Structure Elucidation and Toxicity Analysis of the Byproducts Formed after Biodegradation of Aflatoxins B1 and B2 Using Extracts of Mentha arvensis
Abstract
:1. Introduction
2. Results
2.1. Effect of Temperature on In Vitro Biodegradation of Aflatoxins B1 and B2 by Extracts of Mentha Arvensis
2.2. Effect of pH on In Vitro Biodegradation of Aflatoxins B1 and B2 by Extracts of M. arvensis
2.3. In Vitro Biodegradation of Aflatoxin B1 and B2 in Maize Samples
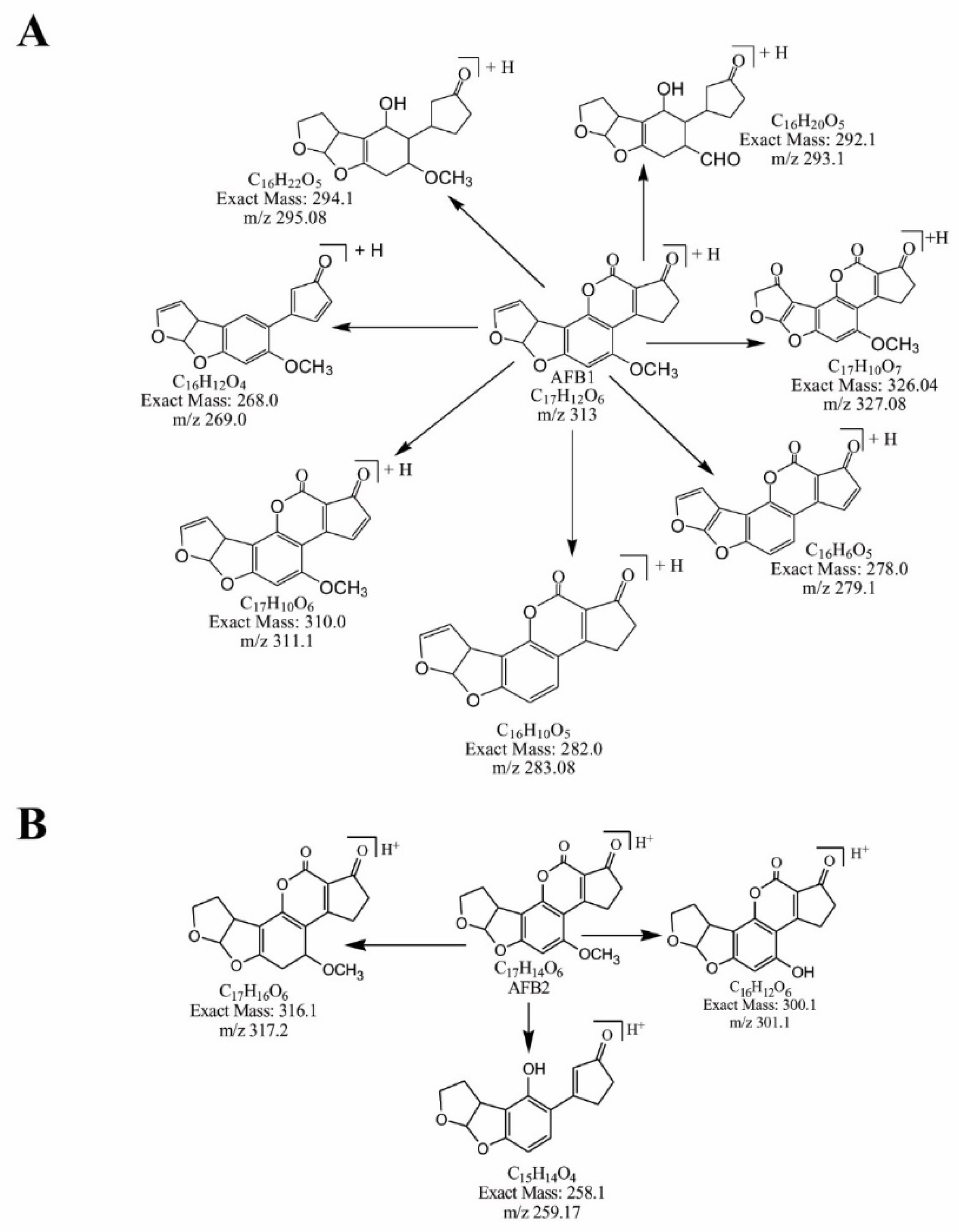
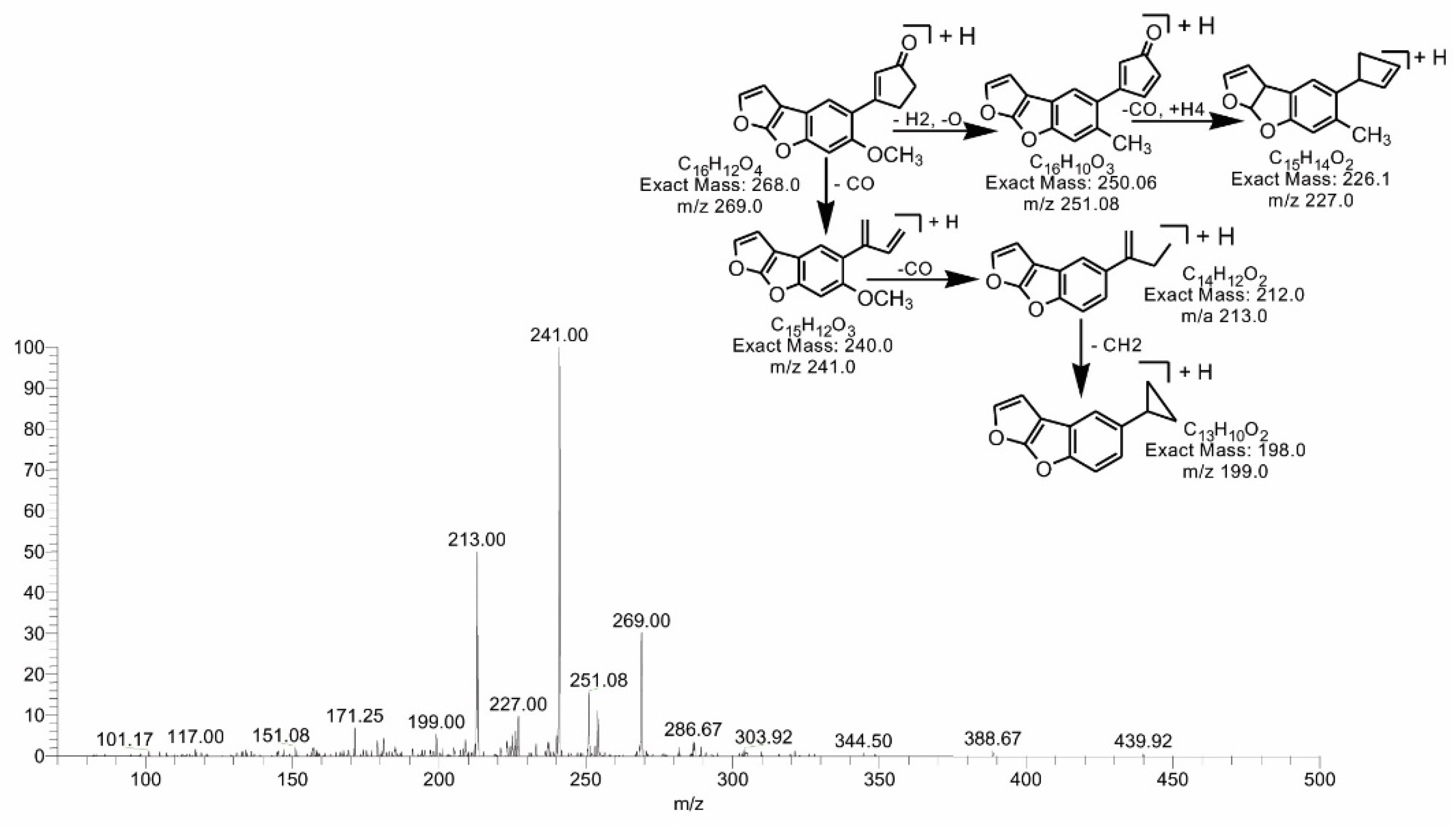
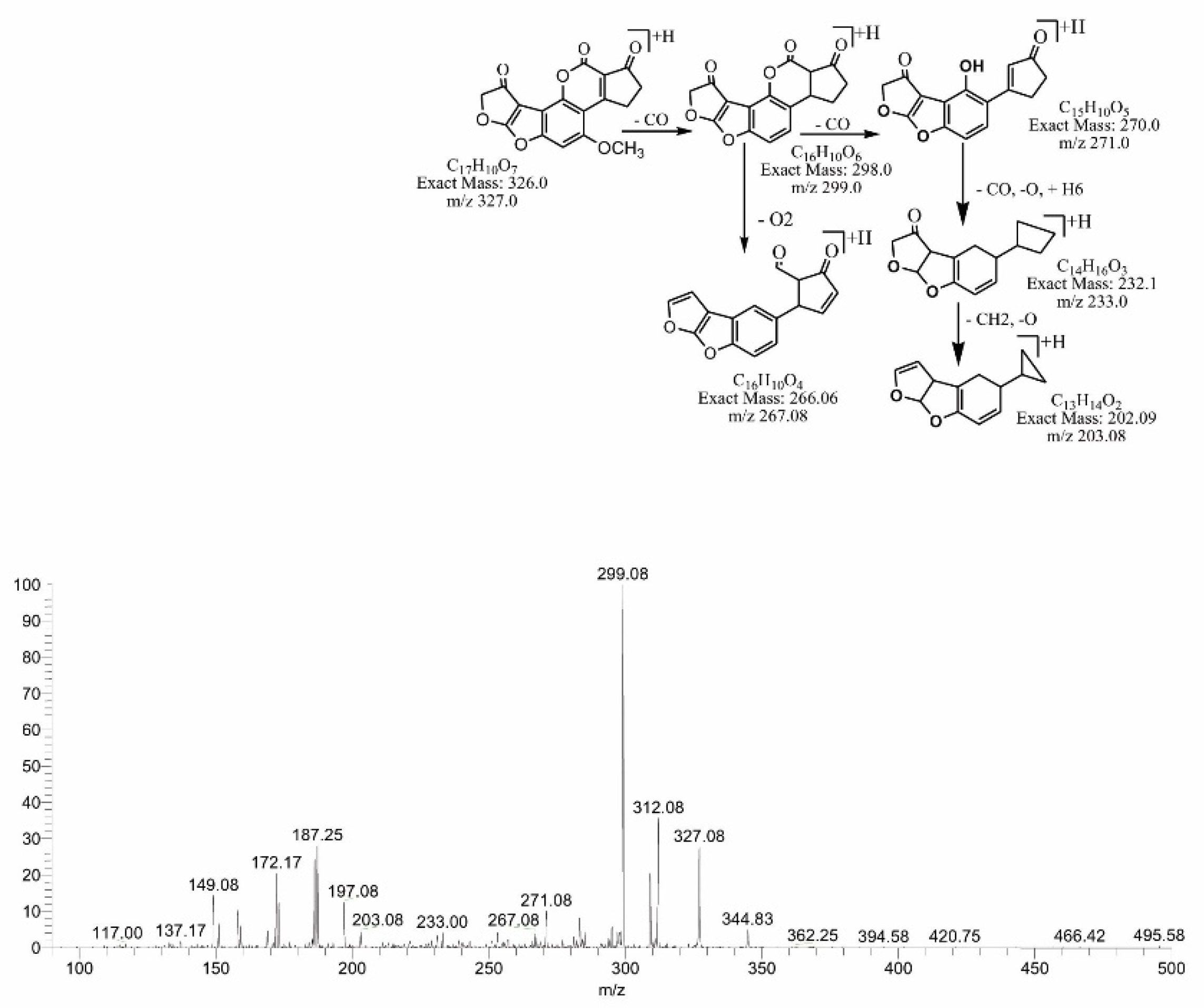
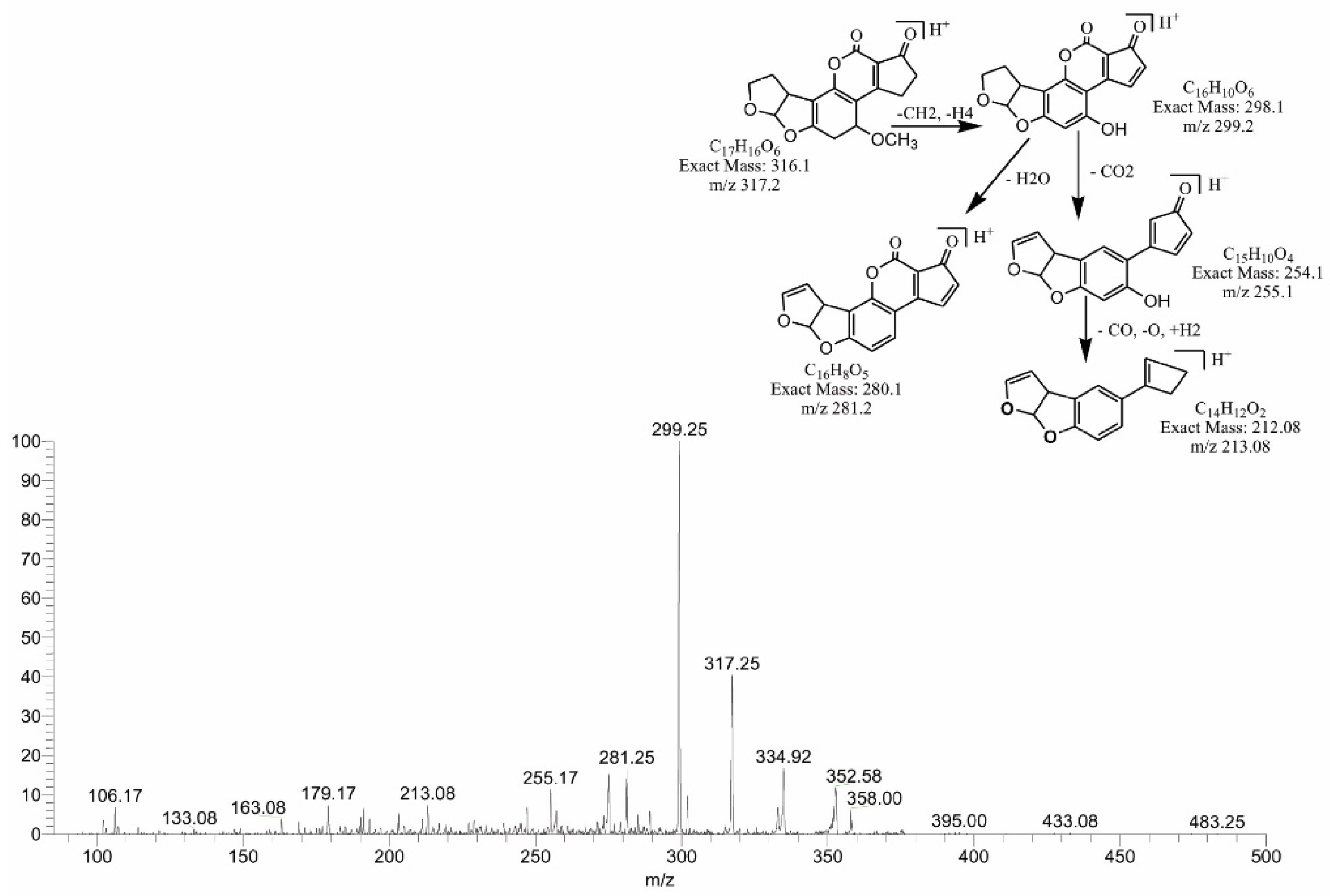
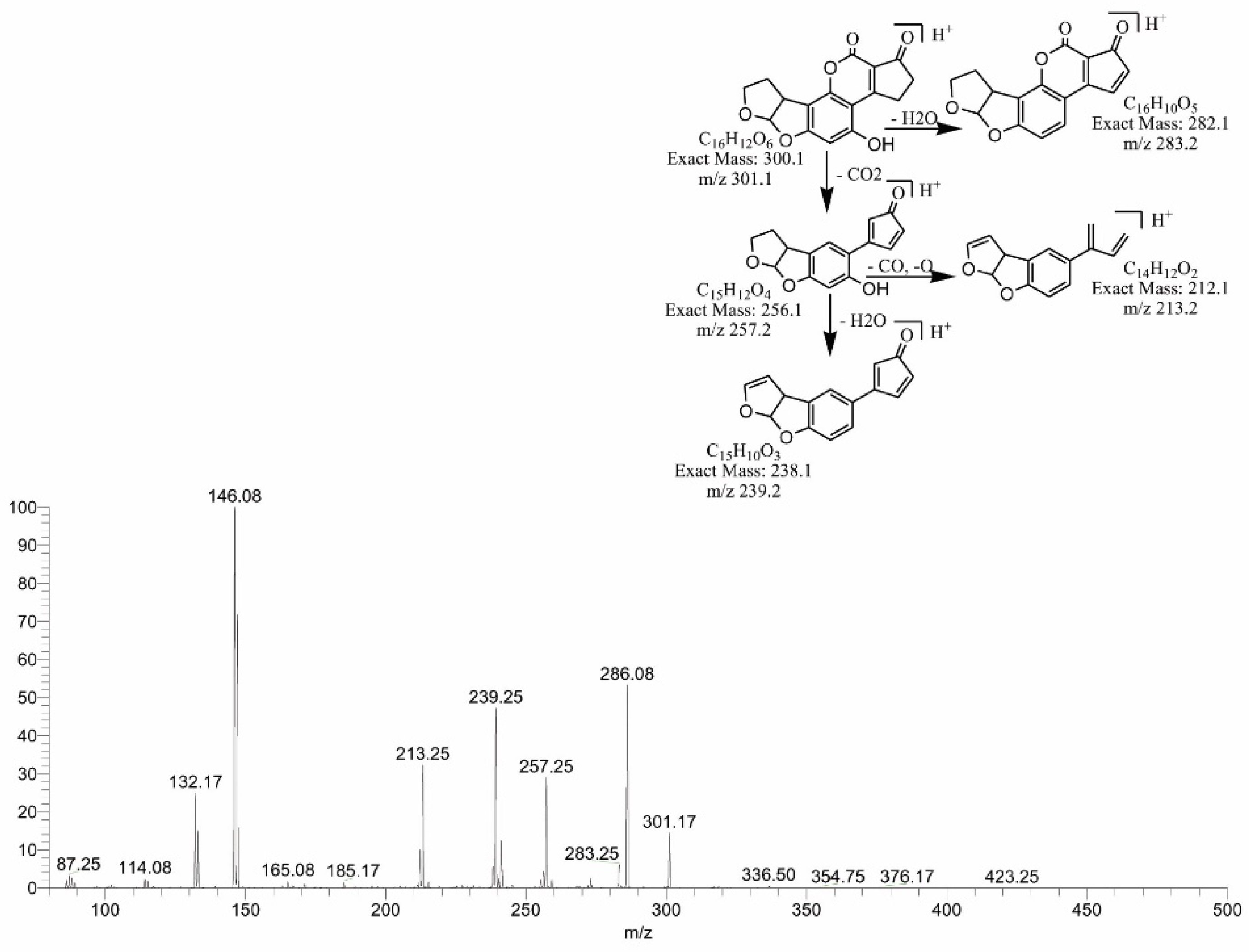
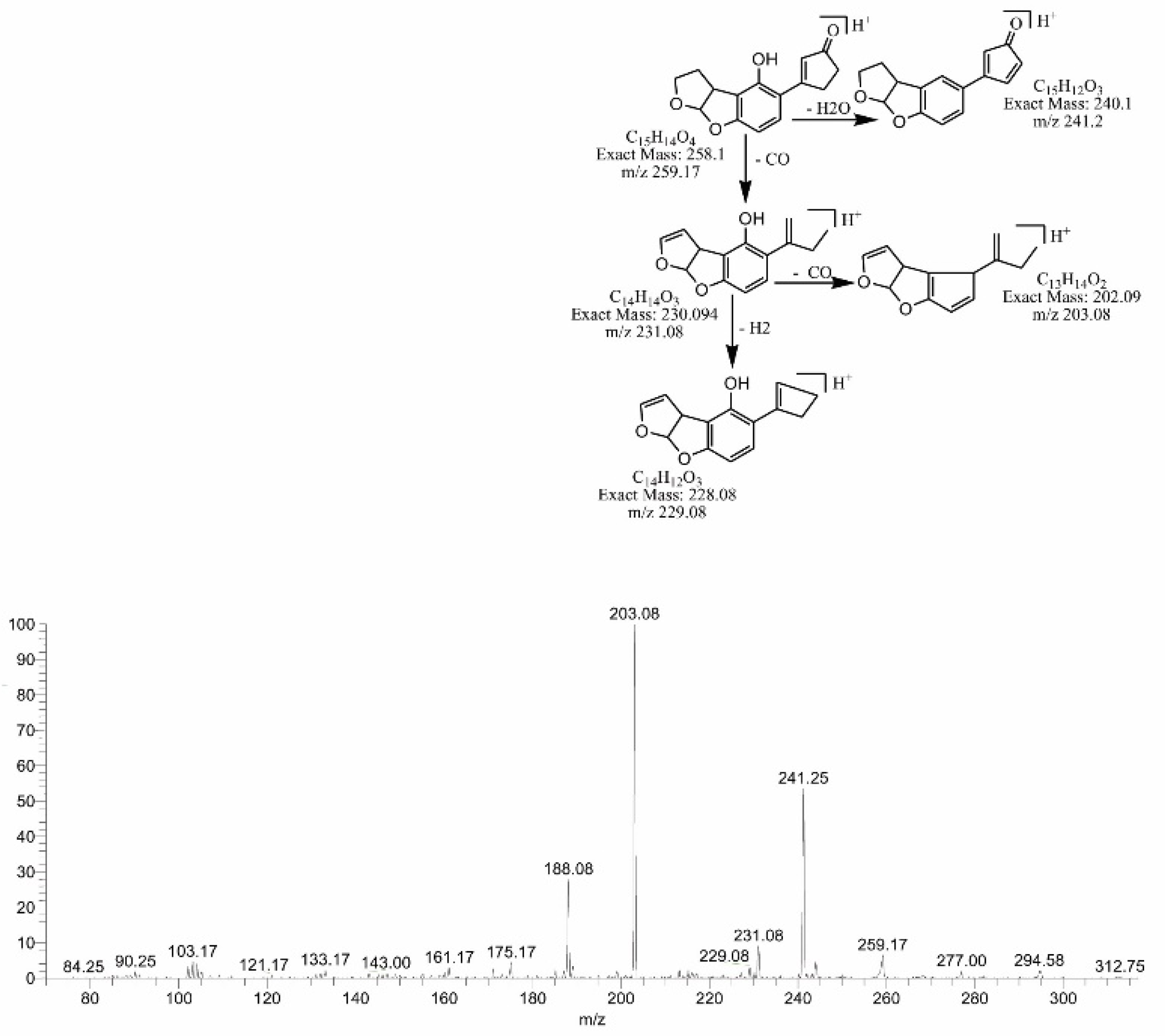
2.4. Mass Spectral Identification of Degraded Products of AFB1 and AFB2 Treated with M. arvensis Leaf Extracts
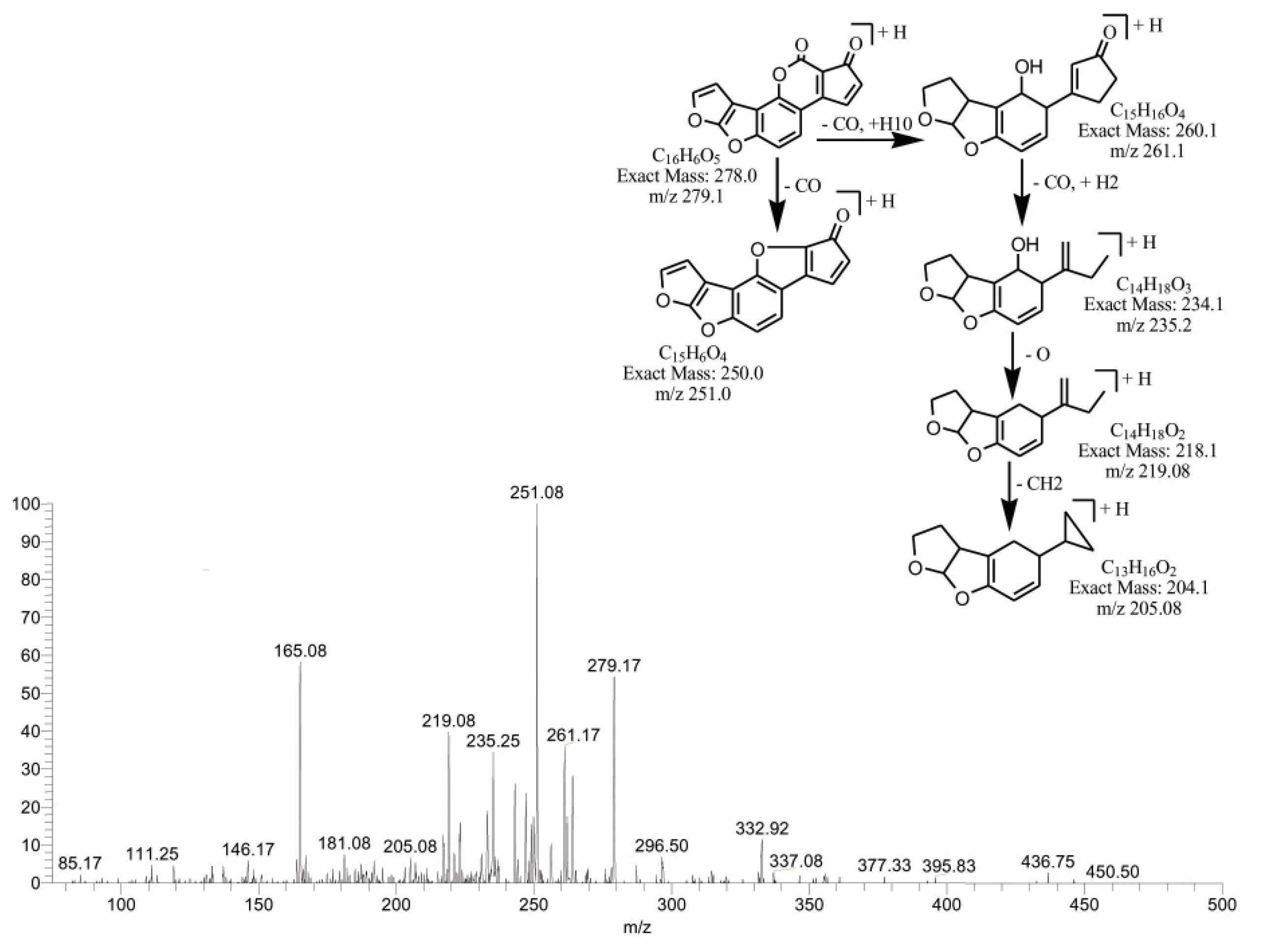
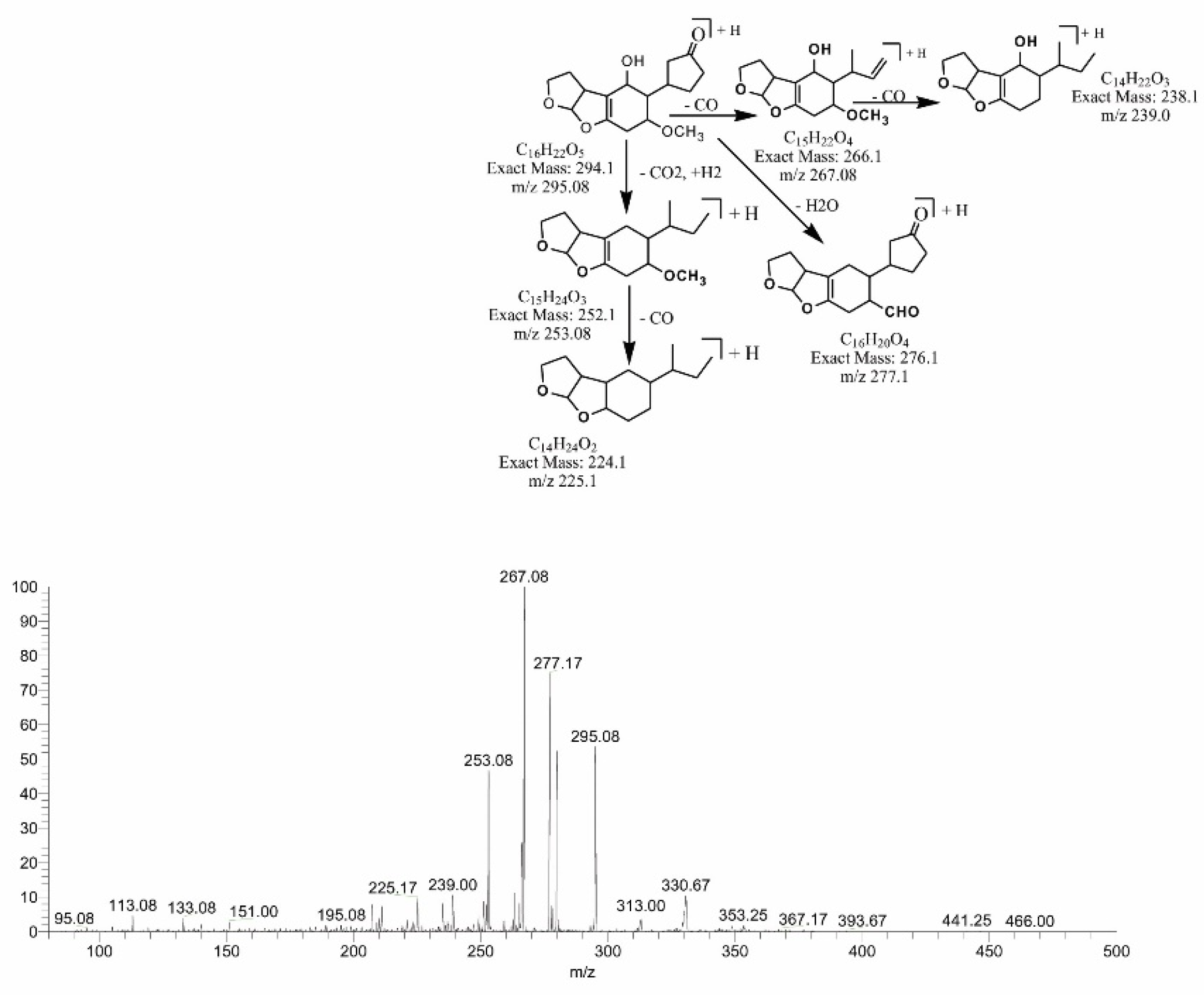
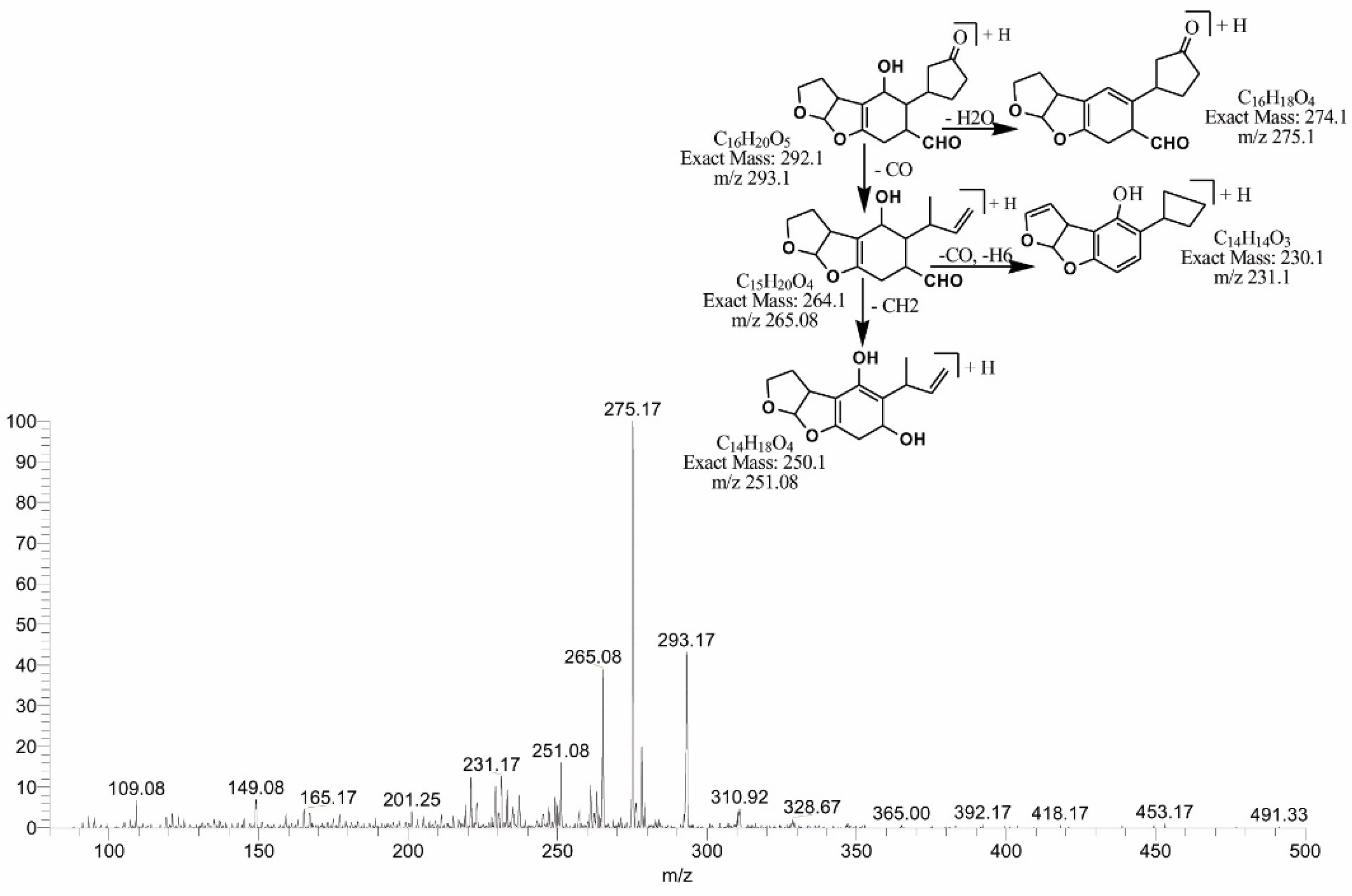
2.5. MS/MS Analysis for Confirmation of AFB1degraded Products
2.6. MS/MS Analysis for Confirmation of Degraded Products of AFB2
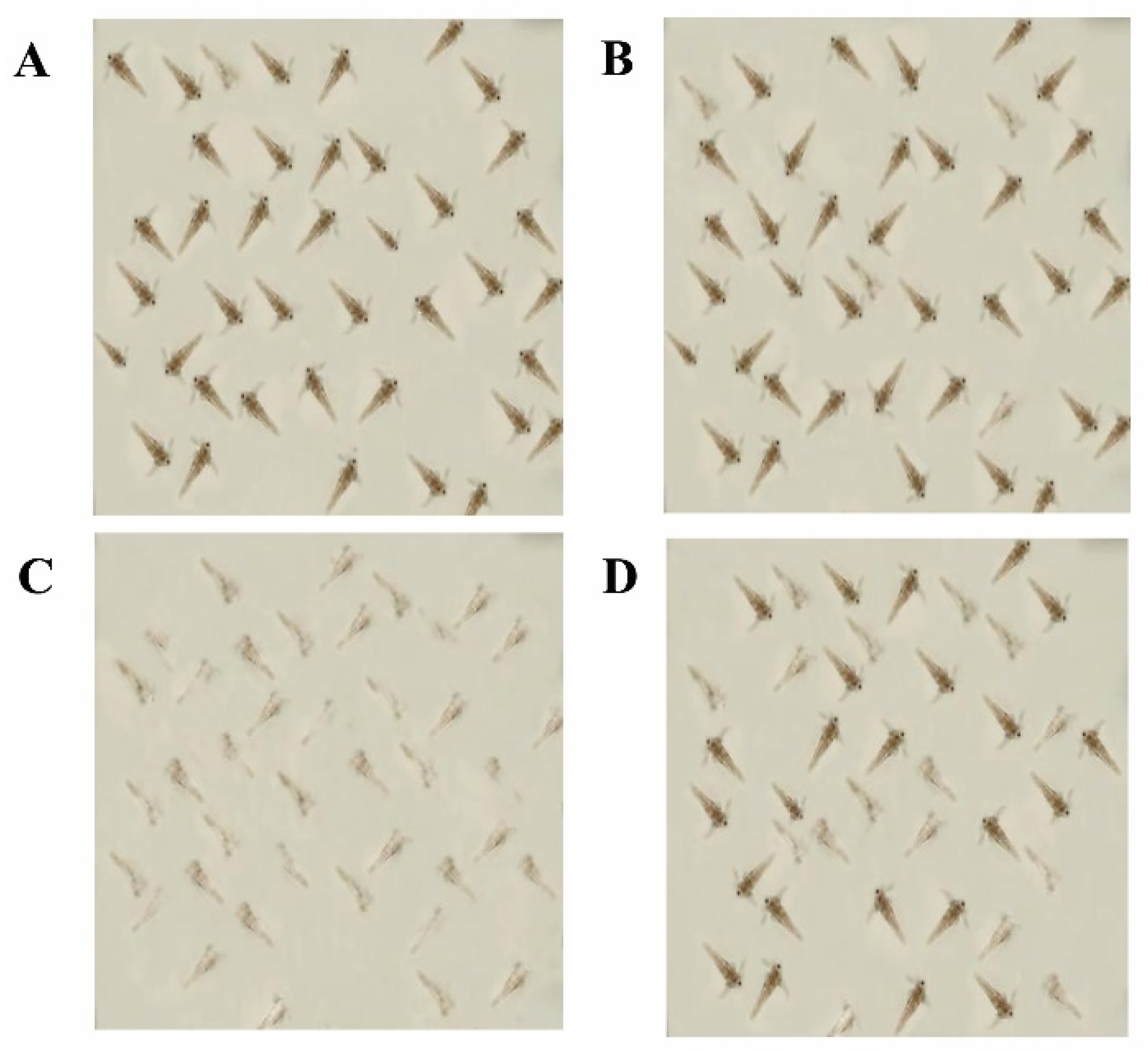
2.7. Assessment of Biological Toxicity of Degraded Products
3. Discussion
4. Materials and Methods
4.1. Preparation of Plant Extract
4.2. In Vitro Toxin Inactivation Assay
4.3. Estimation of Optimal pH for In Vitro Biodegradation Using Plant Extracts
4.4. Estimating Optimal Temperature and Incubation Time for In Vitro Biodegradation Using Plant Extracts
4.5. In Vitro Biodegradation of Toxins in Maize Samples Using Plant Extracts
4.6. Detection and Quantification of Treated Toxins
4.7. LCMS Analysis of Bio-Degraded Toxins
4.8. ESI–MS/MS Conditions for Aflatoxins through Direct Insertion Pump
4.9. Determining Bio-Toxicity of Degraded Products
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Creppy, E.E. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002, 127, 19–28. [Google Scholar] [CrossRef]
- Tabuc, C.; Marin, D.; Guerre, P.; Sesan, T.; Bailly, J.D. Molds and mycotoxin content of cereals in southeastern Romania. J. Food Prot. 2009, 72, 662–665. [Google Scholar] [CrossRef]
- Ghiasian, S.A.; Shephard, G.S.; Yazdanpanah, H. Natural occurrence of aflatoxins from maize in Iran. Mycopathologia 2011, 172, 153–160. [Google Scholar] [CrossRef]
- Khatoon, S.; Hanif, N.Q.; Tahira, I.; Sultana, N.; Sultana, K.; Ayub, N. Natural occurrence of aflatoxins, zearalenone and trichothecenes in maize grown in Pakistan. Pak. J. Bot. 2012, 44, 231–236. [Google Scholar]
- Majeed, S.; Iqbal, M.; Asi, M.R.; Iqbal, S.Z. Aflatoxins and ochratoxin A contamination in rice, corn and corn products from Punjab, Pakistan. J. Cereal Sci. 2013, 58, 446–450. [Google Scholar] [CrossRef]
- Munir, M.A.; Saleem, M.; Malik, Z.R.; Ahmed, M.; Ali, A. Incidence of aflatoxin contamination in non-perishable food commodities. J. Pak. Med. Assoc. 1989, 39, 154–157. [Google Scholar]
- Shah, H.U.; Simpson, T.J.; Alam, S.; Khattak, K.F.; Perveen, S. Mould incidence and mycotoxin contamination in maize kernels from Swat Valley, North West Frontier Province of Pakistan. Food Chem. Toxicol. 2010, 48, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Iram, W.; Anjum, T.; Abbas, M.; Khan, A.M. Aflatoxins and ochratoxin A in maize of Punjab, Pakistan. Food Addit. Contam. Part B 2014, 7, 57–62. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Li, J. Updating techniques on controlling mycotoxins—A review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- Gowda, N.K.S.; Swamy, H.; Mahajan, P. Aflatoxins—Recent Advances and Future Prospects; Recent Advances for Control, Counteraction and Amelioration of Potential Aflatoxins in Animal Feeds; IntechOpen: London, UK, 2013; pp. 129–140. [Google Scholar]
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods 2020, 9, 644. [Google Scholar] [CrossRef]
- Ciegler, A.; Lillehoj, E.B.; Peterson, R.E.; Hall, H.H. Microbial detoxification of aflatoxin. Appl. Microbiol. 1966, 14, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Minuye, M. Aflatoxin Reduction Mechanism of Probiotics. J. Prob. Health 2021, 9, 235. [Google Scholar]
- Al-Rahmah, N.; Mostafa, A.; Abdel-Megeed, A. Antifungal and antiaflatoxigenic activities of some plant extracts. Afr. J. Microbiol. Res. 2011, 5, 1342–1348. [Google Scholar] [CrossRef] [Green Version]
- El-Nagerabi, S.A.F.; Al-Bahry, S.N.; Elshafie, A.E.; AlHilali, S. Effect of Hibiscus sabdariffa extract and Nigella sativa oil on the growth and aflatoxin B1 production of Aspergillus flavus and Aspergillus parasiticus strains. Food Control 2012, 25, 59–63. [Google Scholar] [CrossRef]
- Hamad, G.M.; Mohdaly, A.A.A.; El-Nogoumy, B.A.; Ramadan, M.F.; Hassan, S.A.; Zeitoun, A.M. Detoxification of Aflatoxin B1 and Ochratoxin A Using Salvia farinacea and Azadirachta indica Water Extract and Application in Meat Products. Appl. Biochem. Biotechnol. 2021, 193, 3098–3120. [Google Scholar] [CrossRef] [PubMed]
- Shanakhat, H.; Sorrentino, A.; Raiola, A.; Romano, A.; Masi, P.; Cavella, S. Current methods for mycotoxins analysis and innovative strategies for their reduction in cereals: An overview. J. Sci. Food Agric. 2018, 98, 4003–4013. [Google Scholar] [CrossRef]
- Velazhahan, R.; Vijayanandraj, S.; Vijayasamundeeswari, A.; Paranidharan, V.; Samiyappan, R.; Iwamoto, T.; Friebe, B.; Muthukrishnan, S. Detoxification of aflatoxins by seed extracts of the medicinal plant, Trachyspermum ammi (L.) Sprague ex Turrill–structural analysis and biological toxicity of degradation product of aflatoxin G1. Food Control. 2010, 21, 719–725. [Google Scholar] [CrossRef]
- Rajan, M.; Gupta, P.; Kumar, A. Promising Antiviral Molecules from Ayurvedic Herbs and Spices against COVID-19. Chin. J. Integr. Med. 2021, 27, 243–244. [Google Scholar] [CrossRef]
- Ghani, A. Medicinal Plants of Bangladesh: Chemical Constituents and Uses; Asiatic Society of Bangladesh: Dhaka, Bangladesh, 1998. [Google Scholar]
- Satyavati, G.V.; Raina, M.K.; Sharma, M. Medicinal Plants of India; Indian Council of Medical Research: New Delhi, India, 1987; Volume 2. [Google Scholar]
- Verma, R.S.; Rahman, L.; Verma, R.K.; Chauhan, A.; Yadav, A.K.; Singh, A. Essential oil composition of menthol mint (Mentha arvensis) and peppermint (Mentha piperita) cultivars at different stages of plant growth from Kumaon region of Western Himalaya. Open Access J. Med. Aromat. Plants 2010, 1, 13. [Google Scholar]
- Iram, W.; Anjum, T.; Jabeen, R.; Abbas, M. Isolation of stored maize mycoflora, identification of aflatoxigenic fungi and its inhibition using medicinal plant extracts. Int. J. Agric. Biol. 2018, 20, 2149–2160. [Google Scholar]
- Koka, J.A.; Wani, A.H.; Bhat, M.Y.; Parveen, S. Antifungal activity of ethanolic and aqueous leaf extracts of Taraxicum officinale and Mentha arvensis on the growth of some selected fungal species under in vitro conditions. Int. J. Pure Appl. Biosci. 2017, 5, 1170–1176. [Google Scholar] [CrossRef]
- Afshar, P.; Shokrzadeh, M.; Raeisi, S.N.; Ghorbani-HasanSaraei, A.; Nasiraii, L.R. Aflatoxins biodetoxification strategies based on probiotic bacteria. Toxicon 2020, 178, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Wei, C.; Ma, Q.; Ji, C.; Zhang, J.; Zhao, L. Efficacy of Bacillus subtilis ANSB060 biodegradation product for the reduction of the milk aflatoxin M1 content of dairy cows exposed to aflatoxin B1. Toxins 2019, 11, 161. [Google Scholar] [CrossRef] [Green Version]
- Hassan, Y.I.; Zhou, T. Promising detoxification strategies to mitigate mycotoxins in food and feed. Toxins 2018, 10, 116. [Google Scholar] [CrossRef] [Green Version]
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Song, Z.; Zhang, X.; Zhang, J.; Mei, S. Antifungal, plant growth-promoting, and mycotoxin detoxication activities of Burkholderia sp. strain XHY-12. 3 Biotech 2020, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Anjum, T.; Iram, W.; Iqbal, M.; Ghaffar, A.; Abbas, M. Identification of degradation products of aflatoxin B1 and B2 resulting after their biodetoxification by aqueous extracts of Acacia nilotica. World Mycotoxin J. 2020, 13, 499–514. [Google Scholar] [CrossRef]
- Rustom, I.Y.S. Aflatoxin in food and feed: Occurrence, legislation and inactivation by physical methods. Food Chem. 1997, 59, 57–67. [Google Scholar] [CrossRef]
- Hajare, S.S.; Hajare, S.N.; Sharma, A. Aflatoxin inactivation using aqueous extract of ajowan (Trachyspermum ammi) seeds. J. Food Sci. 2005, 70, C29–C34. [Google Scholar] [CrossRef]
- Vijayanandraj, S.; Brinda, R.; Kannan, K.; Adhithya, R.; Vinothini, S.; Senthil, K.; Chinta, R.R.; Paranidharan, V.; Velazhahan, R. Detoxification of aflatoxin B1 by an aqueous extract from leaves of Adhatoda vasica Nees. Microbiol. Res. 2014, 169, 294–300. [Google Scholar] [CrossRef]
- Méndez-Albores, J.A.; Villa, G.A.; Del Rio-García, J.C.; Martínez, E.M. Aflatoxin-detoxification achieved with Mexican traditional nixtamalization process (MTNP) is reversible. J. Sci. Food Agric. 2004, 84, 1611–1614. [Google Scholar] [CrossRef]
- Jackson, L.W.; Pryor, B.M. Degradation of aflatoxin B 1 from naturally contaminated maize using the edible fungus Pleurotus ostreatus. AMB Express 2017, 7, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Xie, F.; Xue, X.; Wang, Z.; Fan, B.; Ha, Y. Structure elucidation and toxicity analyses of the radiolytic products of aflatoxin B1 in methanol–water solution. J. Hazard. Mater. 2011, 192, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Durakovic, S.; Delas, F.; Durakovic, L. Microbial indicators of safety and quality of foods. In Modern Microbiology of Food; Durakovic, S., Ed.; Kugler: London, UK, 2002; pp. 249–282. [Google Scholar]
- Moretti, A.; Mule, G.; Ritieni, A.; Logrieco, A. Further data on the production of beauvericin, enniatins and fusaproliferin and toxicity to Artemia salina by Fusarium species of Gibberella fujikuroi species complex. Int. J. Food Microbiol. 2007, 118, 158–163. [Google Scholar] [CrossRef]
- Das, C.; Mishra, H. In vitro Degradation of Aflatoxin B1in Groundnut (Arachis hypogea) Meal by Horse Radish Peroxidase. LWT-Food Sci. Technol. 2000, 33, 308–312. [Google Scholar] [CrossRef]
- Stroka, J.; Van Otterdijk, R.; Anklam, E. Immunoaffinity column clean-up prior to thin-layer chromatography for the determination of aflatoxins in various food matrices. J. Chromatogr. A 2000, 904, 251–256. [Google Scholar] [CrossRef]



| Treatments | Temp (°C) | % Degradation of AFB1 | % Degradation of AFB2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 h | 6 h | 12 h | 24 h | 48 h | 72 h | 3 h | 6 h | 12 h | 24 h | 48 h | 72 h | ||
| Toxin | 25 | 0.3 ± 0.0 c | 0.8 ± 0.0 c | 1.6 ± 0.9 b | 1.9 ± 0.1 b | 2.2 ± 0.1 a | 2.9 ± 0.2 a | 0.1 ± 0.0 c | 0.5 ± 0.0 b,c | 0.7 ± 0.0 a–c | 0.8 ± 0.0 a–c | 1.0 ± 0.0 a,b | 1.4 ± 0.1 a |
| 30 | 0.8 ± 0.0 d | 0.8 ± 0.0 d | 1.8 ± 0.6 c | 2.3 ± 0.1 b | 3.1 ± 0.2 a | 3.8 ± 0.1 a | 0.1 ± 0.0 c | 0.6 ± 0.0 b,c | 0.8 ± 0.0 b,c | 0.9 ± 0.1 a,b | 1.1 ± 0.0 a,b | 1.6 ± 0.1 a | |
| 35 | 1.2 ± 0.3 c | 2.5 ± 0.3 b,c | 2.5 ± 0.1 b,c | 3.0 ± 0.2 b | 3.8 ± 0.2 a,b | 4.5 ± 0.3 a | 0.2 ± 0.0 c | 0.8 ± 0.0 b,c | 0.8 ± 0.0 b,c | 1.1 ± 0.1 b | 1.2 ± 0.1 a,b | 1.8 ± 0.2 a | |
| 40 | 2.2 ± 0.1 d | 3.5 ± 0.4 c | 3.8 ± 0.2 c | 4.3 ± 0.5 b | 4.4 ± 0.3 b | 5.2 ± 0.4 a | 0.3 ± 0.0 c | 0.9 ± 0.1 b,c | 0.9 ± 0.0 b,c | 1.3 ± 0.1 b | 1.4 ± 0.1 a,b | 2.0 ± 0.1 a | |
| 45 | 3.2 ± 0.3 c | 4.5 ± 0.3 b | 5.1 ± 0.3 a,b | 5.2 ± 0.4 a,b | 5.6 ± 0.5 a | 5.8 ± 0.3 a | 0.3 ± 0.0 c | 1.0 ± 0.2 b,c | 1.1 ± 0.1 b,c | 1.4 ± 0.1 b | 1.6 ± 0.1 b | 2.3 ± 0.2 a | |
| 50 | 4.2 ± 0.5 c | 5.5 ± 0.2 b | 5.8 ± 0.2 b | 6.5 ± 0.3 a,b | 6.5 ± 0.4 a,b | 6.9 ± 0.4 a | 0.3 ± 0.0 c | 1.1 ± 0.1 b,c | 1.2 ± 0.1 b | 1.6 ± 0.1 b | 1.7 ± 0.1 b | 2.5 ± 0.2 a | |
| 55 | 5.2 ± 0.3 c | 6.4 ± 0.7 b | 6.5 ± 0.4 b | 7.1 ± 0.5 a,b | 7.8 ± 0.6 a | 7.9 ± 0.6 a | 0.4 ± 0.0 d | 1.1 ± 0.1 c | 1.4 ± 0.1 b,c | 1.7 ± 0.1 b | 1.9 ± 0.1 b | 2.7 ± 0.1 a | |
| 60 | 6.2 ± 0.4 b | 7.1 ± 0.5 a,b | 7.5 ± 0.8 a,b | 7.7 ± 0.8 a | 7.8 ± 0.4 a | 7.9 ± 0.5 a | 0.5 ± 0.0 d | 1.2 ± 0.2 c,d | 1.5 ± 0.1 c | 1.8 ± 0.2 b,c | 2.0 ± 0.1 b | 3.0 ± 0.2 a | |
| Toxin + H2O | 25 | 0.2 ± 0.0 d | 1.4 ± 0.1 c | 2.7 ± 0.2 b | 3.4 ± 0.2 a | 3.4 ± 0.2 a | 3.4 ± 0.2 a | 0.3 ± 0.0 c | 0.4 ± 0.0 c | 1.3 ± 0.0 b,c | 1.4 ± 0.1 a–c | 2.2 ± 0.1 a,b | 2.4 ± 0.1 a |
| 30 | 1.1 ± 0.1 d | 2.5 ± 0.1 c | 3.2 ± 0.3 b | 3.5 ± 0.3 b | 3.8 ± 0.2 a,b | 4.2 ± 0.3 a | 0.3 ± 0.0 d | 1.1 ± 0.0 c | 1.5 ± 0.0 c | 2.0 ± 0.1 b,c | 2.3 ± 0.2 b | 3.4 ± 0.2 a | |
| 35 | 2.4 ± 0.2 d | 3.4 ± 0.2 c | 4.7 ± 0.2 b | 5.1 ± 0.3 a,b | 5.5 ± 0.3 a | 5.7 ± 0.3 a | 1.2 ± 0.1 c | 1.2 ± 0.1 c | 2.2 ± 0.1 b,c | 2.7 ± 0.2 a,b | 2.8 ± 0.1 a,b | 3.3 ± 0.2 a | |
| 40 | 3.7 ± 0.2 d | 4.7 ± 0.5 c | 6.1 ± 0.5 b | 6.4 ± 0.4 a,b | 6.8 ± 0.7 a | 6.8 ± 0.5 a | 2.1 ± 0.3 c | 2.7 ± 0.1 b | 3.3 ± 0.2 a,b | 3.4 ± 0.2 a,b | 3.7 ± 0.2 a | 3.8 ± 0.3 a | |
| 45 | 5.1 ± 0.4 d | 5.9 ± 0.3 c | 7.2 ± 0.6 b,c | 7.4 ± 0.5 b,c | 7.8 ± 0.5 b | 8.0 ± 0.4 a | 2.7 ± 0.2 d | 3.9 ± 0.2 c | 4.1 ± 0.3 b,c | 4.1 ± 0.3 b,c | 4.5 ± 0.1 a,b | 4.9 ± 0.2 a | |
| 50 | 6.4 ± 0.3 d | 7.3 ± 0.6 c | 8.3 ± 0.6 b | 8.9 ± 0.6 b | 9.1 ± 0.7 a,b | 9.5 ± 0.8 a | 3.4 ± 0.5 c | 4.4 ± 0.3 c | 4.4 ± 0.1 c | 5.6 ± 0.2 b | 5.6 ± 0.4 b | 6.0 ± 0.4 a | |
| 55 | 7.7 ± 0.6 d | 8.7 ± 0.5 c | 9.3 ± 0.8 b,c | 9.9 ± 0.7 b | 10 ± 1.3 a,b | 10 ± 0.6 a | 4.2 ± 0.3 c | 4.8 ± 0.2 c | 4.9 ± 0.3 c | 6.7 ± 0.4 b | 7.1 ± 0.6 a | 7.1 ± 0.3 a | |
| 60 | 9.1 ± 0.7 d | 10 ± 1.1 c,d | 10 ± 0.9 c,d | 10 ± 0.8 c | 11 ± 1.1 b | 12 ± 1.1 a | 4.9 ± 0.6 d | 5.2 ± 0.4 c,d | 5.4 ± 0.4 c | 7.8 ± 0.7 b | 8.3 ± 0.5 a,b | 8.6 ± 0.5 a | |
| Toxin + leaf extract | 25 | 44 ± 2.3 d | 46 ± 2.3 d | 52 ± 3.6 c,d | 60 ± 4.3 b,c | 66 ± 4.3 a,b | 70 ± 6.3 a | 62 ± 5.6 c | 65 ± 4.3 b,c | 66 ± 4.6 b | 69 ± 5.7 a,b | 70 ± 5.0 a | 71 ± 4.3 a |
| 30 | 49 ± 4.1 d | 52 ± 4.1 d | 58 ± 2.3 c,d | 61 ± 5.1 b,c | 67 ± 5.0 a,b | 72 ± 5.9 a | 63 ± 7.2 d | 65 ± 5.2 c,d | 66 ± 5.2 c | 70 ± 6.1 b | 72 ± 6.2 a,b | 74 ± 6.3 a | |
| 35 | 53 ± 3.2 e | 57 ± 3.2 d,e | 60 ± 4.1 c,d | 64 ± 3.6 b,c | 68 ± 3.3 a,b | 74 ± 5.0 a | 65 ± 4.2 d | 66 ± 5.3 c,d | 67 ± 4.9 c | 72 ± 4.8 b | 74 ± 5.8 a,b | 75 ± 5.2 a | |
| 40 | 61 ± 5.1 e | 64 ± 5.4 d,e | 67 ± 5.2 c,d | 71 ± 4.1 b,c | 77 ± 6.1 a,b | 81 ± 7.1 a | 66 ± 4.0 d | 67 ± 4.0 d | 69 ± 4.8 c | 74 ± 6.2 b | 76 ± 6.3 a,b | 77 ± 4.9 a | |
| 45 | 62 ± 3.7 e | 66 ± 2.9 d,e | 69 ± 3.7 c,d | 73 ± 6.0 b,c | 79 ± 4.9 a,b | 85 ± 6.2 a | 68 ± 5.9 d | 69 ± 5.1 d | 71 ± 6.6 c | 75 ± 5.3 b | 77 ± 4.9 a,b | 78 ± 5.8 a | |
| 50 | 63 ± 4.1 e | 66 ± 4.0 d | 70 ± 6.1 c | 74 ± 5.1 b,c | 80 ± 5.3 b | 86 ± 7.7 a | 69 ± 3.7 d | 72 ± 4.9 c,d | 73 ± 5.8 c | 78 ± 6.1 b | 79 ± 5.7 a,b | 80 ± 6.2 a | |
| 55 | 65 ± 5.5 e | 68 ± 5.1 d | 72 ± 4.5 c,d | 76 ± 6.9 b,c | 82 ± 4.2 a,b | 87 ± 4.9 a | 71 ± 4.8 d | 72 ± 2.6 c,d | 74 ± 4.8 b | 79 ± 5.6 a,b | 79 ± 5.8 a,b | 81 ± 5.4 a | |
| 60 | 67 ± 4.2 d | 68 ± 7.1 d | 73 ± 5.1 c,d | 78 ± 5.1 b,c | 84 ± 6.7 a,b | 88 ± 6.2 a | 72 ± 5.9 d | 73 ± 5.9 c,d | 74 ± 5.7 c | 80 ± 6.2 b | 82 ± 6.3 a,b | 83 ± 7.7 a | |
| Toxin + shoot extract | 25 | 19 ± 1.3 d | 23 ± 1.9 c | 25 ± 2.1 b,c | 28 ± 1.3 b | 30 ± 2.2 a,b | 32 ± 2.6 a | 25 ± 1.9 f | 29 ± 1.6 e | 32 ± 1.9 d | 38 ± 2.7 c | 43 ± 3.3 b | 46 ± 3.6 a |
| 30 | 25 ± 2.2 d,c | 23 ± 1.6 d | 26 ± 1.8 c | 29 ± 1.1 b,c | 31 ± 1.9 b | 34 ± 2.7 a | 26 ± 2.3 f | 30 ± 2.3 e | 33 ± 2.0 d | 39 ± 2.8 c | 45 ± 2.8 b | 48 ± 2.7 a | |
| 35 | 29 ± 1.7 c | 32 ± 2.5 b,c | 33 ± 2.3 a,b | 35 ± 2.4 a | 36 ± 3.4 a | 36 ± 3.2 a | 28 ± 1.7 f | 32 ± 1.9 d,e | 35 ± 2.2 d | 45 ± 3.3 c | 48 ± 3.1 b | 50 ± 4.1 a | |
| 40 | 36 ± 1.6 c | 39 ± 2.3 b | 41 ± 2.9 a,b | 42 ± 3.2 a,b | 42 ± 2.8 a | 43 ± 3.3 a | 29 ± 2.2 e | 32 ± 2.6 d,e | 34 ± 1.7 d | 45 ± 2.1 c | 49 ± 3.6 b | 51 ± 3.8 a | |
| 45 | 37 ± 2.8 c | 41 ± 3.7 b,c | 44 ± 3.6 b | 46 ± 3.1 a,b | 46 ± 3.1 a,b | 47 ± 2.0 a | 31 ± 1.9 e | 33 ± 1.1 d,e | 35 ± 1.8 d | 42 ± 1.9 c | 48 ± 2.9 b | 53 ± 2.7 a | |
| 50 | 37 ± 2.7 c | 41 ± 2.9 b,c | 44 ± 2.8 b | 46 ± 2.7 a,b | 47 ± 2.9 a,b | 48 ± 3.9 a | 32 ± 2.8 e | 33 ± 2.7 d,e | 35 ± 2.6 d | 43 ± 2.6 c | 49 ± 2.7 b | 54 ± 3.9 a | |
| 55 | 40 ± 3.1 c | 44 ± 3.1 b,c | 45 ± 3.7 b | 47 ± 2.9 a,b | 48 ± 3.4 a,b | 49 ± 2.8 a | 34 ± 3.3 e | 35 ± 1.9 d,e | 37 ± 2.2 d | 44 ± 3.1 c | 51 ± 4.1 b | 56 ± 2.5 a | |
| 60 | 42 ± 2.8 c | 45 ± 2.3 b,c | 46 ± 2.6b | 49 ± 3.2 a,b | 50 ± 3.2 a | 50 ± 4.0 a | 35 ± 1.2 e | 35 ± 2.3 d,e | 37 ± 2.9 d | 45 ± 2.3 c | 52 ± 4.4 b | 57 ± 3.7 a | |
| Treatments | pH | 3 h | 6 h | 12 h | 24 h | 48 h | 72 h | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toxin Recovery | D% | Toxin Recovery | D% | Toxin Recovery | D% | Toxin Recovery | D% | Toxin Recovery | D% | Toxin Recovery | D% | ||
| Toxin AFB1 | 99 ± 7.8 r | 0.81 | 99 ± 6.7 r | 0.88 | 98 ± 7.7 r | 1.80 | 97 ± 5.4 r | 2.3 | 96 ± 6.4 q,r | 3.07 | 96 ± 7.5 o–r | 3.81 | |
| Toxin + H2O | pH2 | 96 ± 9.5 p,q,r | 3.2 | 95 ± 7.3n –r | 4.6 | 92 ± 6.9 k–p | 7.8 | 90 ± 6.9 g–l | 9.6 | 89 ± 6.5 e–l | 10.2 | 87 ± 5.9 d–j | 12.7 |
| Toxin + H2O | pH4 | 96 ± 7.6 o–r | 3.8 | 95 ± 7.4 n–r | 4.7 | 90 ± 8.8 h–m | 9.2 | 88 ± 5.9 d–l | 11.6 | 87 ± 8.9 d–i | 13.0 | 86 ± 7.9 c–h | 13.9 |
| Toxin + H2O | pH6 | 94 ± 5.2 m–r | 5.1 | 92 ± 6.8 l–q | 7.5 | 90 ± 6.8 f–l | 9.8 | 88 ± 7.4 c–l | 12.0 | 86 ± 6.1 c–g | 14.0 | 85 ± 6.0 b–f | 14.9 |
| Toxin + H2O | pH8 | 91 ± 6.9 j–o | 8.1 | 89 ± 6.3 e–l | 10.1 | 87 ± 6.1 d–k | 12.3 | 85 ± 5.9 b–c | 14.7 | 84 ± 5.9 a–d | 15.9 | 83 ± 5.7 a–d | 16.5 |
| Toxin + H2O | pH10 | 90 ± 6.8 i–n | 8.6 | 87 ± 6.1 d–k | 12.2 | 85 ± 5.0 b–c | 14.7 | 82 ± 5.0 a,b,c | 17.7 | 81 ± 4.5 a,b | 18.8 | 80 ± 5.4 a | 19.9 |
| Toxin + H2O | WpH | 98 ± 5.7 r | 1.09 | 97 ± 5.6 r | 2.6 | 96 ± 5.9 p,q,r | 3.23 | 96 ± 5.8 o–r | 3.56 | 96 ± 5.9 o–r | 3.97 | 95 ± 7.4 n–r | 4.19 |
| Leaf extract + AFB1 | pH2 | 56 ± 3.3 h–u | 43.8 | 55 ± 4.4 c–u | 44.4 | 52 ± 2.2 a–t | 47.7 | 48 ± 3.0a–o | 51.7 | 42 ± 1.8 a–l | 57.5 | 37 ± 1.6 a–j | 62.2 |
| pH4 | 54 ± 2.5 b–u | 45.7 | 55 ± 3.5 a–u | 44.4 | 52 ± 3.2 a–t | 47.7 | 48 ± 1.9 a–o | 51.7 | 42 ± 2.9 a–j | 57.5 | 37 ± 2.5 a–j | 62.2 | |
| pH6 | 36 ± 1.6 a–q | 64.0 | 34 ± 2.4 a–n | 65.2 | 30 ± 1.6 a–l | 70.0 | 27 ± 1.9 a–l | 72.6 | 24 ± 0.9 a–i | 75.9 | 20 ± 0.6 a–e | 79.6 | |
| pH8 | 38 ± 3.7 a–n | 61.6 | 34 ± 1.3 a–l | 65.7 | 30 ± 1.5 a–k | 69.4 | 28 ± 1.1 a–j | 71.2 | 23 ± 0.7 a–h | 76.8 | 18 ± 0.4 a,b,c | 81.8 | |
| pH10 | 30 ± 1.5 a–j | 69.1 | 28 ± 1.2 a–j | 72.0 | 26 ± 1.9 a–h | 73.9 | 23 ± 0.8 a–g | 76.4 | 17 ± 1.5 a,b | 82.4 | 12 ± 0.3 a | 87.6 | |
| WpH | 57 ± 3.4 i–u | 42.5 | 56 ± 3.3d-u | 43.6 | 53 ± 3.4 b–t | 46.3 | 50 ± 4.2 a–q | 49.8 | 45 ± 3.1 a–m | 54.4 | 40 ± 1.9 a–l | 59.3 | |
| Shoot extract + AFB1 | pH2 | 75 ± 4.7 q–u | 24.7 | 76 ± 4.6 r–u | 23.7 | 75 ± 6.5 q–u | 24.2 | 72 ± 5.5 k–u | 27.2 | 66 ± 4.3 e–u | 34.0 | 61 ± 3.6 d–u | 38.1 |
| pH4 | 77 ± 5.8 p–u | 22.6 | 76 ± 4.7 p–u | 23.4 | 76 ± 4.7 o–u | 24.0 | 69 ± 4.2 f–u | 30.8 | 68 ± 3.5 e–u | 31.2 | 63 ± 5.5 d–u | 36.3 | |
| pH6 | 74 ± 4.3 k–u | 25.9 | 73 ± 6.2 j–u | 26.7 | 71 ± 4.3 g–u | 28.5 | 69 ± 3.2 f–u | 30.3 | 67 ± 3.8 e–u | 32.4 | 62 ± 2.5 d–u | 37.1 | |
| pH8 | 67 ± 3.8 e–u | 32.1 | 66 ± 3.9 e–u | 33.6 | 65 ± 2.6 e–u | 34.6 | 64 ± 4. 1d–u | 35.8 | 60 ± 3.5 b–u | 39.3 | 57 ± 3.2 a–u | 42.8 | |
| pH10 | 61 ± 4.7 d–u | 38.79 | 60 ± 4.5 d–u | 39.2 | 58 ± 3.4 d–u | 42.3 | 56 ± 3.2 d–u | 43.4 | 50 ± 4.1 a–q | 49.4 | 43 ± 2.9 a–m | 56.9 | |
| WpH | 73 ± 4.4 j–u | 26.2 | 71 ± 4.5 g–u | 28.8 | 68 ± 3.8 f–u | 31.4 | 65 ± 5.1 e–u | 34.6 | 62 ± 3.7 f–u | 37.3 | 59 ± 4.3 j–u | 40.2 | |
| Treatments | pH | 3 h | 6 h | 12 h | 24 h | 48 h | 72 h | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toxin Recovery | D% | Toxin Recovery | D% | Toxin Recovery | D% | Toxin Recovery | D% | Toxin Recovery | D% | Toxin Recovery | D% | ||
| Toxin AFB1 | 49 ± 2 n | 0.17 | 49 ± 2 l–n | 0.68 | 49 ± 2 k–n | 0.79 | 49 ± 3 h–n | 0.99 | 49 ± 1 g–n | 1.16 | 49 ± 2 d–l | 1.67 | |
| Toxin + H2O | pH2 | 49 ± 1 n | 1.7 | 49 ± 2 m,n | 1.3 | 49 ± 7 k–n | 4.0 | 49 ± 3 j–n | 4.2 | 49 ± 3 i–n | 5.8 | 49 ± 3 g–n | 5.4 |
| Toxin + H2O | pH4 | 49 ± 3 m,n | 2.8 | 49 ± 3 l–n | 3.3 | 49 ± 3 e–n | 6.8 | 49 ± 4 d–n | 7.1 | 49 ± 2 d–m | 7.6 | 49 ± 2 d–l | 9.7 |
| Toxin + H2O | pH6 | 49 ± 3 f–n | 6.5 | 49 ± 2 f–n | 6.4 | 48 ± 3 c–k | 10.9 | 48 ± 2 c–i | 10.9 | 49 ± 3 c–h | 10.3 | 48 ± 3 c–h | 11.0 |
| Toxin + H2O | pH8 | 49 ± 2 d–m | 7.8 | 48 ± 3 b–g | 11.4 | 48 ± 2 a–g | 13.0 | 48 ± 3 a–e | 12.8 | 48 ± 1 a–e | 11.7 | 48 ± 3 a–d | 12.9 |
| Toxin + H2O | pH10 | 48 ± 4 a–f | 12.3 | 48 ± 3 a–c | 15.4 | 48 ± 4 a–c | 15.5 | 48 ± 3 a–c | 15.6 | 48 ± 2 a,b | 17.5 | 48 ± 3 a | 17.2 |
| Toxin + H2O | WpH | 49 ± 2 m,n | 0.36 | 49 ± 3 g–n | 1.14 | 49 ± 3 d–m | 1.56 | 48 ± 3 c–j | 2.04 | 48 ± 3 a–f | 2.41 | 48 ± 2 a,b | 3.41 |
| Leaf extract + AFB1 | pH2 | 21 ± 1 e–s | 56.9 | 20 ± 1 d–p | 59.9 | 18 ± 1 c–p | 62.9 | 17 ± 1 b–o | 65.8 | 15 ± 1 b–p | 68.8 | 16 ± 1 b–n | 67.7 |
| pH4 | 21 ± 1 e–q | 58.0 | 18 ± 1 c–p | 62.6 | 17 ± 1 b–o | 64.7 | 16 ± 1 b–n | 66.5 | 16 ± 1 b–n | 67.3 | 13 ± 0 a–f | 72.5 | |
| pH6 | 19 ± 2 d–p | 60.3 | 18 ± 1 c–p | 62.6 | 16 ± 1 b–n | 66.7 | 16 ± 1 b–n | 67.4 | 15 ± 0 b–p | 69.6 | 13 ± 1 a–e | 74.1 | |
| pH8 | 17 ± 1 b–o | 65.5 | 16 ± 1 b–n | 67.8 | 13 ±1 a–f | 73.0 | 12 ± 1 a–e | 75.2 | 12 ± 1 a–e | 76.0 | 07 ± 0.6 a,b | 84.1 | |
| pH10 | 15 ± 1 b–l | 69.5 | 13 ± 1 a–f | 72.5 | 10 ± 0 a–d | 79.2 | 9.0 ± 0 a–c | 81.9 | 08 ± 0.3 a,b | 83.6 | 07 ± 0.4 a | 85.2 | |
| WpH | 18 ± 0 c–p | 63.2 | 19 ± 2 d–p | 61.0 | 17 ± 1 b–o | 64.8 | 16 ± 1 b–n | 66.4 | 15 ± 1 b–p | 69.2 | 13 ± 1 a–f | 72.8 | |
| Shoot extract + AFB1 | pH2 | 39 ± 2 y | 21.7 | 37 ± 2 x,y | 24.9 | 35 ± 2 v–y | 28.4 | 35 ± 2 u–y | 29.9 | 34 ± 2 s–y | 30.9 | 32 ± 2 q–y | 35.1 |
| pH4 | 37 ± 3 x,y | 24.6 | 35 ± 1 u–y | 29.3 | 32 ± 2 q–y | 35.0 | 30 ± 3 p–y | 38.8 | 29 ± 1 l–x | 42.0 | 26 ± 1 j–x | 46.2 | |
| pH6 | 36 ± 2 v–y | 27.8 | 33 ± 2 r–y | 32.5 | 30 ± 1 p–y | 38.2 | 29 ± 1 l–x | 41.9 | 27 ± 2 j–x | 45.1 | 25 ± 1 h–x | 49.3 | |
| pH8 | 35 ± 2 u–y | 29.2 | 33 ± 2 q–y | 34.0 | 30 ± 2 n–y | 39.7 | 28 ± 2 k–x | 43.4 | 26 ± 1 i–x | 46.6 | 24 ± 1 g–x | 50.8 | |
| pH10 | 34 ± 1 s–y | 31.9 | 31 ± 1 p–y | 36.7 | 28 ± 1 k–x | 42.4 | 27 ± 1 j–x | 46.1 | 25 ± 1 h–x | 49.3 | 23 ± 2 f–x | 53.5 | |
| WpH | 36 ± 2 w–y | 27.6 | 34 ± 2 s–y | 31.8 | 33 ± 2 q–y | 33.2 | 32 ± 2 q–y | 35.6 | 30 ± 2 p–y | 38.8 | 28 ± 1 k–x | 43.0 | |
| Treatments | Toxin Recovery (µg/L) | |
|---|---|---|
| AFB1 | AFB2 | |
| Unspiked maize | 0.5 ± 0.02 f | 00.3 ± 0.01 e |
| Unspiked maize + leaf extract | 00.0 ± 0.0 g | 00.0 ± 0.0 f |
| Unspiked maize + shoot extract | 00.0 ± 0.0 g | 00.0 ± 0.0 f |
| Spiked maize with AFB1 (100 µg/L) and AFB2 (50 µg/L) | 97.3 ± 6.7 a | 47.6 ± 2.4 b |
| Spiked maize with toxin + leaf extract | 24.9 ± 1.3 e | 09.6 ± 0.8 d |
| Degradation % | 75.1 ± 4.9 b | 80.8 ± 5.9 a |
| Spiked maize with toxin + shoot extract | 60.0 ± 3.8 c | 26.7 ± 1.3 c |
| Degradation % | 40.0 ± 2.5 d | 46.5 ± 1.8 b |
| Treatments | Toxin Conc. µg/L | No. of Living Cells (hours) | No. of Dead Cells (hours) | % Mortality (hours) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFB1 | AFB2 | 24 | 48 | 72 | 96 | 24 | 48 | 72 | 96 | 24 | 48 | 72 | 96 | |
| Seawater + shrimps | - | - | 40 ± 2.1 a | 40 ± 2.5 a | 40 ± 2.8 a | 39 ± 1.3 a | 0.1 ± 0.0 f | 0.6 ± 0.0 e | 0.5 ± 0.0 e | 1.1 ± 0.0 e | 0.2 ± 0.0 f | 0.1 ± 0.0 e | 0.2 ± 0.0 e | 2.5 ± 0.1 e,f |
| Methanol + shrimps | - | - | 38 ± 2.3 a | 38 ± 1.9 a | 37 ± 2.0 b | 36 ± 2.8 a | 1.6 ± 0.0 e | 2.2 ± 0.1 d | 3.1 ± 0.2 d | 3.5 ± 0.1 d | 2.5 ± 0.1 e | 5.3 ± 0.3 d | 7.5 ± 0.4 d | 7.5 ± 0.6 e |
| Untreated toxins + shrimps | 50 | 20 | 10 ± 0.8 b | 07 ± 0.4 b | 5.1 ± 4.1 c | 5.2 ± 2.6 b | 30 ± 1.6 c,d | 33 ± 2.2 b,c | 35 ± 3.1 b,c | 35 ± 2.1 b,c | 75 ± 4.2 c,d | 81 ± 5.2 b,c | 86 ± 6.2 b,c | 87 ± 3.9 c,d |
| 100 | 50 | 7.3 ± 0.6 b,c | 5.4 ± 0.3 c | 4.5 ± 3.4 c | 3.7 ± 1.5 b,c | 33 ± 1.1 b,c | 35 ± 3.1 a,b | 36 ± 1.8 a,b | 37 ± 1.1 a,b | 83 ± 3.8 b,c | 86 ± 7.3 a,b | 89 ± 5.0 a,b | 91 ± 5.2 b,c | |
| 200 | 90 | 5.9 ± 0.2 c,d | 3.2 ± 0.1 c | 2.9 ± 1.2 d | 1.9 ± 1.0 c,d | 35 ± 2.8 a,b | 37 ± 1.6 a | 38 ± 1.7 a | 39 ± 2.5 a | 86 ± 5.9 a,b | 91 ± 5.4 a | 94 ± 6.3 a | 96 ± 5.7 b | |
| 300 | 130 | 2.3 ± 0.1 e | 2.8 ± 0.1 c,d | 1.7 ± 0.8 e | 0.3 ± 0.0 e | 38 ± 1.7 a | 38 ± 2.5 a | 39 ± 2.9 a | 40 ± 3.1 a | 95 ± 7.1 a | 95 ± 3.6 a | 96 ± 5.8 a | 100 ± 7 a | |
| Toxin degraded with M. arvensis Leaf extracts + shrimps | 50 | 20 | 35 ± 2.4 a | 33 ± 2.6 a | 32 ± 2. 1a | 31 ± 1.7 a | 05 ± 0.3 b,c | 07 ± 0.3 b,c | 08 ± 0.6 b,c | 09 ± 0.5 b,c | 13 ± 1.4 d | 17 ± 1.3 d | 19 ± 1.4 d | 22 ± 1.5 c,d |
| 100 | 50 | 32 ± 1.9 a | 31 ± 1.7 a | 31 ± 1.1 a | 29 ± 1.4 a | 08 ± 0.7 b | 09 ± 0.5 b | 09 ± 0.1 b | 11 ± 0.7 b | 19 ± 1.0 c | 21 ± 1.4 c | 23 ± 1.9 c | 28 ± 1.4 c | |
| 200 | 90 | 28 ± 1.3 a,b | 27 ± 1.3 b | 26 ± 1.3 b | 26 ± 0.9 a,b | 12 ± 0.9 a | 13 ± 0.9 a | 14 ± 1.2 a | 14 ± 1.1,a | 30 ± 2.6 a,b | 32 ± 2.2 a,b | 34 ± 2.3 b | 35 ± 2.4 b | |
| 300 | 130 | 26 ± 1.9 b,c | 25 ± 2.2 c | 25 ± 0.9 b | 24 ± 1.5 b,c | 14 ± 1.1 a | 15 ± 1.1 a | 15 ± 1.1 a | 16 ± 1.3 a | 34 ± 2.7 a | 36 ± 2.8 a | 38 ± 2.6 a | 40 ± 2.7 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjum, T.; Iram, W.; Iqbal, M.; Abbas, M.; Akram, W.; Li, G. Structure Elucidation and Toxicity Analysis of the Byproducts Formed after Biodegradation of Aflatoxins B1 and B2 Using Extracts of Mentha arvensis. Toxins 2022, 14, 24. https://doi.org/10.3390/toxins14010024
Anjum T, Iram W, Iqbal M, Abbas M, Akram W, Li G. Structure Elucidation and Toxicity Analysis of the Byproducts Formed after Biodegradation of Aflatoxins B1 and B2 Using Extracts of Mentha arvensis. Toxins. 2022; 14(1):24. https://doi.org/10.3390/toxins14010024
Chicago/Turabian StyleAnjum, Tehmina, Wajiha Iram, Mazhar Iqbal, Mateen Abbas, Waheed Akram, and Guihua Li. 2022. "Structure Elucidation and Toxicity Analysis of the Byproducts Formed after Biodegradation of Aflatoxins B1 and B2 Using Extracts of Mentha arvensis" Toxins 14, no. 1: 24. https://doi.org/10.3390/toxins14010024
APA StyleAnjum, T., Iram, W., Iqbal, M., Abbas, M., Akram, W., & Li, G. (2022). Structure Elucidation and Toxicity Analysis of the Byproducts Formed after Biodegradation of Aflatoxins B1 and B2 Using Extracts of Mentha arvensis. Toxins, 14(1), 24. https://doi.org/10.3390/toxins14010024





