Indoxyl Sulfate Might Play a Role in Sarcopenia, While Myostatin Is an Indicator of Muscle Mass in Patients with Chronic Kidney Disease: Analysis from the RECOVERY Study
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Comparison of Characteristics According to Myostatin Levels
2.3. Comparison of Characteristics According to IS Levels
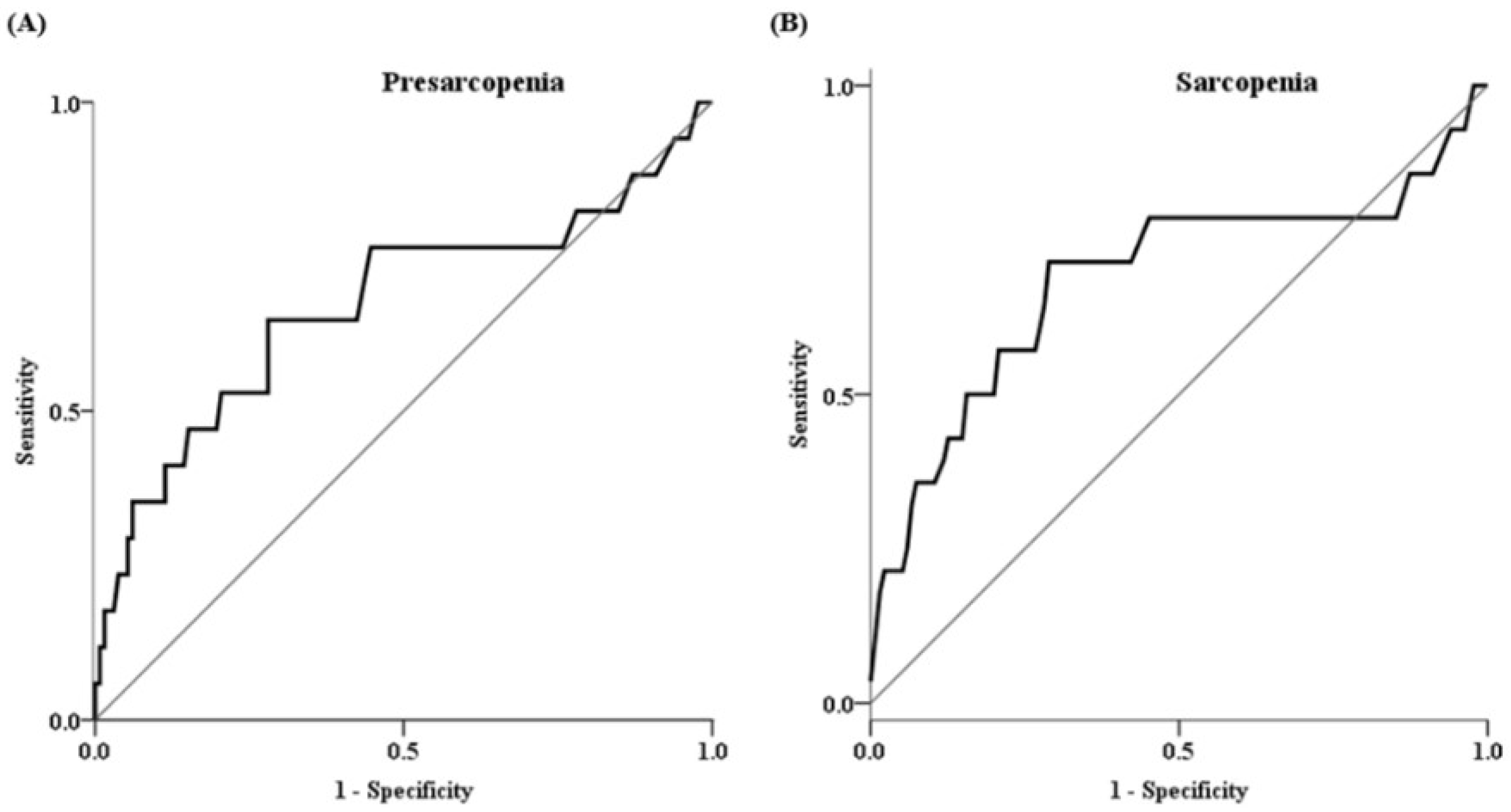
2.4. Factors Associated with Presarcopenia and Sarcopenia
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Design and Patients
5.2. Laboratory Data
5.3. Body Composition and Muscle Strength Measurements
5.4. Definition of Sarcopenia and Myostatin/ASM Ratio
5.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, S.; Muscaritoli, M.; Andreozzi, P.; Sgreccia, A.; De Leo, S.; Mazzaferro, S.; Mitterhofer, A.P.; Pasquali, M.; Protopapa, P.; Spagnoli, A.; et al. Sarcopenia and cardiovascular risk indices in patients with chronic kidney disease on conservative and replacement therapy. Nutrition 2019, 62, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Cordeiro, A.C.; Avesani, C.M.; Carrero, J.J.; Lindholm, B.; Amparo, F.C.; Amodeo, C.; Cuppari, L.; Kamimura, M.A. Sarcopenia in chronic kidney disease on conservative therapy: Prevalence and association with mortality. Nephrol. Dial. Transplant. 2015, 30, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Enoki, Y.; Watanabe, H.; Arake, R.; Sugimoto, R.; Imafuku, T.; Tominaga, Y.; Ishima, Y.; Kotani, S.; Nakajima, M.; Tanaka, M.; et al. Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1. Sci. Rep. 2016, 6, 32084. [Google Scholar] [CrossRef] [PubMed]
- Bataille, S.; Dou, L.; Bartoli, M.; Sallee, M.; Aniort, J.; Ferkak, B.; Chermiti, R.; McKay, N.; Da Silva, N.; Burtey, S.; et al. Mechanisms of myostatin and activin A accumulation in chronic kidney disease. Nephrol. Dial. Transplant. 2022, 37, 1249–1260. [Google Scholar] [CrossRef]
- Yano, S.; Nagai, A.; Isomura, M.; Yamasaki, M.; Kijima, T.; Takeda, M.; Hamano, T.; Nabika, T. Relationship between Blood Myostatin Levels and Kidney Function: Shimane CoHRE Study. PLoS ONE 2015, 10, e0141035. [Google Scholar] [CrossRef]
- Holle, J.; Querfeld, U.; Kirchner, M.; Anninos, A.; Okun, J.; Thurn-Valsassina, D.; Bayazit, A.; Niemirska, A.; Canpolat, N.; Bulut, I.K.; et al. Indoxyl sulfate associates with cardiovascular phenotype in children with chronic kidney disease. Pediatr. Nephrol. 2019, 34, 2571–2582. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, M.S.; Kang, D.H.; Ko, G.J.; Lim, H.S.; Yu, B.C.; Park, M.Y.; Kim, J.K.; Kim, C.H.; Hwang, S.D.; et al. Myostatin/Appendicular Skeletal Muscle Mass (ASM) Ratio, Not Myostatin, Is Associated with Low Handgrip Strength in Community-Dwelling Older Women. Int. J. Environ. Res. Public Health 2021, 18, 7344. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work Group (EUTox). Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef]
- Cheng, T.C.; Huang, S.H.; Kao, C.L.; Hsu, P.C. Muscle Wasting in Chronic Kidney Disease: Mechanism and Clinical Implications—A Narrative Review. Int. J. Mol. Sci. 2022, 23, 6047. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Zimmers, T.A.; Davies, M.V.; Koniaris, L.G.; Haynes, P.; Esquela, A.F.; Tomkinson, K.N.; McPherron, A.C.; Wolfman, N.M.; Lee, S.J. Induction of cachexia in mice by systemically administered myostatin. Science 2002, 296, 1486–1488. [Google Scholar] [CrossRef]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hubner, C.; Riebel, T.; Komen, W.; Braun, T.; Tobin, J.F.; Lee, S.J. Myostatin mutation associated with gross muscle hypertrophy in a child. N. Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.R.; Chen, R.C. Serum myostatin levels and skeletal muscle wasting in chronic obstructive pulmonary disease. Respir. Med. 2012, 106, 102–108. [Google Scholar] [CrossRef] [PubMed]
- George, I.; Bish, L.T.; Kamalakkannan, G.; Petrilli, C.M.; Oz, M.C.; Naka, Y.; Sweeney, H.L.; Maybaum, S. Myostatin activation in patients with advanced heart failure and after mechanical unloading. Eur. J. Heart Fail. 2010, 12, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D.; Cohen, A.H.; Wang, H.; Qing, D.; Tang, Z.; Fournier, M.; Lewis, M.; Casaburi, R.; Storer, T. Effect of exercise on mRNA levels for growth factors in skeletal muscle of hemodialysis patients. J. Ren. Nutr. 2006, 16, 312–324. [Google Scholar] [CrossRef]
- Han, D.S.; Chen, Y.M.; Lin, S.Y.; Chang, H.H.; Huang, T.M.; Chi, Y.C.; Yang, W.S. Serum myostatin levels and grip strength in normal subjects and patients on maintenance haemodialysis. Clin. Endocrinol. 2011, 75, 857–863. [Google Scholar] [CrossRef]
- Yamada, S.; Tsuruya, K.; Yoshida, H.; Tokumoto, M.; Ueki, K.; Ooboshi, H.; Kitazono, T. Factors Associated with the Serum Myostatin Level in Patients Undergoing Peritoneal Dialysis: Potential Effects of Skeletal Muscle Mass and Vitamin D Receptor Activator Use. Calcif. Tissue Int. 2016, 99, 13–22. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, S.E.; Lee, J.Y.; Jeong, H.J.; Son, Y.K.; An, W.S. Serum myostatin levels are associated with abdominal aortic calcification in dialysis patients. Kidney Res. Clin. Pract. 2019, 38, 481–489. [Google Scholar] [CrossRef]
- Willoughby, D.S. Effects of heavy resistance training on myostatin mRNA and protein expression. Med. Sci. Sports Exerc. 2004, 36, 574–582. [Google Scholar] [CrossRef]
- Cha, R.H.; Kang, S.H.; Han, M.Y.; An, W.S.; Kim, S.H.; Kim, J.C. Effects of AST-120 on muscle health and quality of life in chronic kidney disease patients: Results of RECOVERY study. J. Cachexia Sarcopenia Muscle 2021, 13, 397–408. [Google Scholar] [CrossRef]
- Shin, J.; Hwang, J.H.; Han, M.; Cha, R.H.; Kang, S.H.; An, W.S.; Kim, J.C.; Kim, S.H. Phase angle as a marker for muscle health and quality of life in patients with chronic kidney disease. Clin. Nutr. 2022, 41, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Myostatin < 4.5 (pg/mL) (n = 76) | Myostatin ≥ 4.5 (pg/mL) (n = 73) | p Value |
|---|---|---|---|
| Age (years) | 67.0 ± 9.2 | 63.0 ± 11.9 | 0.022 |
| Male, n (%) | 39 (51.3) | 57 (78.1) | 0.001 |
| Diabetes mellitus, n (%) | 37 (48.7) | 38 (52.1) | 0.681 |
| Modified CCI score | 4.0 (3.0, 5.0) | 4.0 (2.0, 5.0) | 0.479 |
| Body mass index (kg/m2) | 24.7 ± 2.8 | 25.8 ± 3.2 | 0.030 |
| Laboratory results | |||
| Hemoglobin (g/dL) | 12.1 ± 2.1 | 12.6 ± 2.0 | 0.103 |
| Total protein (g/dL) | 7.0 ± 0.4 | 6.9 ± 0.5 | 0.365 |
| Albumin (g/dL) | 4.3 (4.1, 4.6) | 4.3 (4.2, 4.5) | 0.978 |
| Blood urea nitrogen (mg/dL) | 31.1 ± 11.9 | 32.8 ± 10.1 | 0.348 |
| Creatinine (mg/dL) | 1.7 (1.5, 2.2) | 2.1 (1.6, 2.5) | 0.048 |
| eGFR (mL/min/1.73 m2) | 34.8 ± 12.7 | 33.0 ± 12.1 | 0.395 |
| C-reactive protein (mg/dL) | 0.1 (0.0, 0.5) | 0.1 (0.0, 0.2) | 0.314 |
| TNFa (pg/mL) | 1.5 (1.3, 2.1) | 1.6 (1.4, 1.9) | 0.271 |
| IL-6 (pg/mL) | 1.9 (1.2, 2.8) | 1.8 (1.2, 3.0) | 0.986 |
| iPTH (pg/mL) | 67.3 (46.9, 109.0) | 68.6 (43.3, 97.8)) | 0.184 |
| 25(OH)D (ng/mL) | 14.3 (10.2, 20.3) | 14.0 (11.1, 18.4) | 0.454 |
| Hemoglobin A1c (%) | 6.3 (5.9, 7.3) | 6.3 (5.9, 7.5) | 0.991 |
| Indoxyl sulfate (mg/dL) | 0.4 (0.2, 0.6) | 0.3 (0.2, 0.6) | 0.141 |
| Myostatin (pg/mL) | 3.3 ± 0.8 | 6.4 ± 1.7 | <0.001 |
| Myostatin/SMI | 0.5 ± 0.1 | 0.8 ± 0.2 | <0.001 |
| Muscle function | |||
| SMI (kg/m2) | 7.3 ± 1.2 | 8.1 ± 1.1 | <0.001 |
| HGS (kg) | 26.2 ± 9.6 | 30.6 ± 7.7 | 0.003 |
| 6 m GS (m/s) | 1.1 (0.9, 1.3) | 1.1 (0.8, 1.4) | 0.580 |
| presarcopenia, n (%) | 13 (17.1) | 4 (5.6) | 0.028 |
| low muscle strength, n (%) | 24 (31.6) | 15 (20.5) | 0.126 |
| low physical performance, n (%) | 34 (44.7) | 31 (42.5) | 0.780 |
| Sarcopenia, n (%) | 11 (14.5) | 3 (4.2) | 0.032 |
| Characteristics | Indoxyl Sulfate (n = 150) | p Value | Myostatin (n = 149) | p Value |
|---|---|---|---|---|
| Age (years) | 0.136 | 0.491 | −0.226 | 0.016 |
| Modified CCI score | 0.136 | 0.292 | −0.096 | 0.738 |
| Body mass index (kg/m2) | −0.169 | 0.119 | 0.147 | 0.221 |
| Laboratory results | ||||
| Hemoglobin (g/dL) | −0.402 | <0.001 | 0.158 | 0.165 |
| Total protein (g/dL) | 0.080 | 0.989 | −0.055 | 1.000 |
| Albumin (g/dL) | 0.009 | 1.000 | −0.055 | 1.000 |
| Blood urea nitrogen (mg/dL) | 0.478 | <0.001 | 0.007 | 1.000 |
| Creatinine (mg/dL) | 0.636 | <0.001 | 0.137 | 0.284 |
| eGFR (mL/min/1.73 m2) | −0.612 | <0.001 | −0.073 | 1.000 |
| C-reactive protein (mg/dL) | 0.079 | 1.000 | −0.109 | 0.727 |
| TNFa (pg/mL) | 0.051 | 1.000 | −0.041 | 1.000 |
| IL-6 (pg/mL) | 0.211 | 0.030 | −0.046 | 1.000 |
| iPTH (pg/mL) | 0.433 | <0.001 | −0.071 | 1.000 |
| 25(OH)D (ng/mL) | −0.141 | 0.253 | −0.023 | 1.000 |
| Hemoglobin A1c (%) | 0.033 | 1.000 | 0.074 | 1.000 |
| Indoxyl sulfate (mg/dL) | −0.237 | 0.011 | ||
| Myostatin (pg/mL) | −0.237 | 0.011 | ||
| Myostatin/SMI | −0.141 | 0.261 | 0.908 | <0.001 |
| Muscle function | ||||
| SMI (kg/m2) | −0.309 | <0.001 | 0.382 | <0.001 |
| HGS (kg) | −0.210 | 0.029 | 0.274 | 0.002 |
| 6 m GS (m/s) | −0.087 | 0.870 | 0.085 | 0.906 |
| Characteristics | IS < 0.365 mg/dL (n = 75) | IS ≥ 0.365 mg/dL (n = 75) | p Value |
|---|---|---|---|
| Age (years) | 64.9 ± 10.6 | 65.2 ± 11.0 | 0.856 |
| Male, n (%) | 58 (77.3) | 39 (52.0) | 0.001 |
| Diabetes mellitus, n (%) | 37 (49.3) | 39 (52.0) | 0.744 |
| Modified CCI score | 4.0 (3.0, 5.0) | 4.0 (3.0, 5.0) | 0.389 |
| Body mass index (kg/m2) | 25.6 ± 3.0 | 24.9 ± 3.1 | 0.217 |
| Laboratory results | |||
| Hemoglobin (g/dL) | 13.3 ± 2.1 | 11.4 ± 1.5 | <0.001 |
| Total protein (g/dL) | 6.9 ± 0.5 | 7.0 ± 0.5 | 0.660 |
| Albumin (g/dL) | 4.3 (4.2, 4.5) | 4.3 (4.1, 4.5) | 0.467 |
| Blood urea nitrogen (mg/dL) | 27.4 ± 9.2 | 36.5 ± 11.0 | <0.001 |
| Creatinine (mg/dL) | 1.6 (1.5, 2.0) | 2.2 (1.7, 2.9) | <0.001 |
| eGFR (mL/min/1.73 m2) | 40.1 ± 10.8 | 27.6 ± 10.8 | <0.001 |
| C-reactive protein (mg/dL) | 0.1 (0.0, 0.4) | 0.1 (0.0, 0.3) | 0.251 |
| TNFa (pg/mL) | 1.4 (1.1, 1.8) | 1.8 (1.5, 2.1) | 0.910 |
| IL-6 (pg/mL) | 1.7 (1.2, 2.6) | 2.2 (1.4, 3.2) | 0.019 |
| iPTH (pg/mL) | 51.7 (37.8, 78.9) | 83.5 (60.1, 125.4) | 0.001 |
| 25(OH)D (ng/mL) | 14.7 (11.6, 21.2) | 13.4 (10.0, 15.9) | 0.016 |
| Hemoglobin A1c (%) | 6.2 (5.9, 7.1) | 6.4 (5.9, 7.5) | 0.116 |
| Indoxyl sulfate (mg/dL) | 0.2 (0.2, 0.3) | 0.6 (0.4, 1.0) | <0.001 |
| Myostatin (pg/mL) | 5.1 ± 2.1 | 4.5 ± 1.9 | 0.070 |
| Myostatin/SMI | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.925 |
| Muscle function | |||
| SMI (kg/m2) | 8.2 ± 1.1 | 7.3 ± 1.2 | <0.001 |
| HGS (kg) | 30.9 ± 9.2 | 26.0 ± 8.2 | 0.001 |
| 6 m GS (m/s) | 1.1 (0.8, 1.3) | 1.1 (0.8, 1.4) | 0.414 |
| presarcopenia, n (%) | 4 (5.4) | 13 (17.3) | 0.022 |
| low muscle strength, n (%) | 14 (18.7) | 25 (33.3) | 0.041 |
| low physical performance, n (%) | 35 (46.7) | 30 (40.0) | 0.410 |
| Sarcopenia, n (%) | 3 (4.1) | 11 (14.7) | 0.026 |
| Presarcopenia | Sarcopenia | |||
|---|---|---|---|---|
| HR a (95% CI) | p Value | HR a (95% CI) | p Value | |
| Age (years) | 1.10 (1.02–1.18) | 0.011 | 1.12 (1.03–1.22) | 0.009 |
| Male, n (%) | 2.21 (0.69–7.11) | 0.183 | 2.94 (0.80–10.77) | 0.104 |
| Diabetes mellitus, n (%) | 1.30 (0.40–4.20) | 0.660 | 1.85 (0.49–6.98) | 0.362 |
| Creatinine (mg/dL) | 0.64 (0.20–2.02) | 0.449 | 0.61 (0.17–2.17) | 0.442 |
| Myostatin/SMI | 4.09 (0.22–75.57) | 0.344 | 5.01 (0.19–135.76) | 0.338 |
| Indoxyl sulfate (mg/dL) | 6.25 (1.14–34.23) | 0.035 | 6.67 (1.08–41.44) | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.M.; Han, M.Y.; Kim, S.H.; Cha, R.H.; Kang, S.H.; Kim, J.C.; An, W.S. Indoxyl Sulfate Might Play a Role in Sarcopenia, While Myostatin Is an Indicator of Muscle Mass in Patients with Chronic Kidney Disease: Analysis from the RECOVERY Study. Toxins 2022, 14, 660. https://doi.org/10.3390/toxins14100660
Lee SM, Han MY, Kim SH, Cha RH, Kang SH, Kim JC, An WS. Indoxyl Sulfate Might Play a Role in Sarcopenia, While Myostatin Is an Indicator of Muscle Mass in Patients with Chronic Kidney Disease: Analysis from the RECOVERY Study. Toxins. 2022; 14(10):660. https://doi.org/10.3390/toxins14100660
Chicago/Turabian StyleLee, Su Mi, Mi Yeun Han, Su Hyun Kim, Ran Hui Cha, Seock Hui Kang, Jun Chul Kim, and Won Suk An. 2022. "Indoxyl Sulfate Might Play a Role in Sarcopenia, While Myostatin Is an Indicator of Muscle Mass in Patients with Chronic Kidney Disease: Analysis from the RECOVERY Study" Toxins 14, no. 10: 660. https://doi.org/10.3390/toxins14100660
APA StyleLee, S. M., Han, M. Y., Kim, S. H., Cha, R. H., Kang, S. H., Kim, J. C., & An, W. S. (2022). Indoxyl Sulfate Might Play a Role in Sarcopenia, While Myostatin Is an Indicator of Muscle Mass in Patients with Chronic Kidney Disease: Analysis from the RECOVERY Study. Toxins, 14(10), 660. https://doi.org/10.3390/toxins14100660






