Tetrodotoxins Secretion and Voltage-Gated Sodium Channel Adaptation in the Ribbon Worm Kulikovia alborostrata (Takakura, 1898) (Nemertea)
Abstract
:1. Introduction
2. Results
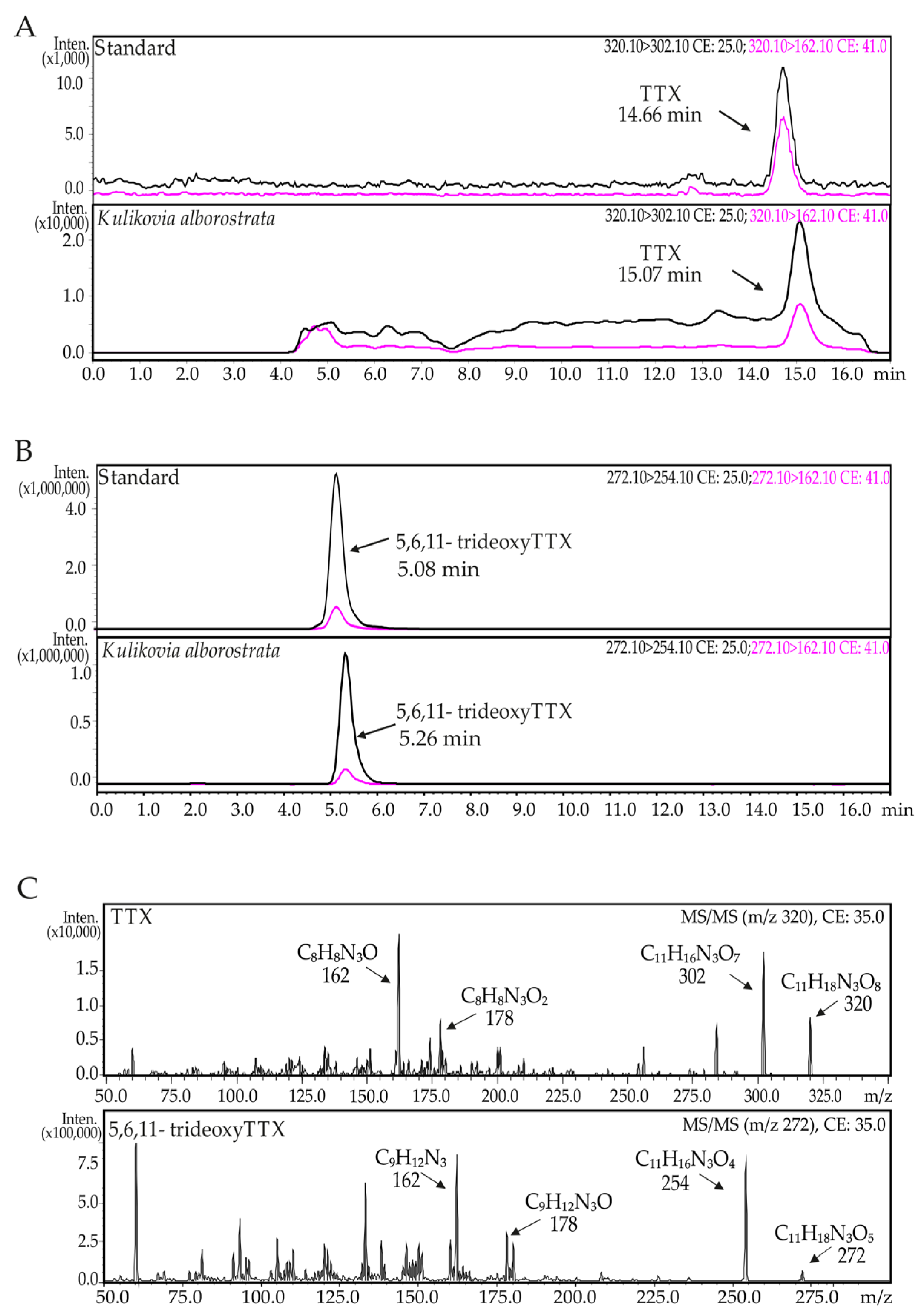
2.1. TTXs
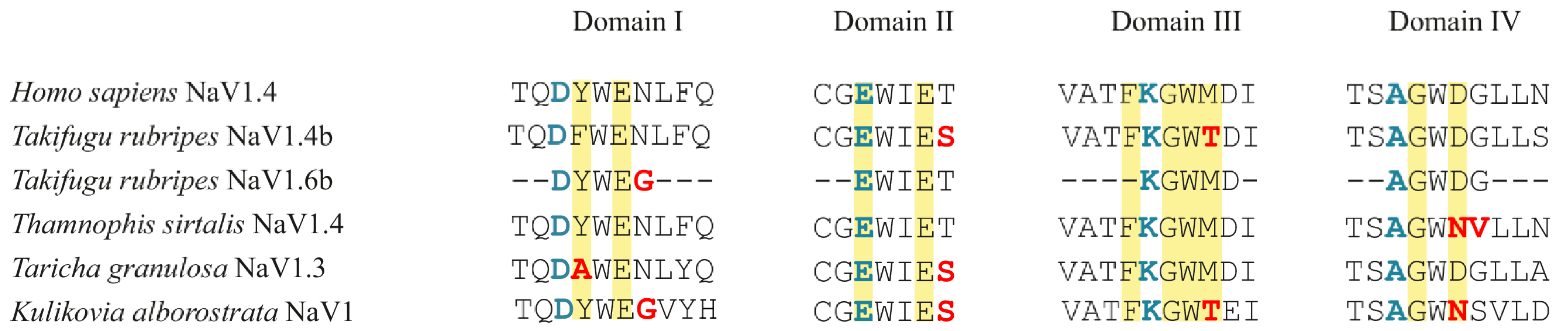
2.2. Nav1 Channel
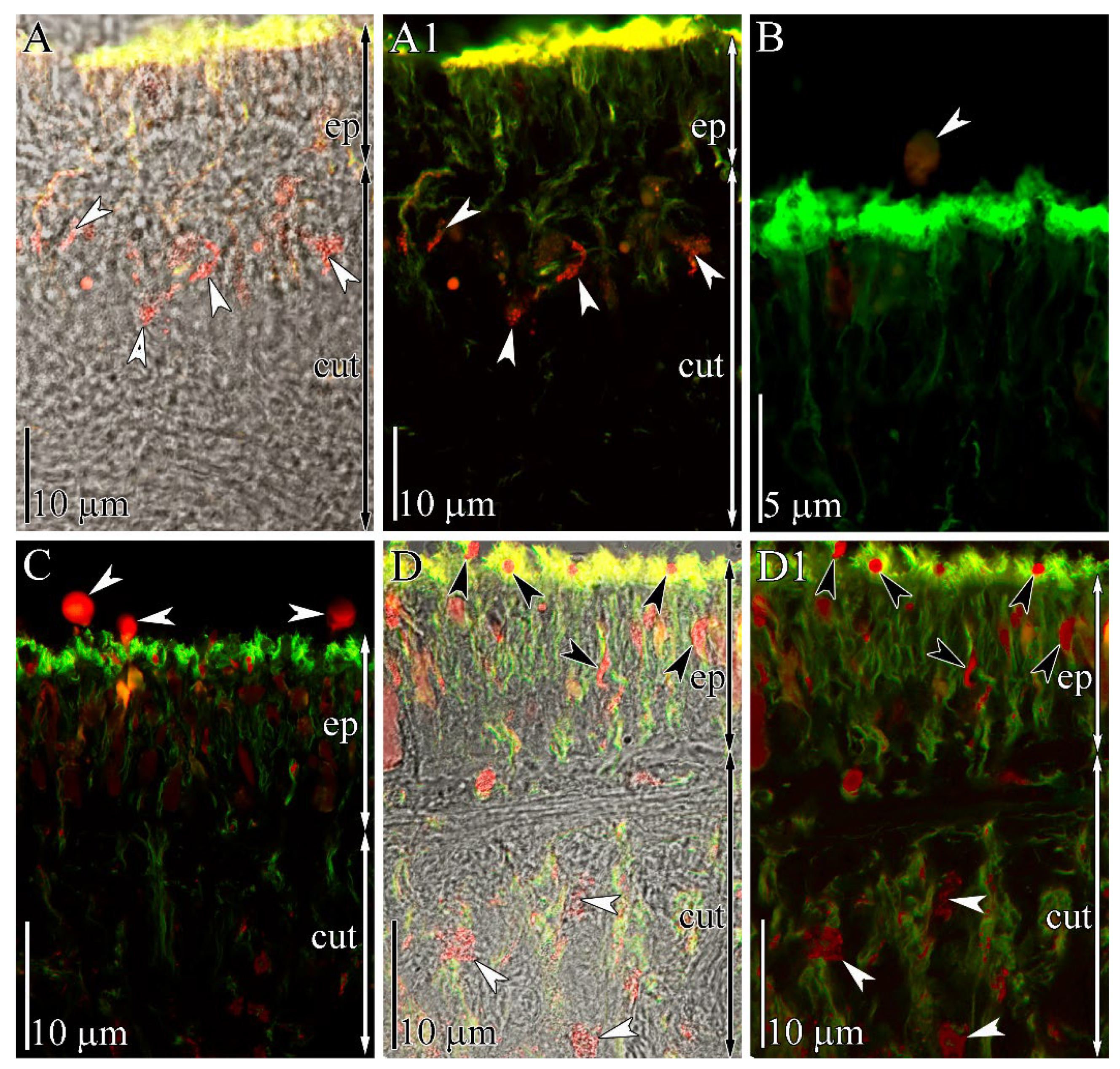
2.3. Immunohistochemical Study
3. Discussion
4. Materials and Methods
4.1. TTXs
4.2. Nav1 Channel
4.2.1. Design of Primers for the Amplification of P-Loop Regions of the Nav1 Channel Domains I–IV
4.2.2. The Isolation of RNA, Synthesis of cDNA, and Amplification of P-Loop Regions of Domains I–IV of the Nav1 Channel
4.2.3. Sanger Sequencing
4.2.4. Search for Mutations in the P-Loop Regions of Domains I–IV of the Nav1 Channel
4.3. Immunohistochemical Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kajihara, H.; Chernyshev, A.V.; Sun, S.-C.; Sundberg, P.; Crandall, F.B. Checklist of nemertean genera and species published between 1995 and 2007. Species Divers. 2008, 13, 245–274. [Google Scholar] [CrossRef] [Green Version]
- Göransson, U.; Jacobsson, E.; Strand, M.; Andersson, H.S. The toxins of nemertean worms. Toxins 2019, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazawa, K.; Higashiyama, M.; Ito, K.; Noguchi, T.; Arakawa, O.; Shida, Y.; Hashimoto, K. Tetrodotoxin in two species of ribbon worm (Nemertini), Lineus fuscoviridis and Tubulanus punctatus. Toxicon 1988, 26, 867–874. [Google Scholar] [CrossRef]
- Asakawa, M.; Toyoshima, T.; Ito, K.; Bessho, K.; Yamaguchi, C.; Tsunetsugu, S.; Shida, Y.; Kajihara, H.; Mawatari, S.F.; Noguchi, T.; et al. Paralytic toxicity in the ribbon worm Cephalothrix species (Nemertea) in Hiroshima Bay, Hiroshima Prefecture, Japan and the isolation of tetrodotoxin as a main component of its toxins. Toxicon 2003, 41, 747–753. [Google Scholar] [CrossRef]
- Asakawa, M.; Ito, K.; Kajihara, H. Highly toxic ribbon worm Cephalothrix simula containing tetrodotoxin in Hiroshima bay, Hiroshima prefecture, Japan. Toxins 2013, 5, 376–395. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Min, S.K.; Yeon, S.J.; Hwang, J.H.; Hong, J.-S.; Shin, H.S. Assessment of neuronal cell-based cytotoxicity of neurotoxins from an estuarine nemertean in the Han River estuary. J. Microbiol. Biotechnol. 2017, 27, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Vlasenko, A.; Velansky, P.; Chernyshev, A.; Kuznetsov, V.; Magarlamov, T.Y. Tetrodotoxin and its analogues profile in nemertean species from the Sea of Japan. Toxicon 2018, 156, 48–51. [Google Scholar] [CrossRef]
- McEvoy, E.G.; Rogers, A.; Gibson, R. Preliminary investigation of Vibrio alginolyticus-like bacteria associated with marine nemerteans. Hydrobiologia 1997, 365, 287–291. [Google Scholar] [CrossRef]
- Carroll, S.; McEvoy, E.G.; Gibson, R. The production of tetrodotoxin-like substances by nemertean worms in conjunction with bacteria. J. Exp. Mar. Biol. Ecol. 2003, 288, 51–63. [Google Scholar] [CrossRef]
- Turner, A.D.; Fenwick, D.; Powell, A.; Dhanji-Rapkova, M.; Ford, C.; Hatfield, R.G.; Santos, A.; Martinez-Urtaza, J.; Bean, T.P.; Baker-Austin, C.; et al. New invasive nemertean species (Cephalothrix simula) in England with high levels of tetrodotoxin and a microbiome linked to toxin metabolism. Mar. Drugs 2018, 16, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.E.; Arakawa, O.; Noguchi, T.; Miyazawa, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin and related substances in a ribbon worm Cephalothrix linearis (Nemertean). Toxicon 1990, 28, 1083–1093. [Google Scholar] [CrossRef]
- Vlasenko, A.E.; Magarlamov, T.Y. Tetrodotoxin and its analogues in Cephalothrix cf. simula (Nemertea: Palaeonemertea) from the sea of Japan (Peter the Great Gulf): Intrabody distribution and secretions. Toxins 2020, 12, 745. [Google Scholar] [CrossRef]
- Durán-Riveroll, L.M.; Cembella, A.D. Guanidinium toxins and their interactions with voltage-gated sodium ion channels. Mar. Drugs 2017, 15, 303. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, B.; Lu, S.Q.; Dandona, N.; See, S.L.; Brenner, S.; Soong, T.W. Genetic basis of tetrodotoxin resistance in pufferfishes. Curr. Biol. 2005, 15, 2069–2072. [Google Scholar] [CrossRef] [Green Version]
- Jost, M.C.; Hillis, D.M.; Lu, Y.; Kyle, J.W.; Fozzard, H.A.; Zakon, H.H. Toxin-resistant sodium channels: Parallel adaptive evolution across a complete gene family. Mol. Biol. Evol. 2008, 25, 1016–1024. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Li, Z.; Jiang, Y.; Pan, X.; Wu, J.; Cristofori-Armstrong, B.; Smith, J.J.; Chin, Y.K.Y.; Lei, J.; Zhou, Q.; et al. Structural basis for the modulation of voltage-gated sodium channels by animal toxins. Science 2018, 362, eaau2596. [Google Scholar] [CrossRef] [Green Version]
- Vaelli, P.M.; Theis, K.R.; Williams, J.E.; O’Connell, L.A.; Foster, J.A.; Eisthen, H.L. The skin microbiome facilitates adaptive tetrodotoxin production in poisonous newts. eLife 2020, 9, 1–29. [Google Scholar] [CrossRef]
- Choudhary, G.; Yotsu-Yamashita, M.; Shang, L.; Yasumoto, T.; Dudley, S.C. Interactions of the C-11 hydroxyl of tetrodotoxin with the sodium channel outer vestibule. Biophys. J. 2003, 84, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Norenburg, J.L. Structure of the nemertine integument with consideration of its ecological and phylogenetic significance. Integr. Comp. Biol. 1985, 25, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Magarlamov, T.Y.; Shokur, O.A.; Chernyshev, A.V. Distribution of tetrodotoxin in the ribbon worm Lineus alborostratus. Toxicon 2016, 112, 29–34. [Google Scholar] [CrossRef]
- Williams, B.L. Behavioral and chemical ecology of marine organisms with respect to tetrodotoxin. Mar. Drugs 2010, 8, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Salvitti, L.R.; Wood, S.A.; Winsor, L.; Cary, S.C. Intracellular immunohistochemical detection of tetrodotoxin in Pleurobranchaea maculata (Gastropoda) and Stylochoplana sp. (Turbellaria). Mar. Drugs 2015, 13, 756–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorentz, M.N.; Stokes, A.N.; Rößler, D.C.; Lötters, S. Tetrodotoxin. Curr. Biol. 2016, 26, R870–R872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, T.; Arakawa, O.; Takatani, T. TTX accumulation in pufferfish. Comp. Biochem. Physiol. Part D Genom. Proteom. 2006, 1, 145–152. [Google Scholar] [CrossRef]
- Noguchi, T.; Arakawa, O. Tetrodotoxin—distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoi, S.; Yoshikawa, S.; Tatsuno, R.; Suzuki, M.; Asahina, K.; Yamamoto, S.; Takanashi, S.; Takatani, T.; Arakawa, O.; Sakakura, Y.; et al. Difference in the localization of tetrodotoxin between the female and male pufferfish Takifugu niphobles, during spawning. Toxicon 2012, 60, 1000–1004. [Google Scholar] [CrossRef]
- Tsuruda, K.; Arakawa, O.; Kawatsu, K.; Hamano, Y.; Takatani, T.; Noguchi, T. Secretory glands of tetrodotoxin in the skin of the Japanese newt Cynops pyrrhogaster. Toxicon 2001, 40, 131–136. [Google Scholar] [CrossRef]
- Mebs, D.; Arakawa, O.; Yotsu-Yamashita, M. Tissue distribution of tetrodotoxin in the red-spotted newt Notophthalmus viridescens. Toxicon 2010, 55, 1353–1357. [Google Scholar] [CrossRef]
- Mebs, D.; Yotsu-Yamashita, M.; Seitz, H.M.; Arakawa, O. Tetrodotoxin does not protect red-spotted newts, Notophthalmus viridescens, from intestinal parasites. Toxicon 2012, 60, 66–69. [Google Scholar] [CrossRef]
- Williams, B.L.; Stark, M.R.; Caldwell, R.L. Microdistribution of tetrodotoxin in two species of blue-ringed octopuses (Hapalochlaena lunulata and Hapalochlaena fasciata) detected by fluorescent immunolabeling. Toxicon 2012, 60, 1307–1313. [Google Scholar] [CrossRef]
- Tanu, M.B.; Mahmud, Y.; Arakawa, O.; Takatani, T.; Kajihara, H.; Kawatsu, K.; Hamano, Y.; Asakawa, M.; Miyazawa, K.; Noguchi, T. Immunoenzymatic visualization of tetrodotoxin (TTX) in Cephalothrix species (Nemertea: Anopla: Palaeonemertea: Cephalotrichidae) and Planocera reticulata (Platyhelminthes: Turbellaria: Polycladida: Planoceridae). Toxicon 2004, 44, 515–520. [Google Scholar] [CrossRef]
- Campbell, M.E.; Schwartz, M. Immunohistological visualization of tetrodotoxin in Micrura verrili and Dushia atra (Phylum Nemertea). In Proceedings of the National Conferences for Undergraduate Research (NCUR), Salisbury, MD, USA, 10–12 April 2008. [Google Scholar]
- Kodama, M.; Sato, S.; Ogata, T.; Suzuki, Y.; Kaneko, T.; Aida, K. Tetrodotoxin secreting glands in the skin of puffer fishes. Toxicon 1986, 24, 819–829. [Google Scholar] [CrossRef]
- Saito, T.; Noguchi, T.; Harada, T.; Murata, O.; Hashimoto, K. Tetrodotoxin as a biological defense agent for puffers. Nippon. Suisan Gakkaishi 1985, 51, 1175–1180. [Google Scholar] [CrossRef]
- Kodama, M.; Ogata, T.; Sato, S. External secretion of tetrodotoxin from puffer fishes stimulated by electric shock. Mar. Biol. 1985, 87, 199–202. [Google Scholar] [CrossRef]
- Ritson-Williams, R.; Yotsu-Yamashita, M.; Paul, V.J. Ecological functions of tetrodotoxin in a deadly polyclad flatworm. Proc. Natl. Acad. Sci. USA 2006, 103, 3176–3179. [Google Scholar] [CrossRef] [Green Version]
- Cardall, B.L.; Brodie, E.D.; Hanifin, C.T. Secretion and regeneration of tetrodotoxin in the rough-skin newt (Taricha granulosa). Toxicon 2004, 44, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Yotsu-Yamashita, M.; Sugimoto, A.; Takai, A.; Yasumoto, T. Effects of specific modifications of several hydroxyls of tetrodotoxin on its affinity to rat brain membrane. J. Pharmacol. Exp. Ther. 1999, 289, 1688–1696. [Google Scholar] [PubMed]
- Dellafiora, L.; Galaverna, G.; Dall’Asta, C. An in-silico perspective on the toxicodynamic of tetrodotoxin and analogues—A tool for supporting the hazard identification. Toxicon 2017, 138, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Zou, S. Comparative transcriptome analysis of toxic and non-toxic Nassarius communities and identification of genes involved in TTX-adaptation. Toxins 2020, 12, 761. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Zhihui, Y.; Xun, X.; Zhang, J.; Guo, X.; Jiao, W.; Zhang, L.; Liu, W.; Wang, J.; et al. Scallop genome reveals molecular adaptations to semi-sessile life and neurotoxins. Nat. Commun. 2017, 8, 1721. [Google Scholar] [CrossRef]
- Du, Y.; Nomura, Y.; Liu, Z.; Huang, Z.Y.; Dong, K. Functional expression of an arachnid sodium channel reveals residues responsible for tetrodotoxin resistance in invertebrate sodium channels. J. Biol. Chem. 2009, 284, 33869–33875. [Google Scholar] [CrossRef] [Green Version]
- Hanifin, C.T.; Gilly, W.F.; Campus, B.; Station, M.; Grove, P. Evolutionary history of a complex adaptation: Tetrodotoxin resistance in salamanders. Evolution 2015, 69, 232–244. [Google Scholar] [CrossRef] [Green Version]
- McGlothlin, J.W.; Chuckalovcak, J.P.; Janes, D.E.; Edwards, S.V.; Feldman, C.R.; Brodie, E.D.; Pfrender, M.E. Parallel evolution of tetrodotoxin resistance in three voltage-gated sodium channel genes in the garter snake Thamnophis sirtalis. Mol. Biol. Evol. 2014, 31, 2836–2846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geffeney, S.L.; Fujimoto, E.; Brodie, E.D.; Ruben, P.C. Evolutionary diversification of TTX-resistant sodium channels in a predator–prey interaction. Nature 2005, 434, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Yotsu-Yamashita, M. Chemistry of puffer fish toxin. J. Toxicol. Toxin Rev. 2001, 20, 51–66. [Google Scholar] [CrossRef]
- Soong, T.W.; Venkatesh, B. Adaptive evolution of tetrodotoxin resistance in animals. Trends Genet. 2006, 22, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-J.; Kanda, M.; Koyanagi, R.; Hisata, K.; Akiyama, T.; Sakamoto, H.; Sakamoto, T.; Satoh, N. Nemertean and phoronid genomes reveal lophotrochozoan evolution and the origin of bilaterian heads. Nat. Ecol. Evol. 2017, 2, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmead, B.; Salzberg, S. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| №. of Specimen | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Secretion (TTXs, ng/g) | Day 1 | TTX | + | + | + | - | + | - | + | - | - | - | - | - | - | - | - | - | - |
| 5,6,11-trideoxyTTX | 0.675 | 0.264 | 0.229 | - | - | - | - | - | - | - | - | - | 0.144 | - | - | - | - | ||
| Day 2 | TTX | 0.340 | 0.357 | + | 0.556 | + | + | + | + | + | - | + | + | - | - | - | - | + | |
| 5,6,11-trideoxyTTX | 1.905 | 3.171 | 0.156 | 6.244 | 0.399 | - | 1.690 | 0.774 | - | 0.440 | - | 0.835 | - | - | - | - | 0.199 | ||
| Day 3 | TTX | - | - | - | - | - | - | - | + | - | - | - | - | - | - | + | - | - | |
| 5,6,11-trideoxyTTX | - | - | - | - | - | - | - | - | - | - | - | 0.287 | 0.115 | - | 0.093 | - | - | ||
| Day 30 | TTX | + | + | - | 0.306 | - | - | - | - | - | + | - | + | + | - | + | - | - | |
| 5,6,11-trideoxyTTX | - | + | + | - | 0.338 | + | 0.862 | 0.413 | + | 0.253 | 0.178 | 0.547 | 0.140 | + | 0.068 | - | + | ||
| Day 210 | TTX | + | + | + | + | + | + | + | + | 0.305 | + | + | + | + | + | + | + | + | |
| 5,6,11-trideoxyTTX | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Body (TTXs, ng/g) | TTX | + | + | + | 1.166 | 0.492 | 0.146 | + | + | 1.286 | + | 0.306 | 0.441 | + | + | + | + | + | |
| 5,6,11-trideoxyTTX | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Primer | Sequence | T Annealing, °C | PCR Product Length, bp |
|---|---|---|---|
| DI forward | ATGCGCCTTTCGCCTTATGAC | 61.7 | 233 |
| DI reverse | CGGCGTTCTTCCTCTTCCTTT | 60.6 | |
| DII forward | GTCCT(Y)CGAACATTCAGATTG | 61.1 | 431 |
| DII reverse | AGATTGGAGATTTTCAGCCCC | 59.9 | |
| DIII forward | GTCTTCTGGCTCATCTTCAGTATCA | 59.8 | 348 |
| DIII reverse | TCAGCGTGAAGAAAGAACCGA | 60.9 | |
| DIV forward | AACATGCTGCCGGGATAGA | 58.8 | 193 |
| DIV reverse | TTGCCGCAGTTACCCTTGAC | 60.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasenko, A.E.; Kuznetsov, V.G.; Malykin, G.V.; Pereverzeva, A.O.; Velansky, P.V.; Yakovlev, K.V.; Magarlamov, T.Y. Tetrodotoxins Secretion and Voltage-Gated Sodium Channel Adaptation in the Ribbon Worm Kulikovia alborostrata (Takakura, 1898) (Nemertea). Toxins 2021, 13, 606. https://doi.org/10.3390/toxins13090606
Vlasenko AE, Kuznetsov VG, Malykin GV, Pereverzeva AO, Velansky PV, Yakovlev KV, Magarlamov TY. Tetrodotoxins Secretion and Voltage-Gated Sodium Channel Adaptation in the Ribbon Worm Kulikovia alborostrata (Takakura, 1898) (Nemertea). Toxins. 2021; 13(9):606. https://doi.org/10.3390/toxins13090606
Chicago/Turabian StyleVlasenko, Anna E., Vasiliy G. Kuznetsov, Grigorii V. Malykin, Alexandra O. Pereverzeva, Peter V. Velansky, Konstantin V. Yakovlev, and Timur Yu. Magarlamov. 2021. "Tetrodotoxins Secretion and Voltage-Gated Sodium Channel Adaptation in the Ribbon Worm Kulikovia alborostrata (Takakura, 1898) (Nemertea)" Toxins 13, no. 9: 606. https://doi.org/10.3390/toxins13090606
APA StyleVlasenko, A. E., Kuznetsov, V. G., Malykin, G. V., Pereverzeva, A. O., Velansky, P. V., Yakovlev, K. V., & Magarlamov, T. Y. (2021). Tetrodotoxins Secretion and Voltage-Gated Sodium Channel Adaptation in the Ribbon Worm Kulikovia alborostrata (Takakura, 1898) (Nemertea). Toxins, 13(9), 606. https://doi.org/10.3390/toxins13090606





