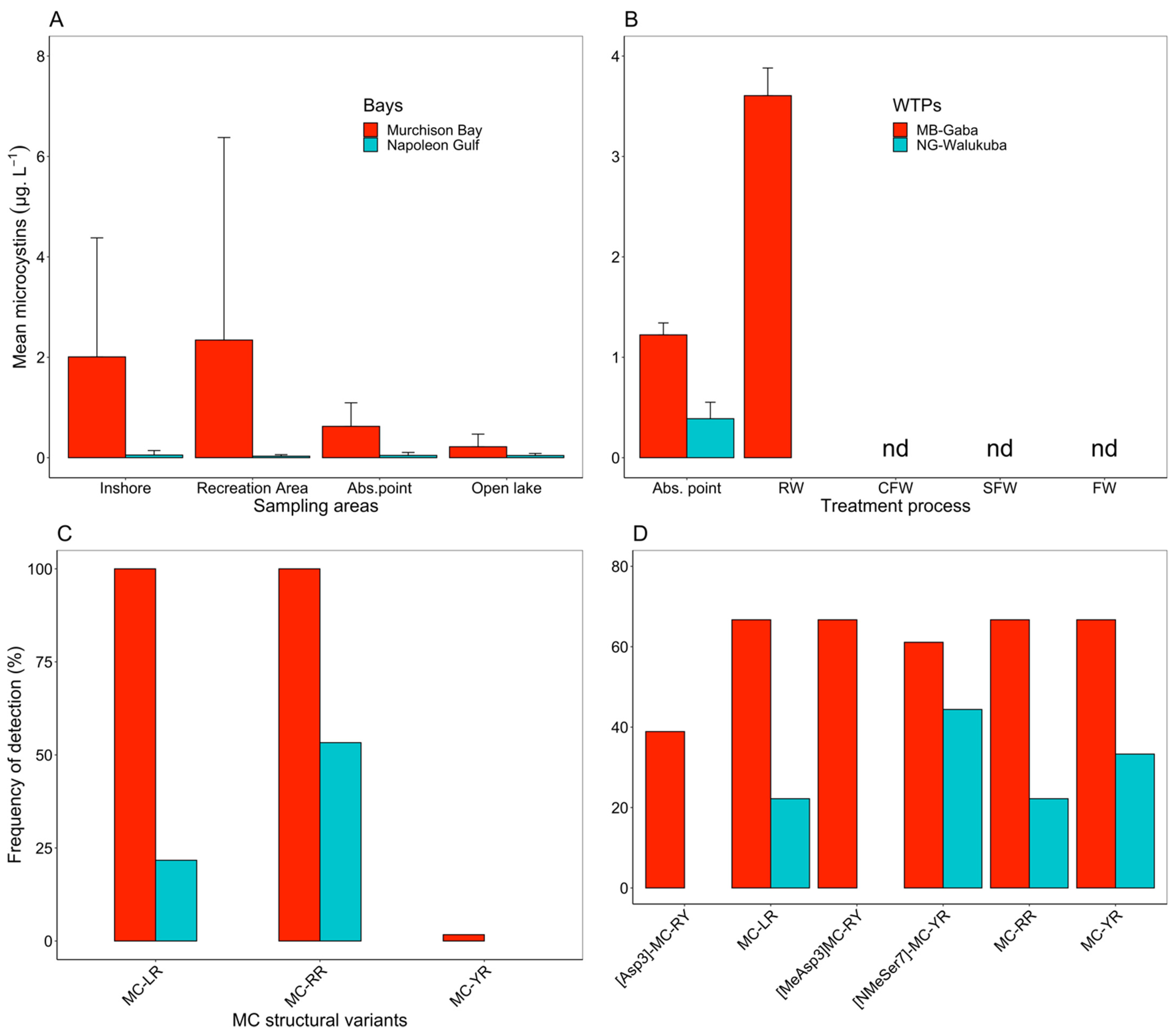
2.2. Cyanotoxins Occurrence in the Lake Victoria Embayments and during Water Treatment
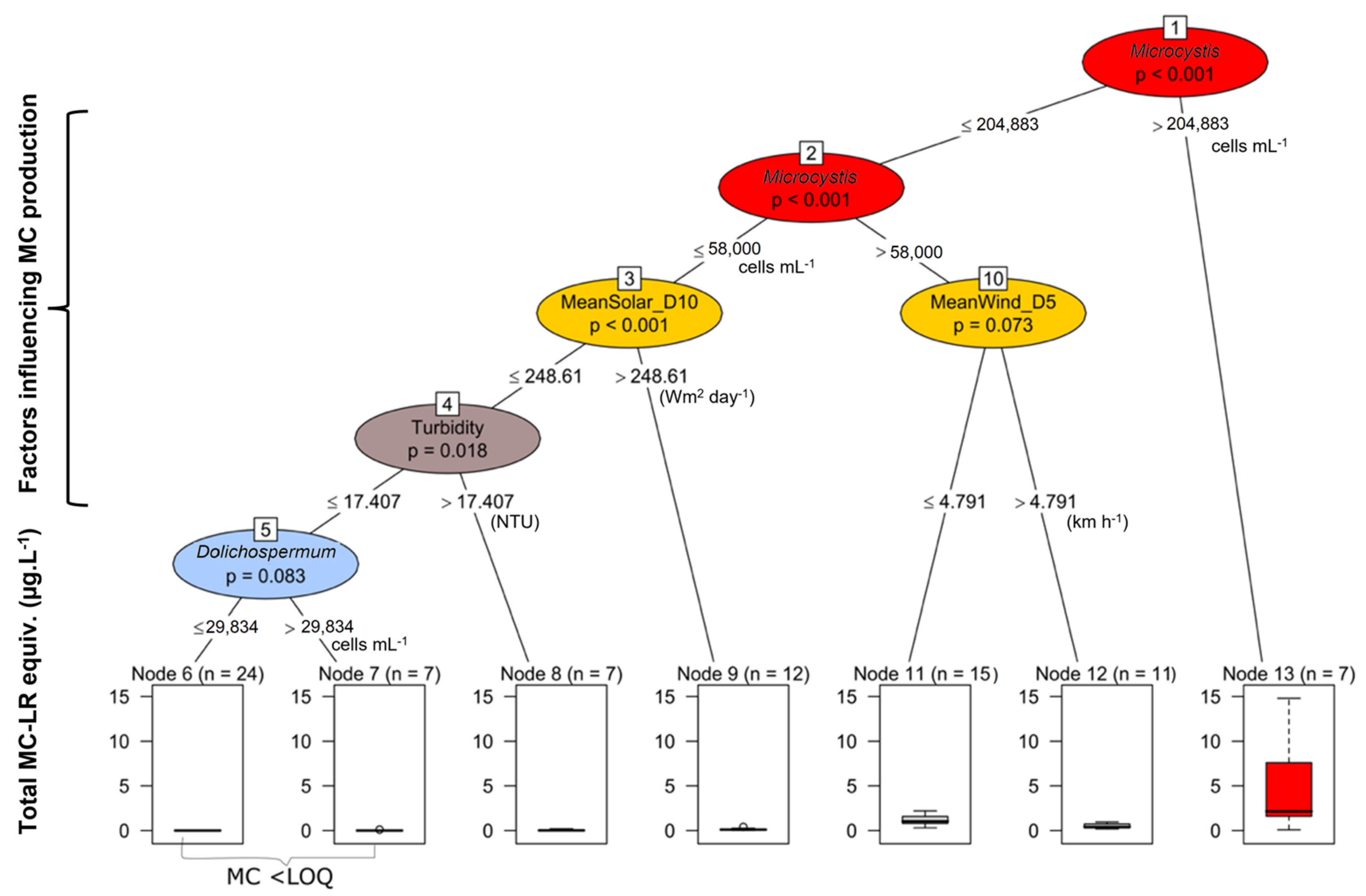
2.3. Relationships between Predictive Variables and Microcystins
3. Discussion
3.1. Toxigenic Cyanobacteria and Cyanotoxins in the Lake Victoria Embayments
3.2. Factors Explaining MC Concentrations
3.3. The Fate of MC during Water Treatment
3.4. Multiple Exposure Routes to Toxigenic Cyanobacteria and Cyanotoxins
4. Conclusions
5. Materials and Methods
5.1. Study Sites and Sampling Design
5.2. Meteorological, Physical-Chemical and Biological Variables
5.3. Cyanotoxins Analysis
Field Survey (Quantification of Intracellular MC)
5.4. Abstraction Points and Water Treatment (Quantification of Intracellular MC)
5.5. Abstraction Points and Water Treatment (Quantification of Dissolved MC Using ELISA)
5.6. Abstraction Points and Water Treatment (Quantification of Dissolved MC Using LC-MS)
5.7. Statistical Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.R.; Lv, H.; Isabwe, A.; Liu, L.; Yu, X.; Chen, H.; Yang, J. Disturbance-induced phytoplankton regime shifts and recovery of cyanobacteria dominance in two subtropical reservoirs. Water Res. 2017, 120, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef]
- Gray, E.; Elliott, J.A.; Mackay, E.B.; Folkard, A.M.; Keenan, P.O.; Jones, I.D. Modelling lake cyanobacterial blooms: Disentangling the climate-driven impacts of changing mixed depth and water temperature. Freshw. Biol. 2019, 64, 2141–2155. [Google Scholar] [CrossRef]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: New York, NY, USA, 2021. [Google Scholar]
- Codd, G.A.; Meriluoto, J.; Metcalf, J.S. Introduction: Cyanobacteria, Cyanotoxins, Their Human Impact, and Risk Management. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis, 1st ed.; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons: London, UK, 2017; pp. 1–8. [Google Scholar]
- Nyamweya, C.S.; Natugonza, V.; Taabu-Munyaho, A.; Aura, C.M.; Njiru, J.M.; Ongore, C.; Mangeni-Sande, R.; Kashindye, B.B.; Odoli, C.O.; Ogari, Z.; et al. A century of drastic change: Human-induced changes of Lake Victoria fisheries and ecology. Fish. Res. 2020, 230, 105564. [Google Scholar] [CrossRef]
- Ochumba, P.B.O.; Kibaara, D.I. Observations on blue-green algal blooms in the open waters of Lake Victoria, Kenya. Afr. J. Ecol. 1989, 27, 23–34. [Google Scholar] [CrossRef]
- Olokotum, M.; Mitroi, V.; Troussellier, M.; Semyalo, R.; Bernard, C.; Montuelle, B.; Okello, W.; Quiblier, C.; Humbert, J.-F. A review of the socioecological causes and consequences of cyanobacterial blooms in Lake Victoria. Harmful Algae 2020, 96, 101829. [Google Scholar] [CrossRef]
- Kotut, K.; Ballot, A.; Krienitz, L. Toxic cyanobacteria and their toxins in standing waters of Kenya: Implications for water resource use. J. Water Health 2006, 4, 233–245. [Google Scholar] [CrossRef]
- McHau, G.J.; Machunda, R.; Kimanya, M.; Makule, E.; Gong, Y.Y.; Mpolya, E.; Meneely, J.P.; Elliott, C.T.; Greer, B. First Report of the co-occurrence of cylindrospermopsin, nodularin and microcystins in the Freshwaters of Lake Victoria, Tanzania. Expo. Health 2020, 13, 185–194. [Google Scholar] [CrossRef]
- Krienitz, L.; Ballot, A.; Wiegand, C.; Kotut, K.; Codd, G.A.; Pflugmacher, S. Cyanotoxin-producing bloom of Anabaena flos-aquae, Anabaena discoidea and Microcystis aeruginosa (Cyanobacteria) in Nyanza Gulf of Lake Victoria, Kenya. J. Appl. Bot.-Angwandte Bot. 2002, 76, 179–183. [Google Scholar]
- Simiyu, B.M.; Oduor, S.O.; Rohrlack, T.; Sitoki, L.; Kurmayer, R. Microcystin content in phytoplankton and in small fish from eutrophic Nyanza Gulf, Lake Victoria, Kenya. Toxins 2018, 10, 275. [Google Scholar] [CrossRef]
- Okello, W.; Kurmayer, R. Seasonal development of cyanobacteria and microcystin production in Ugandan freshwater lakes. Lakes Reserv. Res. Manag. 2011, 16, 123–135. [Google Scholar] [CrossRef]
- Mbonde, A.S.; Sitoki, L.; Kurmayer, R. Phytoplankton composition and microcystin concentrations in open and closed bays of Lake Victoria, Tanzania. Aquat. Ecosyst. Health Manag. 2015, 18, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Meriluoto, J.; Spoof, L.; Codd, G.A. (Eds.) Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons: Chichester, UK, 2017; p. 576. [Google Scholar]
- Sivonen, K.; Jones, G. Cyanobacterial toxins. In Toxic Cyanobacteria in Water. A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Bartram, J., Eds.; E & FN Spon: London, UK, 1999; pp. 41–112. [Google Scholar]
- Rücker, J.; Stüken, A.; Nixdorf, B.; Fastner, J.; Chorus, I.; Wiedner, C. Concentrations of particulate and dissolved cylindrospermopsin in 21 Aphanizomenon-dominated temperate lakes. Toxicon 2007, 50, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Svirčev, Z.; Lalić, D.; Bojadžija Savić, G.; Tokodi, N.; Drobac Backović, D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef] [PubMed]
- Olokotum, M.; Troussellier, M.; Escalas, A.; Hamlaoui, S.; Okello, W.; Semyalo, R.; Humbert, J.-F.; Bernard, C. High nutrient loading and climatic parameters influence the dominance and dissimilarity of toxigenic cyanobacteria in northern bays of Lake Victoria. J. Great Lakes Res. 2021, 47, 985–996. [Google Scholar] [CrossRef]
- Haande, S.; Rohrlack, T.; Semyalo, R.P.; Brettum, P.; Edvardsen, B.; Lyche-Solheim, A.; Sørensen, K.; Larsson, P. Phytoplankton dynamics and cyanobacterial dominance in Murchison Bay of Lake Victoria (Uganda) in relation to environmental conditions. Limnol.-Ecol. Manag. Inland Waters 2011, 41, 20–29. [Google Scholar] [CrossRef]
- Okello, W.; Ostermaier, V.; Portmann, C.; Gademann, K.; Kurmayer, R. Spatial isolation favours the divergence in microcystin net production by Microcystis in Ugandan freshwater lakes. Water Res. 2010, 44, 2803–2814. [Google Scholar] [CrossRef]
- Bruno, M.; Ploux, O.; Metcalf, J.S.; Mejean, A.; Pawlik-Skowronska, B.; Furey, A. Anatoxin-a, Homoanatoxin-a, and Natural Analogues. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, L.S., Codd, G.A., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2017; pp. 138–147. [Google Scholar]
- Mann, S.; Cohen, M.; Chapuis-Hugon, F.; Pichon, V.; Mazmouz, R.; Méjean, A.; Ploux, O. Synthesis, configuration assignment, and simultaneous quantification by liquid chromatography coupled to tandem mass spectrometry, of dihydroanatoxin-a and dihydrohomoanatoxin-a together with the parent toxins, in axenic cyanobacterial strains and in environmental samples. Toxicon 2012, 60, 1404–1414. [Google Scholar]
- Semyalo, R.; Rohrlack, T.; Naggawa, C.; Nyakairu, G.W. Microcystin concentrations in Nile tilapia (Oreochromis niloticus) caught from Murchison Bay, Lake Victoria and Lake Mburo: Uganda. Hydrobiologia 2010, 638, 235–244. [Google Scholar] [CrossRef]
- Poste, A.; Hecky, R.; Guildford, S. Phosphorus enrichment and carbon depletion contribute to high Microcystis biomass and microcystin concentrations in Ugandan lakes. Limnol. Oceanogr. 2013, 58, 1075–1088. [Google Scholar] [CrossRef]
- Bernard, C.; Ballot, A.; Thomazeau, S.; Maloufi, S.; Furey, A.; Mankiewicz-Boczek, J.; Pawlik-Skowrońska, B.; Capelli, C.; Salmaso, N. Appendix 2: Cyanobacteria associated with the production of cyanotoxins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons: Chichester, UK, 2017; pp. 501–525. [Google Scholar]
- Sitoki, L.; Kurmayer, R.; Rott, E. Spatial variation of phytoplankton composition, biovolume, and resulting microcystin concentrations in the Nyanza Gulf (Lake Victoria, Kenya). Hydrobiologia 2012, 691, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Ndlela, L.L.; Oberholster, P.J.; Van Wyk, J.H.; Cheng, P.H. An overview of cyanobacterial bloom occurrences and research in Africa over the last decade. Harmful Algae 2016, 60, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Oberholster, P.J.; Myburgh, J.G.; Govender, D.; Bengis, R.; Botha, A.-M. Identification of toxigenic Microcystis strains after incidents of wild animal mortalities in the Kruger National Park, South Africa. Ecotoxicol. Environ. Saf. 2009, 72, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Orr, P.T.; Jones, G.J. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 1998, 43, 1604–1614. [Google Scholar] [CrossRef]
- Monchamp, M.-E.; Pick, F.R.; Beisner, B.E.; Maranger, R. Nitrogen forms influence microcystin concentration and composition via changes in cyanobacterial community structure. PLoS ONE 2014, 9, e85573. [Google Scholar] [CrossRef]
- Mowe, M.A.D.; Mitrovic, S.M.; Lim, R.P.; Furey, A.; Yeo, D.C.J. Tropical cyanobacterial blooms: A review of prevalence, problem taxa, toxins and influencing environmental factors. J. Limnol. 2014, 2014, 74. [Google Scholar] [CrossRef]
- Krausfeldt, L.E.; Farmer, A.T.; Castro, H.F.; Boyer, G.L.; Campagna, S.R.; Wilhelm, S.W. Nitrogen flux into metabolites and microcystins changes in response to different nitrogen sources in Microcystis aeruginosa NIES-843. Environ. Microbiol. 2020, 22, 2419–2431. [Google Scholar] [CrossRef]
- Dai, R.; Wang, P.; Jia, P.; Zhang, Y.; Chu, X.; Wang, Y. A review on factors affecting microcystins production by algae in aquatic environments. World J. Microbiol. Biotechnol. 2016, 32, 51. [Google Scholar] [CrossRef]
- Roegner, A.; Sitoki, L.; Weirich, C.; Corman, J.; Owage, D.; Umami, M.; Odada, E.; Miruka, J.; Ogari, Z.; Smith, W.; et al. Harmful algal blooms threaten the health of peri-urban fisher communities: A case study in Kisumu Bay, Lake Victoria, Kenya. Expo. Health 2020, 12, 835–848. [Google Scholar] [CrossRef]
- Zhu, W.; Li, M.; Luo, Y.; Dai, X.; Guo, L.; Xiao, M.; Huang, J.; Tan, X. Vertical distribution of Microcystis colony size in Lake Taihu: Its role in algal blooms. J. Great Lakes Res. 2014, 40, 949–955. [Google Scholar] [CrossRef]
- Walsby, A. The nuisance algae: Curiosities in the biology of planktonic blue-green algae. Water Treat. Exam. 1970, 19, 359–373. [Google Scholar]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Simiyu, B.M.; Amukhuma, H.S.; Sitoki, L.; Okello, W.; Kurmayer, R. Interannual variability of water quality conditions in the Nyanza Gulf of Lake Victoria, Kenya. J. Great Lakes Res. 2022, 48, 97–109. [Google Scholar] [CrossRef]
- Marshall, B.E.; Ezekiel, C.N.; Gichuki, J.; Mkumbo, O.C.; Sitoki, L.; Wanda, F. Has climate change disrupted stratification patterns in Lake Victoria, East Africa? Afr. J. Aquat. Sci. 2013, 38, 249–253. [Google Scholar] [CrossRef]
- Sevilla, E.; Martin-Luna, B.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. An active photosynthetic electron transfer chain required for mcyD transcription and microcystin synthesis in Microcystis aeruginosa PCC7806. Ecotoxicology 2012, 21, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Kaebernick, M.; Neilan, B.A.; Börner, T.; Dittmann, E. Light and the transcriptional response of the microcystin biosynthesis gene cluster. Appl. Environ. Microbiol. 2000, 66, 3387–3392. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Bartram, J. (Eds.) Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; E&FN Spon: London UK; New York, NY, USA, 1999; p. 416. [Google Scholar]
- Tarczyńska, M.; Romanowska-Duda, Z.; Jurczak, T.; Zalewski, M. Toxic cyanobacterial blooms in a drinking water reservoir—Causes, consequences and management strategy. Water Supply 2001, 1, 237–246. [Google Scholar] [CrossRef]
- Lahti, K.; Rapala, J.; Kivimäki, A.-L.; Kukkonen, J.; Niemelä, M.; Sivonen, K. Occurrence of microcystins in raw water sources and treated drinking water of Finnish waterworks. Water Sci. Technol. 2001, 43, 225–228. [Google Scholar] [CrossRef]
- Ho, L.; Meyn, T.; Keegan, A.; Hoefel, D.; Brookes, J.; Saint, C.P.; Newcombe, G. Bacterial degradation of microcystin toxins within a biologically active sand filter. Water Res. 2006, 40, 768–774. [Google Scholar] [CrossRef]
- Hrudey, S.; Lambert, T.; Kenefick, S. Health risk assessment of microcystins in drinking water supplies. In Toxic Cyanobacteria—A Global Perspective; Australian Centre for Water Quality Research: Adelaide, Australia, 1994; pp. 7–12. [Google Scholar]
- Zong, W.; Sun, F.; Pei, H.; Hu, W.; Pei, R. Microcystin-associated disinfection by-products: The real and non-negligible risk to drinking water subject to chlorination. Chem. Eng. J. 2015, 279, 498–506. [Google Scholar] [CrossRef]
- Gupta, N.; Pant, S.; Vijayaraghavan, R.; Rao, P.L. Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology 2003, 188, 285–296. [Google Scholar] [CrossRef]
- Chernoff, N.; Hill, D.; Lang, J.; Schmid, J.; Le, T.; Farthing, A.; Huang, H. The Comparative Toxicity of 10 Microcystin Congeners Administered Orally to Mice: Clinical Effects and Organ Toxicity. Toxins 2020, 12, 403. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cyanobacterial Toxins: Microcystins; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ibelings, B.W.; Backer, L.C.; Kardinaal, W.E.A.; Chorus, I. Current approaches to cyanotoxin risk assessment and risk management around the globe. Harmful Algae 2014, 40, 63–74. [Google Scholar] [CrossRef]
- Utermöhl, H. Toward the improvement of the quantitative phytoplankton method. Mitt.-Int. Ver. Limnol. 1958, 9, 1–38. [Google Scholar]
- Sun, J.; Liu, D. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef]
- Talling, J.F. The phytoplankton of Lake Victoria (East Africa). Arch. Hydrobiol. Beih. Ergeb. Limnol. 1987, 25, 229–256. [Google Scholar]
- Komarek, J.; Anagnostidis, K. Cyanoprokaryota, 1.Teil Chroococcales; Gustav Fischer Verlag: Jena, Germany, 1999; Volume 19, 548p. [Google Scholar]
- Komarek, J.; Anagnostidis, K. Cyanoprokaryota, 2.Teil Oscillatoriales; Spektrum Akademischer Verlag: Heidelberg, Germany, 2005; Volume 19, 759p. [Google Scholar]
- Komarek, J. Cyanoprokaryota, 3. Teil Heterocytous Genera; Springer: Heidelberg, Germany, 2013; Volume 19, 1130p. [Google Scholar]
- Cronberg, G.; Annadotter, H. Manual on Aquatic Cyanobacteria: A Photo Guide and a Synopsis of Their Toxicology; Intergovernmental Oceanographic Commission, UNESCO: Paris, France, 2006. [Google Scholar]
- Cerasino, L.; Meriluoto, J.; Bláha, L.; Carmeli, S.; Kaloudis, T.; Mazur-Marzec, H. Extraction of cyanotoxins from cyanobacterial Biomass. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis, 1st ed.; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 350–353. [Google Scholar]
- Fastner, J.; Flieger, I.; Neumann, U. Optimised extraction of microcystins from field samples—A comparison of different solvents and procedures. Water Res. 1998, 32, 3177–3181. [Google Scholar] [CrossRef]
- Lawton, L.A.; Edwards, C.; Codd, G.A. Extraction and high-performance liquid chromatographic method for the determination of microcystins in raw and treated waters. Analyst 1994, 119, 1525–1530. [Google Scholar] [CrossRef]
- Dean, J.R. Extraction Methods for Environmental Analysis; John Wiley & Sons: Chichester, UK, 1998; p. 225. [Google Scholar]


| Parameters | Min–Max (Mean ± SD) | Regression Coefficient (Log10) | Correlation Coefficient |
|---|---|---|---|
| Intercept | −2.044 | ||
| SRP (µg L−1) | 0.56–380.3 (13.4 ± 38.8) | 0.055 | 0.31 |
| NH4 (µg L−1) | 0.01–1206.8 (49.3 ± 150.9) | 0.041 | 0.32 |
| NO3− (µg L−1) # | 1.11–947.4 (73.5 ± 127) | - | 0.02 |
| NO2− (µg L−1) # | 0.00–47.2 (4.7 ± 7.1) | - | 0.24 |
| N:P ratio | 0.16–149.1 (18.2 ± 22.3) | -0.093 | 0.02 |
| Turbidity (NTU) | 0.02–197.2 (18.2 ± 22.3) | - | 0.42 |
| Rainfall (mm) | 0–61.1 (11.2 ± 13.9) | 0.004 | 0.06 |
| Wind speed (km h−1) | 3.1–8.1 (4.5 ± 1.1) | −0.703 * | 0.31 |
| Wind direction | N-S, E-W, S-N, N-NE (S-SE, E-W) | 0.035 * | 0.18 |
| Solar radiation (Wm2 day−1) | 181.7–394.7 (290.9 ± 80.9) | 1.021 *** | 0.60 |
| Microcystis biovolume (mm3 L−1) | 0.27 -69 (7.3 ± 9.4) | - | 0.62 |
| Microcystis cell number | 39,000–1,061,206 (106,277 ± 148,826) | 0.031 ** | 0.57 |
| Dolichospermum biovolume (mm3 L−1) | 0.07–20.6 (3.9 ± 4.1) | - | 0.22 |
| Dolichospermum cell number | 645–182,627 (33,822 ± 34,975) | 0.008 | 0.24 |
| R2 | 0.49 |
| Capacity (m3.day−1) | Piped Water Connection | Demand (m3/day) | Treatment Steps | Physical-Chemical Parameters during the Treatment Process | |||||
|---|---|---|---|---|---|---|---|---|---|
| Depth-Integrated Lake Water (at WTP Abstraction Point) | Raw Water (Entry Point of the WTP) | After Flocculation | After Sand Filtration | Finished Water | |||||
| MB-Gaba | |||||||||
| 80,000, but supplementing Gaba I and II water treatment plants | 315,897 connections supplying between 1,421,536 and 2 million people in Kampala (including lake shore residents) | 300,000 | Pre-chlorination, coagulation, clarification, rapid sand filtration, pH correction and chlorination | pH | 8.0 ± 0.3 | 7.6 ± 0.1 | 7.2 ± 0.3 | 7.2 ± 0.3 | 7.0 ± 0.2 |
| Conductivity (μS cm−1) | 118 ± 2.1 | 119 ± 3.7 | 129 ± 5.5 | 129 ± 5.9 | 131 ± 5.3 | ||||
| Temperature (°C) | 26.1 ± 0.3 | 26.1 ± 0.4 | 26.0 ± 0.4 | 26.0 ± 0.3 | 26.4 ± 0.4 | ||||
| Dissolved oxygen (mg L−1) | 5.0 ± 0.7 | 5.9 ± 0.4 | 7.1 ± 0.1 | 7.4 ± 0.1 | 7.1 ± 0.1 | ||||
| Dissolved oxygen (%) | 71.0 ± 9.6 | 85.0 ± 8.3 | 102 ± 1.7 | 104 ± 1.2 | 101 ± 1.2 | ||||
| NG-Walukuba | |||||||||
| 50,000, but producing only 30,100 | 28,881 connections supplying between 129,964 and 500,000 people (including the neighboring districts of Iganga, Buikwe and Mayuge) | 30,000 | Coagulation, clarification, rapid sand filtration and chlorination | pH | 8.9 ± 0.3 | 7.8 ± 0.2 | 7.5 ± 0.4 | 7.4 ± 0.2 | 7.6 ± 0.2 |
| Conductivity (μS cm−1) | 102 ± 1.2 | 104 ± 6.7 | 105 ± 6.8 | 103 ± 2.8 | 109 ± 4.3 | ||||
| Temperature (°C) | 27.1 ± 0.3 | 27.3 ± 0.6 | 27.3 ± 0.4 | 27.0 ± 0.4 | 27.2 ± 0.2 | ||||
| Dissolved oxygen (mg L−1) | 6.6 ± 1.0 | 3.8 ± 0.9 | 3.9 ± 0.6 | 3.2 ± 0.8 | 6.4 ± 0.6 | ||||
| Dissolved oxygen (%) | 94.3 ± 15.1 | 55.6 ± 13 | 56.7 ± 9.5 | 47 ± 11.8 | 92.1 ± 8.9 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olokotum, M.; Humbert, J.-F.; Quiblier, C.; Okello, W.; Semyalo, R.; Troussellier, M.; Marie, B.; Baumann, K.; Kurmayer, R.; Bernard, C. Characterization of Potential Threats from Cyanobacterial Toxins in Lake Victoria Embayments and during Water Treatment. Toxins 2022, 14, 664. https://doi.org/10.3390/toxins14100664
Olokotum M, Humbert J-F, Quiblier C, Okello W, Semyalo R, Troussellier M, Marie B, Baumann K, Kurmayer R, Bernard C. Characterization of Potential Threats from Cyanobacterial Toxins in Lake Victoria Embayments and during Water Treatment. Toxins. 2022; 14(10):664. https://doi.org/10.3390/toxins14100664
Chicago/Turabian StyleOlokotum, Mark, Jean-François Humbert, Catherine Quiblier, William Okello, Ronald Semyalo, Marc Troussellier, Benjamin Marie, Kathrin Baumann, Rainer Kurmayer, and Cécile Bernard. 2022. "Characterization of Potential Threats from Cyanobacterial Toxins in Lake Victoria Embayments and during Water Treatment" Toxins 14, no. 10: 664. https://doi.org/10.3390/toxins14100664
APA StyleOlokotum, M., Humbert, J.-F., Quiblier, C., Okello, W., Semyalo, R., Troussellier, M., Marie, B., Baumann, K., Kurmayer, R., & Bernard, C. (2022). Characterization of Potential Threats from Cyanobacterial Toxins in Lake Victoria Embayments and during Water Treatment. Toxins, 14(10), 664. https://doi.org/10.3390/toxins14100664






