Pyrolysis of High-Ash Natural Microalgae from Water Blooms: Effects of Acid Pretreatment
Abstract
:1. Introduction
2. Results
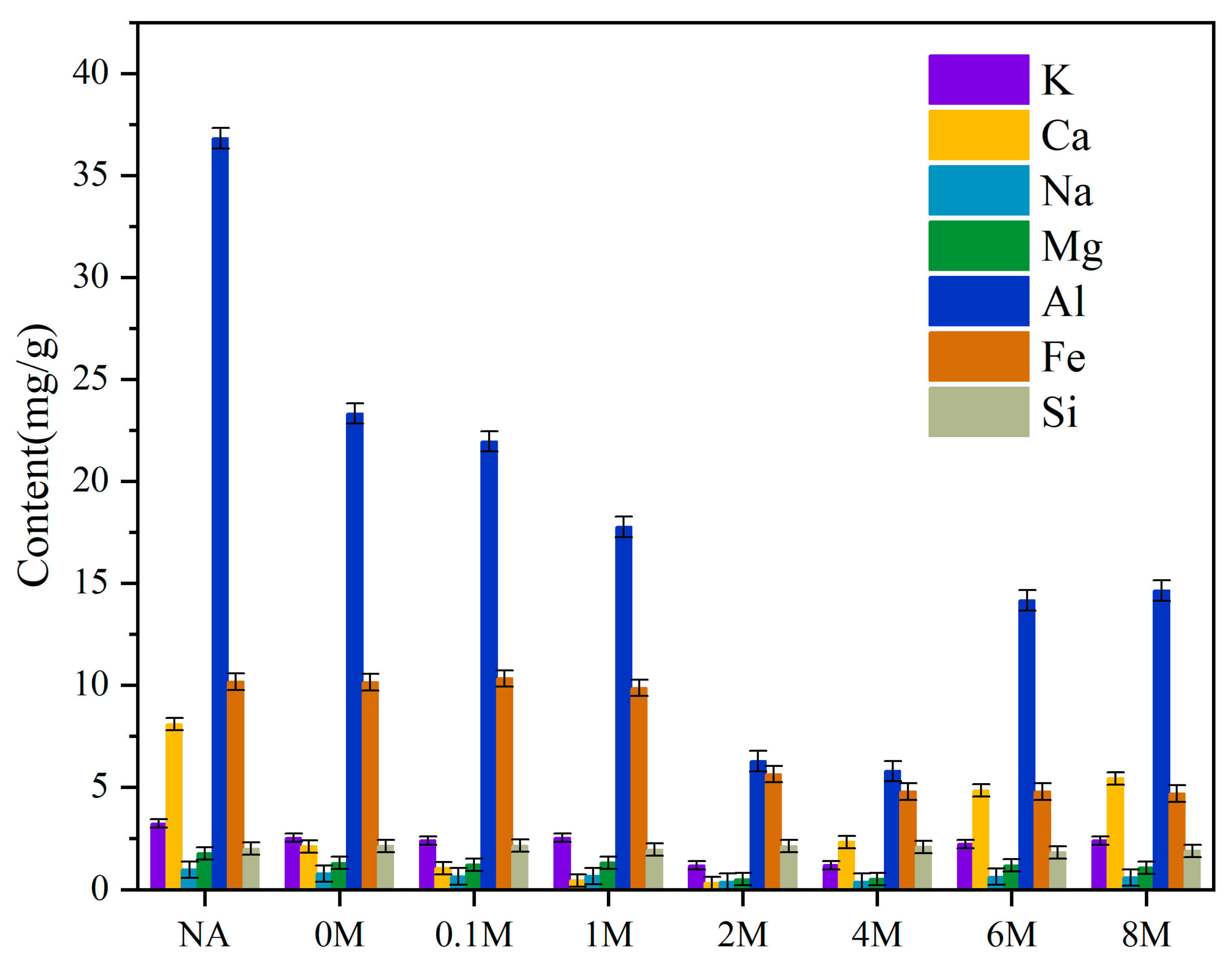
2.1. The Ash Content and Metal Content of Microalgae Samples
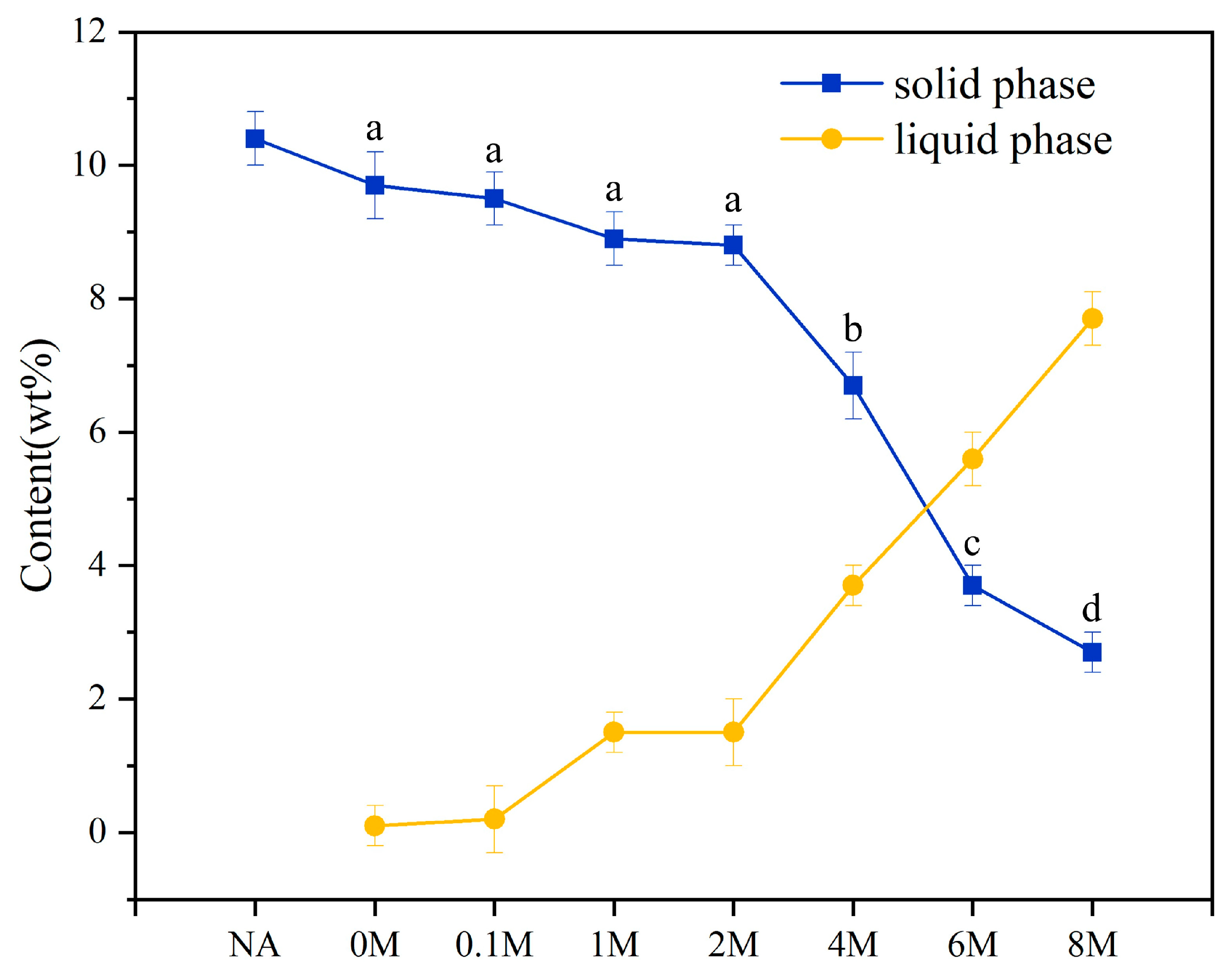
2.2. Dissolution Conversion of Carbohydrates
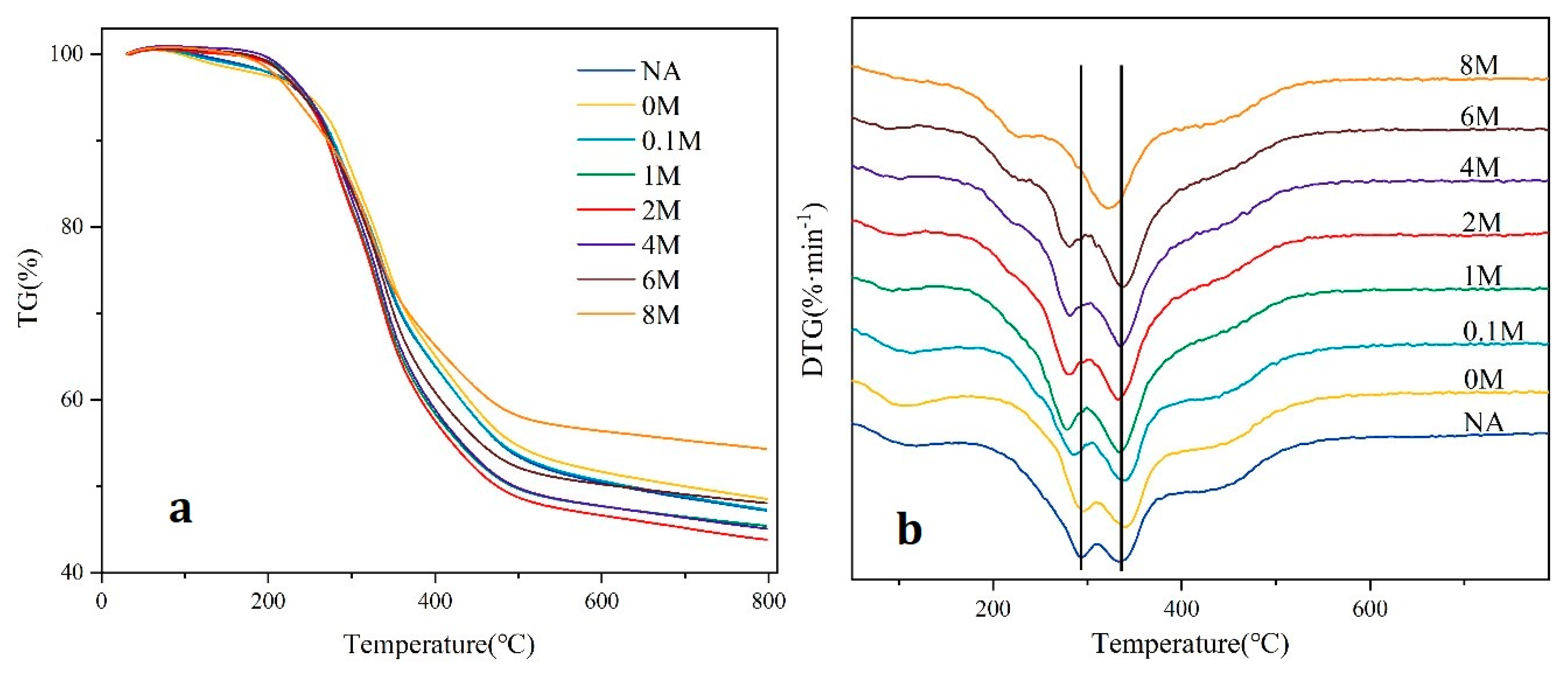
2.3. TG Analysis
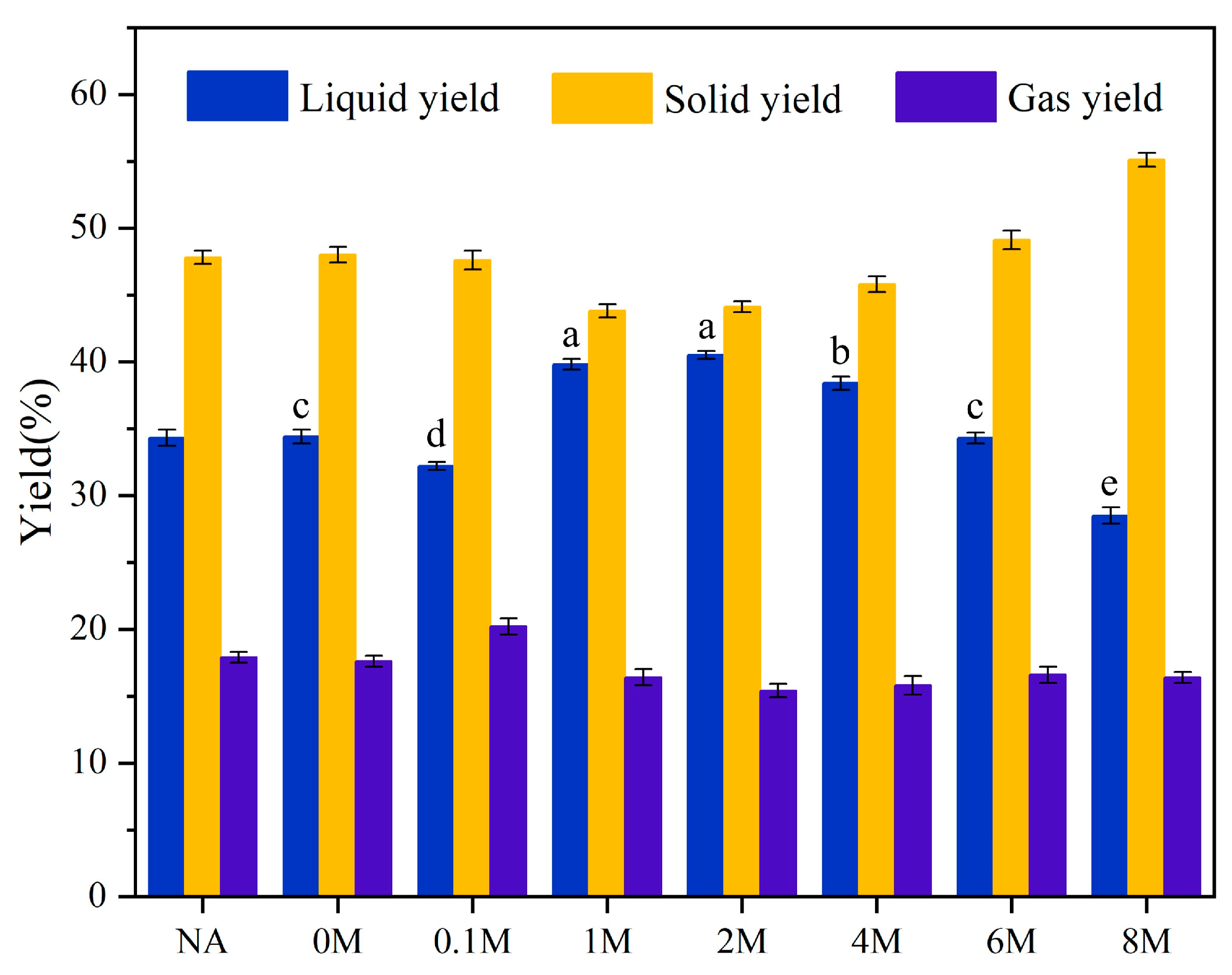
2.4. Yields of Pyrolysis Products
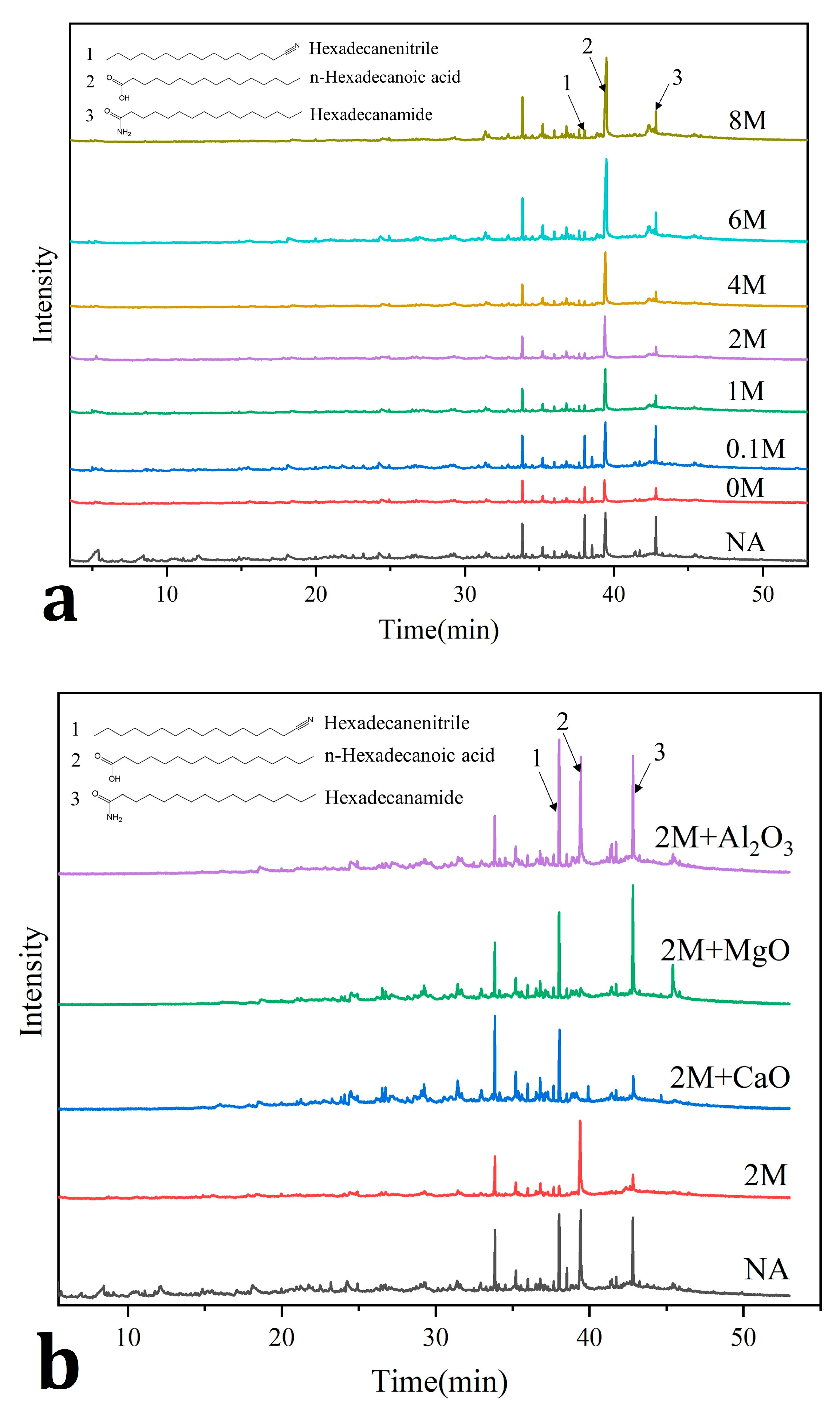
2.5. GC-MS Analysis of Liquid Products
2.6. GPC Analysis of the Bio-Oil
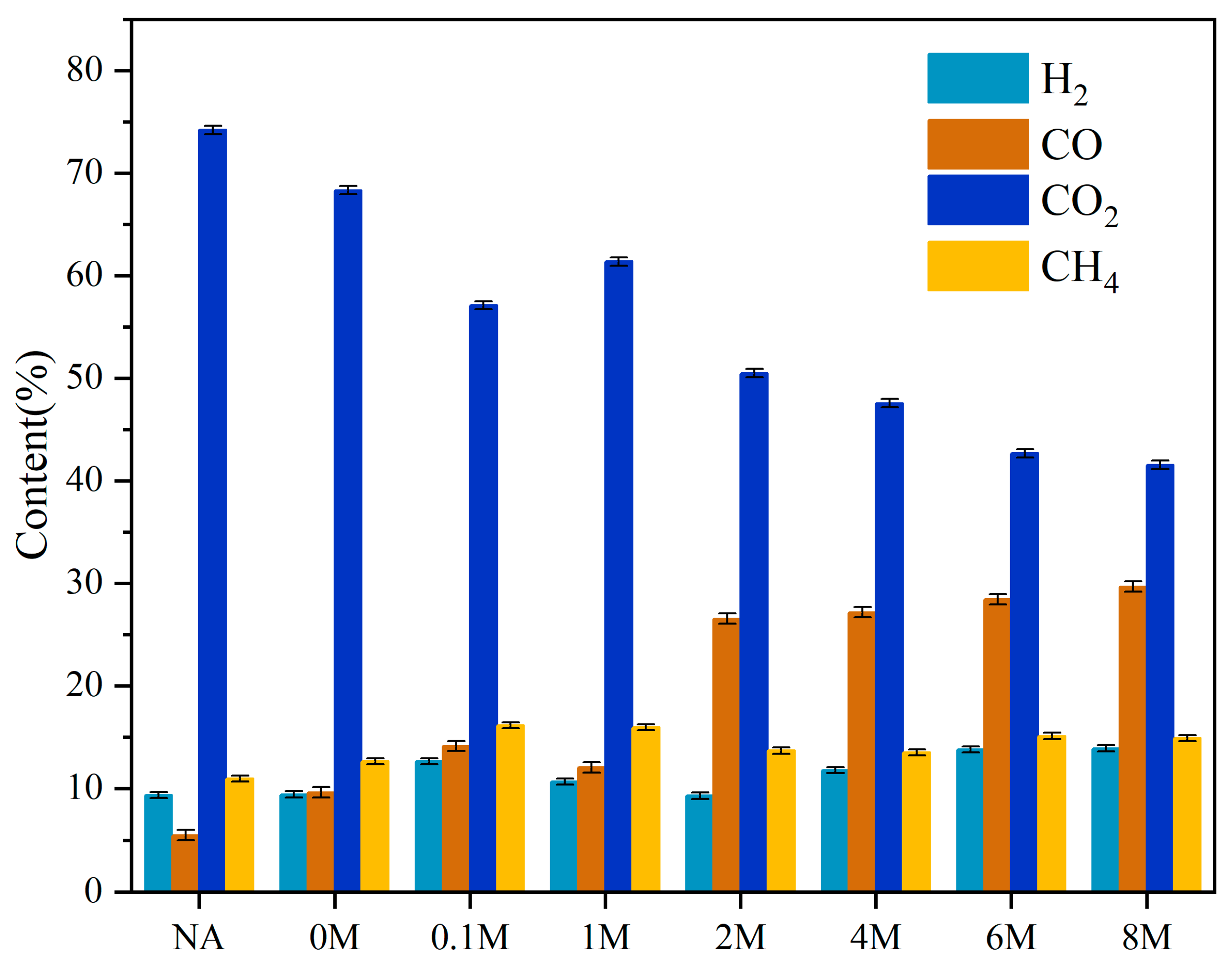
2.7. The Characteristics of Gaseous Products
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Pretreatment
5.3. Pyrolysis Experiment
5.4. Analysis Method
5.4.1. Characterization of Raw and Pretreated Feedstocks
5.4.2. Characterization of the Liquid Products
5.4.3. Characterization of the Gaseous Products
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bhushan, S.; Kalra, A.; Simsek, H.; Kumar, G.; Prajapati, S.K. Current Trends and Prospects in Microalgae-Based Bioenergy Production. J. Environ. Chem. Eng. 2020, 8, 104025. [Google Scholar] [CrossRef]
- Bennion, E.P.; Ginosar, D.M.; Moses, J.; Agblevor, F.; Quinn, J.C. Lifecycle Assessment of Microalgae to Biofuel: Comparison of Thermochemical Processing Pathways. Appl. Energy 2015, 154, 1062–1071. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Chen, Y.; Li, M.; Hu, C. Production of High-Quality Biofuel via Ethanol Liquefaction of Pretreated Natural Microalgae. Renew. Energy 2020, 147, 293–301. [Google Scholar] [CrossRef]
- Li, F.; Srivatsa, S.C.; Bhattacharya, S. A Review on Catalytic Pyrolysis of Microalgae to High-Quality Bio-Oil with Low Oxygeneous and Nitrogenous Compounds. Renew. Sustain. Energy Rev. 2019, 108, 481–497. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Zhang, R.; Tong, D.; Hu, C. Fractional Pyrolysis of Cyanobacteria from Water Blooms over HZSM-5 for High Quality Bio-Oil Production. J. Energy Chem. 2014, 23, 732–741. [Google Scholar] [CrossRef]
- Zeng, Y.; Tang, J.; Lian, S.; Tong, D.; Hu, C. Study on the Conversion of Cyanobacteria of Taihu Lake Water Blooms to Biofuels. Biomass Bioenergy 2015, 73, 95–101. [Google Scholar] [CrossRef]
- Karpagam, R.; Jawaharraj, K.; Gnanam, R. Review on Integrated Biofuel Production from Microalgal Biomass through the Outset of Transesterification Route: A Cascade Approach for Sustainable Bioenergy. Sci. Total Environ. 2021, 766, 144236. [Google Scholar] [CrossRef]
- Mo, L.; Dai, H.; Feng, L.; Liu, B.; Li, X.; Chen, Y.; Khan, S. In-Situ Catalytic Pyrolysis Upgradation of Microalgae into Hydrocarbon Rich Bio-Oil: Effects of Nitrogen and Carbon Dioxide En-Vironment. Bioresour. Technol. 2020, 314, 123758. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, Y.; Peng, Y.; Yao, L.; Dong, Y.; Yang, B.; Song, R. Catalytic Pyrolysis and Liquefaction Behavior of Microalgae for Bio-Oil Production. Bioresour. Technol. 2020, 300, 122665. [Google Scholar] [CrossRef]
- Nejati, B.; Adami, P.; Bozorg, A.; Tavasoli, A.; Mirzahosseini, A.H. Catalytic Pyrolysis and Bio-Products Upgrading Derived from Chlorella vulgaris over Its Biochar and Activated Bio-Char-Supported Fe Catalysts. J. Anal. Appl. Pyrolysis 2020, 152, 104799. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Z.; Zhang, L.; Fan, G.; Wu, Y.; Yang, M. Catalytic Hydrothermal Liquefaction of Microalgae over Different Biochars. Catal. Commun. 2021, 149, 106236. [Google Scholar] [CrossRef]
- Adeniyi, O.M.; Azimov, U.; Burluka, A. Algae Biofuel: Current Status and Future Applications. Renew. Sustain. Energy Rev. 2018, 90, 316–335. [Google Scholar] [CrossRef]
- Azizi, K.; Keshavarz Moraveji, M.; Abedini, N.H. A Review on Bio-Fuel Production from Microalgal Biomass by Using Pyrolysis Method. Renew. Sustain. Energy Rev. 2018, 82, 3046–3059. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Tian, A.; Guo, Q.; Huang, K. Influence of CaO and HZSM-5 on Oxygen Migration in Chlorella vulgaris Polysaccharide Pyrolysis. Carbon Resour. Convers. 2019, 2, 111–116. [Google Scholar] [CrossRef]
- Chen, W.; Yang, H.; Chen, Y.; Xia, M.; Chen, X.; Chen, H. Transformation of Nitrogen and Evolution of N-Containing Species during Algae Pyrolysis. Environ. Sci. Technol. 2017, 51, 6570–6579. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, R.; Parra Cruz, R.A.; Pakalapati, H.; Show, P.L.; Ling, T.C.; Chen, W.H.; Tao, Y. Recent Advances in the Pretreatment of Microalgal and Lignocellulosic Biomass: A Comprehensive Review. Bioresour. Technol. 2020, 298, 122476. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.C.; Magalhaes, A.I., Jr.; de Melo Pereira, G.V.; Medeiros, A.B.P.; Sydney, E.B.; Rodrigues, C.; Aulestia, D.T.M.; de Souza Vandenberghe, L.P.; Soccol, V.T.; Soccol, C.R. Microalgal Biomass Pretreatment for Integrated Processing into Biofuels, Food, and Feed. Bioresour. Technol. 2020, 300, 122719. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.Y.; Chen, W.-H.; Ong, H.C.; Sheen, H.-K.; Chang, J.-S.; Hsieh, T.-H.; Ling, T.C. Effects of Dry and Wet Torrefaction Pretreatment on Microalgae Pyrolysis Analyzed by TG-FTIR and Double-Shot Py-GC/MS. Energy 2020, 210, 118579. [Google Scholar] [CrossRef]
- Ha, G.S.; El-Dalatony, M.M.; Kim, D.H.; Salama, E.S.; Kurade, M.B.; Roh, H.S.; El-Fatah Abomohra, A.; Jeon, B.H. Biocomponent-Based Microalgal Transformations into Biofuels during the Pretreatment and Fermentation Process. Bioresour. Technol. 2020, 302, 122809. [Google Scholar] [CrossRef]
- Nagarajan, D.; Chang, J.S.; Lee, D.J. Pretreatment of Microalgal Biomass for Efficient Biohydrogen Production-Recent Insights and Future Perspectives. Bioresour. Technol. 2020, 302, 122871. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Choi, J.-W.; Suh, D.J.; Ha, J.-M.; Hwang, J.W.; Jung, H.W.; Lee, K.-Y.; Woo, H.-C. Production of Brown Algae Pyrolysis Oils for Liquid Biofuels Depending on the Chemical Pretreatment Methods. Energy Convers. Manag. 2014, 86, 371–378. [Google Scholar] [CrossRef]
- Yu, K.L.; Chen, W.-H.; Sheen, H.-K.; Chang, J.-S.; Lin, C.-S.; Ong, H.C.; Show, P.L.; Ling, T.C. Bioethanol Production from Acid Pretreated Microalgal Hydrolysate Using Microwave-Assisted Heating Wet Torrefaction. Fuel 2020, 279, 118435. [Google Scholar] [CrossRef]
- Yildiz, G.; Ronsse, F.; Venderbosch, R.; Duren, R.V.; Kersten, S.R.A.; Prins, W. Effect of Biomass Ash in Catalytic Fast Pyrolysis of Pine Wood. Appl. Catal. B Environ. 2015, 168, 203–211. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Influence of Inorganic Salts on the Primary Pyrolysis Products of Cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Yang, H.; Gentili, F.G.; Söderlind, U.; Wang, X.; Zhang, W.; Chen, H. Conversion of High-Ash Microalgae through Hydrothermal Liquefaction. Sustain. Energy Fuels 2020, 4, 2782–2791. [Google Scholar] [CrossRef]
- Liu, G.; Mba Wright, M.; Zhao, Q.; Brown, R.C. Hydrocarbon and Ammonia Production from Catalytic Pyrolysis of Sewage Sludge with Acid Pretreatment. ACS Sustain. Chem. Eng. 2016, 4, 1819–1826. [Google Scholar] [CrossRef]
- Hu, Z.; Zheng, Y.; Yan, F.; Xiao, B.; Liu, S. Bio-Oil Production through Pyrolysis of Blue-Green Algae Blooms (BGAB): Product Distribution and Bio-Oil Characterization. Energy 2013, 52, 119–125. [Google Scholar] [CrossRef]
- Du, Z.; Hu, B.; Ma, X.; Cheng, Y.; Liu, Y.; Lin, X.; Wan, Y.; Lei, H.; Chen, P.; Ruan, R. Catalytic Pyrolysis of Microalgae and Their Three Major Components: Carbohydrates, Proteins, and Lipids. Bioresour. Technol. 2013, 130, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sheng, L.; Yang, X. Pyrolysis Characteristics and Pathways of Protein, Lipid and Carbohydrate Isolated from Microalgae Nannochloropsis sp. Bioresour. Technol. 2017, 229, 119–125. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Chen, S.; Zhang, X.; Chen, F.; Ye, N. Comparative Evaluation of the Pyrolytic and Kinetic Characteristics of a Macroalga (Sargassum thunbergii) and a Freshwater Plant (Potamogeton crispus). Fuel 2012, 96, 185–191. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Luo, Z.; Zhu, X. Investigation on the Catalytic Behavior of Alkali Metals and Alkaline Earth Metals on the Biomass Pyrolysis Assisted with Real-Time Monitoring. Energy Fuels 2020, 34, 12654–12664. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Yang, X.; Xia, S.; Zheng, A.; Zeng, K.; Zhao, Z.; Li, H.; Sobek, S.; Werle, S. Comparative Assessment of Pretreatment Options for Biomass Pyrolysis: Linking Biomass Compositions to Resulting Pyrolysis Behaviors, Kinetics, and Product Yields. Energy Fuels 2021, 35, 3186–3196. [Google Scholar] [CrossRef]
- Li, Y.; Xing, X.; Ma, P.; Zhang, X.; Wu, Y.; Huang, L. Effect of Alkali and Alkaline Earth Metals on Co-Pyrolysis Characteristics of Municipal Solid Waste and Biomass Briquettes. J. Therm. Anal. Calorim. 2019, 139, 489–498. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, S.; Yuan, R.; Wang, P. Biomass Pyrolysis with Alkaline-Earth-Metal Additive for Co-Production of Bio-Oil and Biochar-Based Soil Amendment. Sci. Total Environ. 2020, 743, 140760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiong, Y. Simultaneous Improvement in Qualities of Bio-Oil and Syngas from Catalytic Pyrolysis of Rice Husk by Demineralization. Energy Sources Part. A 2020, 42, 1–14. [Google Scholar]
- Laurens, L.M.L.; Nagle, N.; Davis, R.; Sweeney, N.; Van Wychen, S.; Lowell, A.; Pienkos, P.T. Acid-Catalyzed Algal Biomass Pretreatment for Integrated Lipid and Carbohydrate-Based Biofuels Production. Green Chem. 2015, 17, 1145–1158. [Google Scholar] [CrossRef] [Green Version]
- Katahira, R.; Sluiter, J.B.; Schell, D.J.; Davis, M.F. Degradation of Carbohydrates during Dilute Sulfuric Acid Pretreatment Can Interfere with Lignin Measurements in Solid Residues. J. Agric. Food Chem. 2013, 61, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Mekki-Berrada, A.; Bennici, S.; Gillet, J.P.; Couturier, J.L.; Dubois, J.L.; Auroux, A. Fatty Acid Methyl Esters into Nitriles: Acid–Base Properties for Enhanced Catalysts. J. Catal. 2013, 306, 30–37. [Google Scholar] [CrossRef]
- Shirazi, Y.; Tafazolian, H.; Viamajala, S.; Varanasi, S.; Song, Z.; Heben, M.J. High-Yield Production of Fatty Nitriles by One-Step Vapor-Phase Thermocatalysis of Triglycerides. ACS Omega 2017, 2, 9013–9020. [Google Scholar] [CrossRef] [PubMed]
- Mekki-Berrada, A.; Bennici, S.; Gillet, J.P.; Couturier, J.L.; Dubois, J.L.; Auroux, A. Ammoniation-Dehydration of Fatty Acids into Nitriles: Heterogeneous or Homogeneous Catalysis? ChemSusChem 2013, 6, 1478–1489. [Google Scholar] [CrossRef]
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-Value Molecules Recovery and Biofuels Production from Spent Coffee Grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- Beneroso, D.; Bermudez, J.M.; Arenillas, A.; Menendez, J.A. Microwave Pyrolysis of Microalgae for High Syngas Production. Bioresour. Technol. 2013, 144, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Chaiwong, K.; Kiatsiriroat, T.; Vorayos, N.; Thararax, C. Study of Bio-Oil and Bio-Char Production from Algae by Slow Pyrolysis. Biomass Bioenergy 2013, 56, 600–606. [Google Scholar] [CrossRef]
- Torri, C.; Samori, C.; Adamiano, A.; Fabbri, D.; Faraloni, C.; Torzillo, G. Preliminary Investigation on the Production of Fuels and Bio-Char from Chlamydomonas reinhardtii Biomass Residue after Bio-Hydrogen Production. Bioresour. Technol. 2011, 102, 8707–8713. [Google Scholar] [CrossRef]
- Bordoloi, N.; Narzari, R.; Sut, D.; Saikia, R.; Chutia, R.S.; Kataki, R. Characterization of Bio-Oil and Its Sub-Fractions from Pyrolysis of Scenedesmus dimorphus. Renew. Energy 2016, 98, 245–253. [Google Scholar] [CrossRef]
- Wang, K.; Brown, R.C.; Homsy, S.; Martinez, L.; Sidhu, S.S. Fast Pyrolysis of Microalgae Remnants in a Fluidized Bed Reactor for Bio-Oil and Biochar Production. Bioresour. Technol. 2013, 127, 494–499. [Google Scholar] [CrossRef]
- Pan, P.; Hu, C.; Yang, W.; Li, Y.; Dong, L.; Zhu, L.; Tong, D.; Qing, R.; Fan, Y. The Direct Pyrolysis and Catalytic Pyrolysis of Nannochloropsis sp. Residue for Renewable Bio-Oils. Bioresour. Technol. 2010, 101, 4593–4599. [Google Scholar] [CrossRef]
- Lv, X.; Li, Q.; Jiang, Z.; Wang, Y.; Li, J.; Hu, C. Structure Characterization and Pyrolysis Behavior of Organosolv Lignin Isolated from Corncob Residue. J. Anal. Appl. Pyrolysis 2018, 136, 115–124. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–918. [Google Scholar] [CrossRef] [Green Version]
- Collins, E.M.; Shelton, R.W. A K-jeldahl Determination of Protein for General Chemistry. J. Chem. Educ. 1946, 23, 549–550. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q.; Yang, C. Fast Pyrolysis of Microalgae to Produce Renewable Fuels. J. Anal. Appl. Pyrolysis 2004, 71, 855–863. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Wang, W.; Liu, L.; Hu, C. Torrefaction at 200 Degrees C of Pubescens Pretreated with AlCl3 Aqueous Solution at Room Temperature. ACS Omega 2020, 5, 27709–27722. [Google Scholar] [CrossRef]

| Ultimate Analysis (wt%) | Proximate Analysis (wt%) | Components Analysis (wt%) | |||
|---|---|---|---|---|---|
| C | 33.01 ± 0.05 | Ash | 33.2 ± 0.3 | Carbohydrates | 10.4 ± 0.4 |
| H | 5.14 ± 0.02 | Moisture | 5.8 ± 0.3 | Proteins | 41.2 ± 0.5 |
| N | 6.59 ± 0.03 | Volatile matter | 45.6 ± 0.2 | Lipids | 6.6 ± 0.3 |
| O a | 22.06 ± 0.02 | Fixed carbon | 15.4 ± 0.2 | Others b | 8.6 ± 0.3 |
| HHV (MJ/kg) c | 14.90 ± 0.04 | ||||
| NA a | 0 M | 0.1 M | 1 M | 2 M | 4 M | 6 M | 8 M | |
|---|---|---|---|---|---|---|---|---|
| Ash content (wt%) | 33.2 ± 0.1 | 33.7 ± 0.4 | 33.6 ± 0.5 | 28.6 ± 0.3 | 21.1 ± 0.5 | 30.9 ± 0.5 | 32.9 ± 0.2 | 43.3 ± 0.3 |
| S.no. | Algae Species | HHV MJ/kg | Temperature, Conditions | Reference |
|---|---|---|---|---|
| 1 | Taihu microalgae (Cyanobacteria) | 30.4 | 500 °C, slow pyrolysis | - |
| 2 | Arthrospira platensis | 15.8 | 550 °C, slow pyrolysis | [43] |
| 3 | Chlamydomonas reinhardtii | 13 | 350 °C, fast pyrolysis | [44] |
| 4 | Scenedesmus dimorphus | 28.5 | 500 °C, slow pyrolysis | [45] |
| 5 | C. vulgaris remnants | 24.6 | 500 °C, fast pyrolysis | [46] |
| 6 | Nannochloropsis sp. residue | 24.4 | 400 °C, slow pyrolysis | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Liu, Y.; Wang, W.; Wang, Y.; Li, G.; Hu, C. Pyrolysis of High-Ash Natural Microalgae from Water Blooms: Effects of Acid Pretreatment. Toxins 2021, 13, 542. https://doi.org/10.3390/toxins13080542
Liu L, Liu Y, Wang W, Wang Y, Li G, Hu C. Pyrolysis of High-Ash Natural Microalgae from Water Blooms: Effects of Acid Pretreatment. Toxins. 2021; 13(8):542. https://doi.org/10.3390/toxins13080542
Chicago/Turabian StyleLiu, Longfei, Yichen Liu, Wenli Wang, Yue Wang, Guiying Li, and Changwei Hu. 2021. "Pyrolysis of High-Ash Natural Microalgae from Water Blooms: Effects of Acid Pretreatment" Toxins 13, no. 8: 542. https://doi.org/10.3390/toxins13080542
APA StyleLiu, L., Liu, Y., Wang, W., Wang, Y., Li, G., & Hu, C. (2021). Pyrolysis of High-Ash Natural Microalgae from Water Blooms: Effects of Acid Pretreatment. Toxins, 13(8), 542. https://doi.org/10.3390/toxins13080542





