Microcystin Toxicokinetics, Molecular Toxicology, and Pathophysiology in Preclinical Rodent Models and Humans
Abstract
:1. Introduction
2. Human Microcystin Exposure
3. Microcystin Toxicokinetics
3.1. Absorption
3.2. Distribution
3.3. Metabolism
3.4. Excretion
4. Microcystin Molecular Toxicology
4.1. Protein Phosphatases
4.2. Oxidative Stress
4.3. Cell Death
4.4. Cytoskeletal Disruption
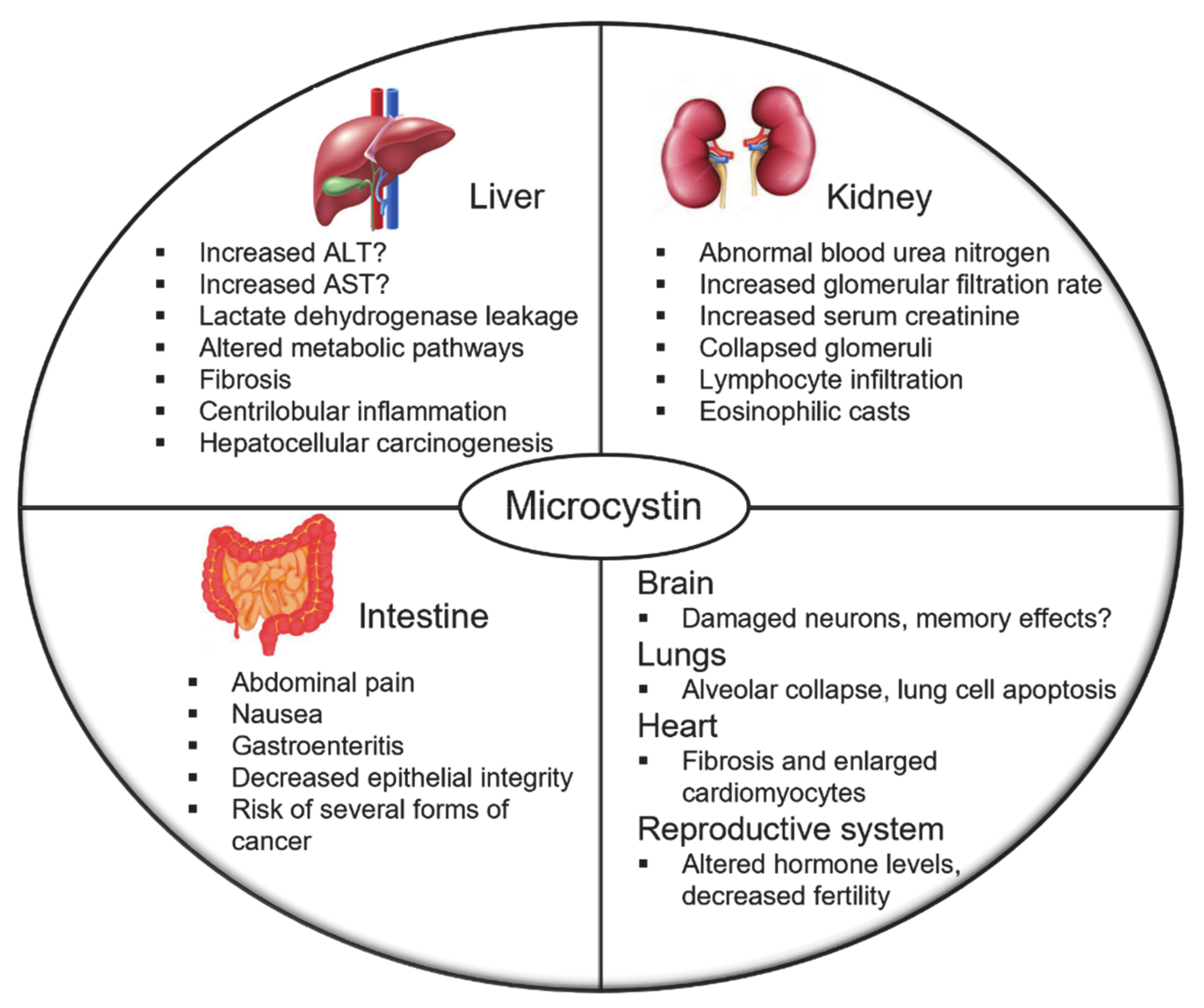
5. Microcystin Pathophysiology
5.1. Liver
5.2. Kidney
5.3. Intestine
5.4. Other Organ Targets
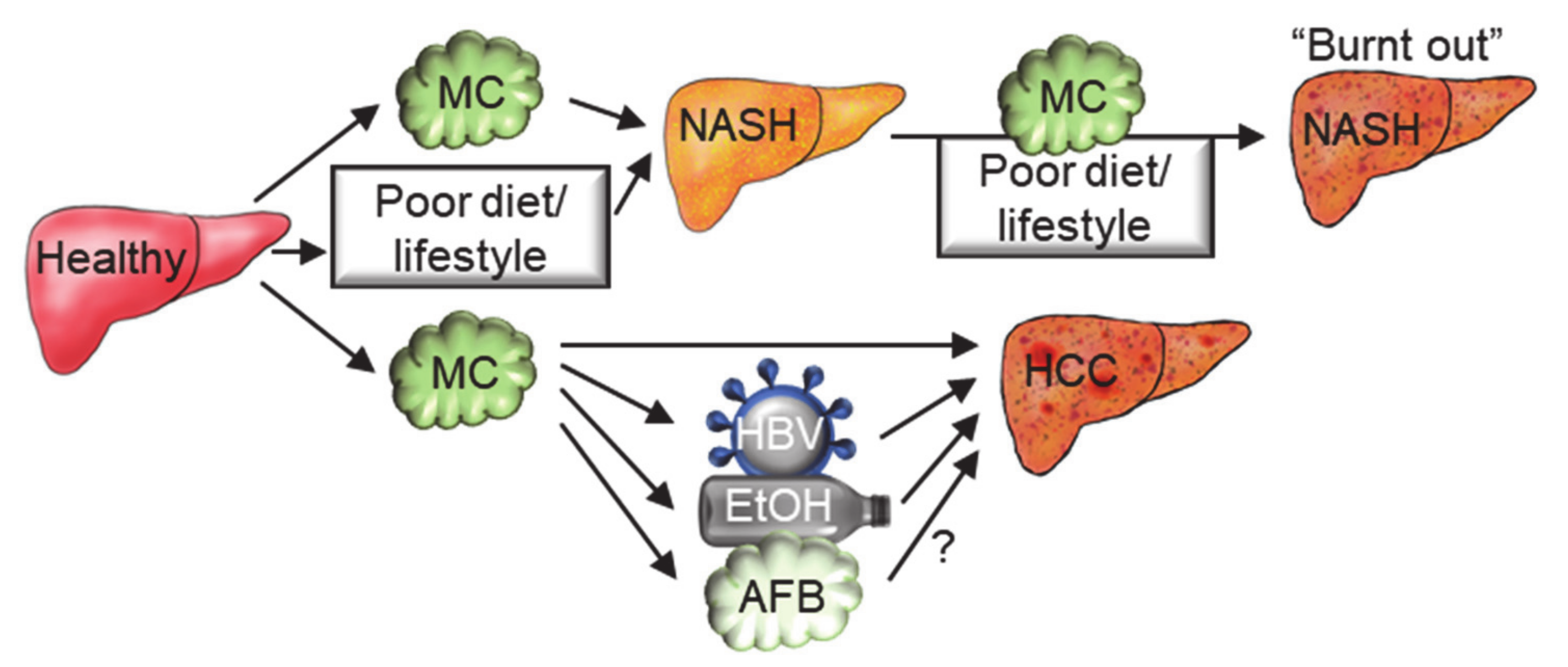
6. Microcystins in the Multifactorial Etiology of Chronic Diseases
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sessions, A.L.; Doughty, D.M.; Welander, P.V.; Summons, R.E.; Newman, D.K. The Continuing Puzzle of the Great Oxidation Event. Curr. Biol. 2009, 19, R567–R574. [Google Scholar] [CrossRef] [Green Version]
- Schirrmeister, B.E.; Gugger, M.; Donoghue, P.C.J. Cyanobacteria and the Great Oxidation Event: Evidence from genes and fossils. Palaeontology 2015, 58, 769–785. [Google Scholar] [CrossRef] [Green Version]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Buick, R. When did oxygenic photosynthesis evolve? Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2731–2743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Mijović, B.; Codd, G.A.; Meriluoto, J. Toxicology of microcystins with reference to cases of human intoxications and epidemiological investigations of exposures to cyanobacteria and cyanotoxins. Arch. Toxicol. 2017, 91, 621–650. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Svirčev, Z.; Lalić, D.; Bojadžija Savić, G.; Tokodi, N.; Drobac Backović, D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Hashtroudi, M.S.; Ghassempour, A.; Riahi, H.; Shariatmadari, Z.; Khanjir, M. Endogenous auxins in plant growth-promoting Cyanobacteria-Anabaena vaginicola and Nostoc calcicola. J. Appl. Phycol. 2013, 25, 379–386. [Google Scholar] [CrossRef]
- Hellier, P.; Al-Haj, L.; Talibi, M.; Purton, S.; Ladommatos, N. Combustion and emissions characterization of terpenes with a view to their biological production in cyanobacteria. Fuel 2013, 111, 670–688. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, P.F.; Palmieri, D.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Palombo, D. TNFα-induced endothelial activation is counteracted by polyphenol extract from UV-stressed cyanobacterium Arthrospira platensis. Med. Chem. Res. 2015, 24, 275–282. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A Precious Bio-resource in Agriculture, Ecosystem, and Environmental Sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [Green Version]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef]
- Carmichael, W.W. Cyanobacteria secondary metabolites-the cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Bláha, L.; Babica, P.; Maršálek, B. Toxins produced in cyanobacterial water blooms-toxicity and risks. Interdiscip. Toxicol. 2009, 2, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Yunes, J.S. Cyanobacterial Toxins. In Cyanobacteria; Elsevier: Amsterdam, The Netherlands, 2019; pp. 443–458. ISBN 9780128146682. [Google Scholar]
- de Figueiredo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Gonçalves, F.J.M.; Pereira, M.J. Microcystin-producing blooms—A serious global public health issue. Ecotoxicol. Environ. Saf. 2004, 59, 151–163. [Google Scholar] [CrossRef]
- Kondo, F.; Matsumoto, H.; Yamada, S.; Ishikawa, N.; Ito, E.; Nagata, S.; Ueno, Y.; Suzuki, M.; Harada, K. Detection and Identification of Metabolites of Microcystins Formed in Vivo in Mouse and Rat Livers. Chem. Res. Toxicol. 1996, 9, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki-Matsushima, R.; Ohta, T.; Nishiwaki, S.; Suganuma, M.; Kohyama, K.; Ishikawa, T.; Carmichael, W.W.; Fujiki, H. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J. Cancer Res. Clin. Oncol. 1992, 118, 420–424. [Google Scholar] [CrossRef]
- Sanseverino, I.; António, D.C.; Loos, R.; Lettieri, T. Cyanotoxins: Methods and Approaches for Their Analysis and Detection; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Massey, I.Y.; Yang, F. A Mini Review on Microcystins and Bacterial Degradation. Toxins 2020, 12, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef] [Green Version]
- Dawson, R.M. The toxicology of microcystins. Toxicon 1998, 36, 953–962. [Google Scholar] [CrossRef]
- Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J.; Spoof, L.; Codd, G.A. (Eds.) John Wiley & Sons, Ltd.: Chichester, UK, 2016; ISBN 9781119068761. [Google Scholar]
- Nicole McLellan, S.L.; Manderville, R.A.; McLellan, N.L. Toxic mechanisms of microcystins in mammals. Toxicol. Res. Camb. 2017, 6, 383–562. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, D.; Hoeger, S. Guidance values for microcystins in water and cyanobacterial supplement products (blue-green algal supplements): A reasonable or misguided approach? Toxicol. Appl. Pharmacol. 2005, 203, 273–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Y.; Xu, Y.; Chen, Y.; Jeffrey, P.D.; Chao, Y.; Lin, Z.; Li, Z.; Strack, S.; Stock, J.B.; Shi, Y. Structure of Protein Phosphatase 2A Core Enzyme Bound to Tumor-Inducing Toxins. Cell 2006, 127, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Linville, R.; Butler, N.; Washburn, B. Microcystins: A Brief Overview of Their Toxicity and Effects, with Special Reference to Fish, Wildlife, and Livestock; Office of Environmental Health Hazard Assessment: Sacramento, CA, USA, 2009. [Google Scholar]
- Falconer, I.R.; Humpage, A.R. Health Risk Assessment of Cyanobacterial (Blue-green Algal) Toxins in Drinking Water. Int. J. Environ. Res. Public Health 2005, 2, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cyanobacterial Toxins: Microcystins Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020.
- Codd, G.; Bell, S.; Kaya, K.; Ward, C.; Beattie, K.; Metcalf, J.; Codd, G.A.; Bell, S.G.; Ward, C.J.; Beattie, K.A.; et al. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999, 34, 405–415. [Google Scholar] [CrossRef]
- Backer, L.C. Recreational Exposure to Low Concentrations of Microcystins during an Algal Bloom in a Small Lake. Mar. Drugs 2008, 6, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Biotechnol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Xiang, L.; Li, Y.-W.; Liu, B.-L.; Zhao, H.-M.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H.; Li, Q.X. High ecological and human health risks from microcystins in vegetable fields in southern China. Environ. Int. 2019, 133, 105142. [Google Scholar] [CrossRef] [PubMed]
- Greer, B.; Maul, R.; Campbell, K.; Elliott, C.T. Detection of freshwater cyanotoxins and measurement of masked microcystins in tilapia from Southeast Asian aquaculture farms. Anal. Bioanal. Chem. 2017, 409, 4057–4069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poste, A.E.; Hecky, R.E.; Guildford, S.J. Evaluating microcystin exposure risk through fish consumption. Environ. Sci. Technol. 2011, 45, 5806–5811. [Google Scholar] [CrossRef] [Green Version]
- Massey, I.Y.; Yang, F.; Ding, Z.; Yang, S.; Guo, J.; Tezi, C.; Al-Osman, M.; Kamegni, R.B.; Zeng, W. Exposure routes and health effects of microcystins on animals and humans: A mini-review. Toxicon 2018, 151, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Li, Y.W.; Wang, Z.R.; Liu, B.L.; Zhao, H.M.; Li, H.; Cai, Q.Y.; Mo, C.H.; Li, Q.X. Bioaccumulation and phytotoxicity and human health risk from microcystin-LR under various treatments: A pot study. Toxins 2020, 12, 523. [Google Scholar] [CrossRef]
- Smith, J.L.; Haney, J.F. Foodweb transfer, accumulation, and depuration of microcystins, a cyanobacterial toxin, in pumpkinseed sunfish (Lepomis gibbosus). Toxicon 2006, 48, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Rohrlack, T.; Dittmann, E.; Rner, T.B.; Christoffersen, K. Effects of Cell-Bound Microcystins on Survival and Feeding of Daphnia spp. Appl. Environ. Microbiol. 2001, 67, 3523–3529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.A.; Kudela, R.M.; Mekebri, A.; Crane, D.; Oates, S.C.; Tinker, M.T.; Staedler, M.; Miller, W.A.; Toy-Choutka, S.; Dominik, C.; et al. Evidence for a Novel Marine Harmful Algal Bloom: Cyanotoxin (Microcystin) Transfer from Land to Sea Otters. PLoS ONE 2010, 5, e12576. [Google Scholar] [CrossRef]
- Mccarty, C.L.; Nelson, L.; Eitniear, S.; Zgodzinski, E.; Zabala, A.; Billing, L.; Diorio, M. Morbidity and Mortality Weekly Report Community Needs Assessment after Microcystin Toxin Contamination of a Municipal Water Supply-Lucas County, Ohio, September 2014. Morbid. Mortal. Week. Rep. 2016, 65, 925–929. [Google Scholar] [CrossRef] [Green Version]
- Falconer, I.R.; Runnegar, M.T.C.; Beresford, A.M. Evidence of liver damage by toxin from a bloom of the blue-green alga, Microcystis aeruginosa. Med. J. Aust. 1983, 1, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.L.; Rodrigues, J.A.; Azevedo, J.; Vasconcelos, V.; Eiras, E.; Campos, M.G. Hepatotoxicity induced by paclitaxel interaction with turmeric in association with a microcystin from a contaminated dietary supplement. Toxicon 2018, 150, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Kozdęba, M.; Borowczyk, J.; Zimoląg, E.; Wasylewski, M.; Dziga, D.; Madeja, Z.; Drukala, J. Microcystin-LR affects properties of human epidermal skin cells crucial for regenerative processes. Toxicon 2014, 80, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yue, Z.; Irvin, C.; Kirkpatrick, B.; Backer, L. Characterization of Aerosols Containing Microcystin. Mar. Drugs 2007, 5, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; McNeel, S.V.; Barber, T.; Kirkpatrick, B.; Williams, C.; Irvin, M.; Zhou, Y.; Johnson, T.B.; Nierenberg, K.; Aubel, M.; et al. Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon 2010, 55, 909–921. [Google Scholar] [CrossRef]
- Vidal, F.; Sedan, D.; D’Agostino, D.; Cavalieri, M.; Mullen, E.; Parot Varela, M.; Flores, C.; Caixach, J.; Andrinolo, D. Recreational Exposure during Algal Bloom in Carrasco Beach, Uruguay: A Liver Failure Case Report. Toxins 2017, 9, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Microcystin Guidelines: NOAA Great Lakes Environmental Research Laboratory—Ann Arbor, MI, USA. Available online: https://www.glerl.noaa.gov/res/HABs_and_Hypoxia/microcystinGuidelines.html (accessed on 3 June 2021).
- Giannuzzi, L.; Sedan, D.; Echenique, R.; Andrinolo, D. An Acute Case of Intoxication with Cyanobacteria and Cyanotoxins in Recreational Water in Salto Grande Dam, Argentina. Mar. Drugs 2011, 9, 2164–2175. [Google Scholar] [CrossRef] [Green Version]
- Pouria, S.; De Andrade, A.; Barbosa, J.; Cavalcanti, R.L.; Barreto, V.T.S.; Ward, C.J.; Preiser, W.; Poon, G.K.; Neild, G.H.; Codd, G.A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 1998, 352, 21–26. [Google Scholar] [CrossRef]
- Chen, J.; Xie, P.; Li, L.; Xu, J. First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. Toxicol. Sci. 2009, 108, 81–89. [Google Scholar] [CrossRef]
- Zheng, C.; Zeng, H.; Lin, H.; Wang, J.; Feng, X.; Qiu, Z.; Chen, J.A.; Luo, J.; Luo, Y.; Huang, Y.; et al. Serum microcystin levels positively linked with risk of hepatocellular carcinoma: A case-control study in southwest China. Hepatology 2017, 66, 1519–1528. [Google Scholar] [CrossRef]
- Zhao, Y.; Xue, Q.; Su, X.; Xie, L.; Yan, Y.; Wang, L.; Steinman, A.D. First Identification of the Toxicity of Microcystins on Pancreatic Islet Function in Humans and the Involved Potential Biomarkers. Environ. Sci. Technol. 2016, 50, 3137–3144. [Google Scholar] [CrossRef]
- Hilborn, E.D.; Soares, R.M.; Servaites, J.C.; Delgado, A.G.; Magalhães, V.F.; Carmichael, W.W.; Azevedo, S.M.F.O. Sublethal Microcystin Exposure and Biochemical Outcomes among Hemodialysis Patients. PLoS ONE 2013, 8, 69518. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, J.; Zhao, Q.; Pu, C.; Qiu, Z.; Zhang, R.; Shu, W. A cross-sectional investigation of chronic exposure to microcystin in relationship to childhood liver damage in the Three Gorges Reservoir Region, China. Environ. Health Perspect. 2011, 119, 1483–1488. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Chen, J.; Zhang, X.; Xie, P. A review of reproductive toxicity of microcystins. J. Hazard. Mater. 2016, 301, 381–399. [Google Scholar] [CrossRef]
- Fischer, W.J.; Altheimer, S.; Cattori, V.; Meier, P.J.; Dietrich, D.R.; Hagenbuch, B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol. Appl. Pharmacol. 2005, 203, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Fahrner, R.; Wittmann, V.; Stieger, B.; Dietrich, D.R. Human MRP2 exports MC-LR but not the glutathione conjugate. Chem. Biol. Interact. 2019, 311, 108761. [Google Scholar] [CrossRef]
- Brózman, O.; Kubickova, B.; Babica, P.; Laboha, P. Microcystin-LR Does Not Alter Cell Survival and Intracellular Signaling in Human Bronchial Epithelial Cells. Toxins 2020, 12, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raska, J.; Ctverackova, L.; Dydowiczova, A.; Sovadinova, I.; Blaha, L.; Babica, P. Tumor-promoting cyanotoxin microcystin-LR does not induce procarcinogenic events in adult human liver stem cells. Toxicol. Appl. Pharmacol. 2018, 345, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Henri, J.; Huguet, A.; Delmas, J.-M.; Besson, A.; Sanders, P.; Fessard, V. Low in vitro permeability of the cyanotoxin microcystin-LR across a Caco-2 monolayer: With identification of the limiting factors using modelling. Toxicon 2014, 91, 5–14. [Google Scholar] [CrossRef]
- Henri, J.; Lanceleur, R.; Delmas, J.; Fessard, V.; Huguet, A. Permeability of the Cyanotoxin Microcystin-RR across a Caco-2 Cells Monolayer. Toxins 2021, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Zeller, P.; Clément, M.; Fessard, V. Similar uptake profiles of microcystin-LR and -RR in an in vitro human intestinal model. Toxicology 2011, 290, 7–13. [Google Scholar] [CrossRef]
- Gupta, N.; Pant, S.C.; Vijayaraghavan, R.; Rao, P.V.L. Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology 2003, 188, 285–296. [Google Scholar] [CrossRef]
- Nishiwaki, R.; Ohta, T.; Sueoka, E.; Suganuma, M.; Harada, K.i.; Watanabe, M.F.; Fujiki, H. Two significant aspects of microcystin-LR: Specific binding and liver specificity. Cancer Lett. 1994, 83, 283–289. [Google Scholar] [CrossRef]
- Dahlem, A.M.; Hassan, A.S.; Swanson, S.P.; Carmichael, W.W.; Beasley, V.R. A Model System for Studying the Bioavailability of Intestinally Administered Microcystin-LR, A Hepatotoxic Peptide from the Cyanobacterium Microcystis aeruginosa. Pharmacol. Toxicol. 1989, 64, 177–181. [Google Scholar] [CrossRef]
- Greer, B.; Meneely, J.P.; Elliott, C.T. Uptake and accumulation of Microcystin-LR based on exposure through drinking water: An animal model assessing the human health risk. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.D.; Dzierlenga, A.; Arman, T.; Toth, E.; Li, H.; Lynch, K.D.; Tian, D.-D.; Goedken, M.; Paine, M.F.; Cherrington, N. Nonalcoholic fatty liver disease alters microcystin-LR toxicokinetics and acute toxicity. Toxicon 2019, 162, 1–8. [Google Scholar] [CrossRef]
- Ito, E.; Kondo, F.; Harada, K. First report on the distribution of orally administered microcystin-LR in mouse tissue using an immunostaining method. Toxicon 2000, 38, 37–48. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, P.; Chen, J.; Liang, G. Distribution of microcystins in various organs (heart, liver, intestine, gonad, brain, kidney and lung) of Wistar rat via intravenous injection. Toxicon 2008, 52, 721–727. [Google Scholar] [CrossRef]
- Falconer, I.R.; Buckley, T.; Runnegar, M.T. Biological half-life, organ distribution and excretion of 125-I-labelled toxic peptide from the blue-green alga Microcystis aeruginosa. Aust. J. Biol. Sci. 1986, 39, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.A.; Pace, J.G.; Matson, C.F.; Miura, G.A.; Lawrence, W.B. Tissue distribution, excretion and hepatic biotransformation of microcystin-LR in mice. J. Pharmacol. Exp. Ther. 1991, 256, 176–182. [Google Scholar] [PubMed]
- Guo, X.; Chen, L.; Chen, J.; Xie, P.; Li, S.; He, J.; Li, W.; Fan, H.; Yu, D.; Zeng, C. Quantitatively evaluating detoxification of the hepatotoxic microcystin-LR through the glutathione (GSH) pathway in SD rats. Environ. Sci. Pollut. Res. 2015, 22, 19273–19284. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Choudhuri, S.; Ogura, K.; Csanaky, I.L.; Lei, X.; Cheng, X.; Song, P.Z.; Klaassen, C.D. Characterization of organic anion transporting polypeptide 1b2-null mice: Essential role in hepatic uptake/toxicity of phalloidin and microcystin-LR. Toxicol. Sci. 2008, 103, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Liang, G.; Wu, L.; Tuo, X.; Wang, W.; Chen, J.; Xie, P. Why mammals more susceptible to the hepatotoxic microcystins than fish: Evidences from plasma and albumin protein binding through equilibrium dialysis. Ecotoxicology 2013, 22, 1012–1019. [Google Scholar] [CrossRef]
- Bowman, C.M.; Okochi, H.; Benet, L.Z. The Presence of a Transporter-Induced Protein Binding Shift: A New Explanation for Protein-Facilitated Uptake and Improvement for In Vitro-In Vivo Extrapolation. Drug Metab. Dispos. 2019, 47, 358–363. [Google Scholar] [CrossRef]
- Schmidt, J.; Wilhelm, S.; Boyer, G. The Fate of Microcystins in the Environment and Challenges for Monitoring. Toxins 2014, 6, 3354–3387. [Google Scholar] [CrossRef] [Green Version]
- Pflugmacher, S.; Wiegand, C.; Oberemm, A.; Beattie, K.A.; Krause, E.; Codd, G.A.; Steinberg, C.E.W. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxication. Biochim. Biophys. Acta Gen. Subj. 1998, 1425, 527–533. [Google Scholar] [CrossRef]
- Kondo, F.; Ikai, Y.; Oka, H.; Okumura, M.; Ishikawa, N.; Harada, K.; Matsuura, K.; Murata, H.; Suzuki, M. Formation, characterization, and toxicity of the glutathione and cysteine conjugates of toxic heptapeptide microcystins. Chem. Res. Toxicol. 1992, 5, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Buratti, F.M.; Scardala, S.; Funari, E.; Testai, E. Human Glutathione Transferases Catalyzing the Conjugation of the Hepatoxin Microcystin-LR. Chem. Res. Toxicol. 2011, 24, 926–933. [Google Scholar] [CrossRef]
- Li, W.; He, J.; Chen, J.; Xie, P. Excretion pattern and dynamics of glutathione detoxification of microcystins in Sprague Dawley rat. Chemosphere 2018, 191, 357–364. [Google Scholar] [CrossRef]
- He, J.; Chen, J.; Wu, L.; Li, G.; Xie, P. Metabolic Response to Oral Microcystin-LR Exposure in the Rat by NMR-Based Metabonomic Study. J. Proteome Res. 2012, 11, 5934–5946. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, J.; Xie, P.; Guo, X.; Fan, H.; Yu, D.; Zeng, C.; Chen, L. The role of glutathione detoxification pathway in MCLR-induced hepatotoxicity in SD rats. Environ. Toxicol. 2015, 30, 1470–1480. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Beattie, K.A.; Pflugmacher, S.; Codd, G.A. Immuno-crossreactivity and toxicity assessment of conjugation products of the cyanobacterial toxin, microcystin-LR. FEMS Microbiol. Lett. 2000, 189, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Arman, T.; Lynch, K.D.; Goedken, M.; Clarke, J.D. Sub-chronic microcystin-LR renal toxicity in rats fed a high fat/high cholesterol diet. Chemosphere 2021, 269, 128773. [Google Scholar] [CrossRef]
- Pace, J.G.; Robinson, N.A.; Miura, G.A.; Matson, C.F.; Geisbert, T.W.; White, J.D. Toxicity and kinetics of [3H]microcystin-LR in isolated perfused rat livers. Toxicol. Appl. Pharmacol. 1991, 107, 391–401. [Google Scholar] [CrossRef]
- Botha, N.; Van De Venter, M.; Downing, T.G.; Shephard, E.G.; Gehringer, M.M. The effect of intraperitoneally administered microcystin-LR on the gastrointestinal tract of Balb/c mice. Toxicon 2004, 43, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Y. The role of PP2A-associated proteins and signal pathways in microcystin-LR toxicity. Toxicol. Lett. 2015, 236, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zegura, B. An Overview of the Mechanisms of Microcystin-LR Genotoxicity and Potential Carcinogenicity. Mini Rev. Med. Chem. 2016, 16, 1042–1062. [Google Scholar] [CrossRef]
- Toivola, D.M.; Eriksson, J.E.; Brautigan, D.L. Identification of protein phosphatase 2A as the primary target for microcystin-LR in rat liver homogenates. FEBS Lett. 1994, 344, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Runnegar, M.T.; Kong, S.; Berndt, N. Protein phosphatase inhibition and in vivo hepatotoxicity of microcystins. Am. J. Physiol. Liver Physiol. 1993, 265, G224–G230. [Google Scholar] [CrossRef] [PubMed]
- Runnegar, M.; Berndt, N.; Shou-Ming Kong, E.Y.C. In vivo and in vitro binding of Microcystin to Protein Phosphatases 1 and 2A. Biochem. Biophys. Res. Commun. 1995, 216, 162–169. [Google Scholar] [CrossRef]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Ito, E.; Takai, A.; Kondo, F.; Masui, H.; Imanishi, S.; Harada, K.-I. Comparison of protein phosphatase inhibitory activity and apparent toxicity of microcystins and related compounds. Toxicon 2002, 40, 1017–1025. [Google Scholar] [CrossRef]
- Chen, Y.M.; Lee, T.H.; Lee, S.J.; Huang, H.B.; Huang, R.; Chou, H.N. Comparison of protein phosphatase inhibition activities and mouse toxicities of microcystins. Toxicon 2006, 47, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Maynes, J.T.; Bateman, K.S.; Cherney, M.M.; Das, A.K.; Luu, H.A.; Holmes, C.F.B.; James, M.N.G. Crystal Structure of the Tumor-promoter Okadaic Acid Bound to Protein Phosphatase-1. J. Biol. Chem. 2001, 276, 44078–44082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKintosh, R.W.; Dalby, K.N.; Campbell, D.G.; Cohen, P.T.; Cohen, P.; MacKintosh, C. The cyanobacterial toxin microcystin binds covalently to cysteine-273 on protein phosphatase 1. FEBS Lett. 1995, 371, 236–240. [Google Scholar] [PubMed] [Green Version]
- Zong, W.; Wang, X.; Du, Y.; Zhang, S.; Zhang, Y.; Teng, Y. Molecular Mechanism for the Regulation of Microcystin Toxicity to Protein Phosphatase 1 by Glutathione Conjugation Pathway. BioMed Res. Int. 2017, 2017, 9676504. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Virshup, D.M. Protein serine/threonine phosphatases: Life, death, and sleeping. Curr. Opin. Cell Biol. 2005, 17, 197–202. [Google Scholar] [CrossRef]
- Shi, Y. Serine/Threonine Phosphatases: Mechanism through Structure. Cell 2009, 139, 468–484. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, H.; Wang, B.; Chen, T.; Wang, X.; Huang, P.; Xu, L.; Guo, Z. Microcystin-LR promotes proliferation by activating Akt/S6K1 pathway and disordering apoptosis and cell cycle associated proteins phosphorylation in HL7702 cells. Toxicol. Lett. 2016, 240, 214–225. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Huang, P.; Wang, H.; Xu, K.; Wang, X.; Xu, L.; Guo, Z. Microcystin-LR promotes cell proliferation in the mice liver by activating Akt and p38/ERK/JNK cascades. Chemosphere 2016, 163, 14–21. [Google Scholar] [CrossRef]
- Wang, H.; Xu, K.; Wang, B.; Liu, J.; Wang, X.; Xing, M.; Huang, P.; Guo, Z.; Xu, L. Microcystin-LR induces a wide variety of biochemical changes in the A549 human non-small cell lung cancer cell line: Roles for protein phosphatase 2A and its substrates. Environ. Toxicol. 2017, 32, 1065–1078. [Google Scholar] [CrossRef]
- Wen, C.; Yang, S.; Zheng, S.; Feng, X.; Chen, J.; Yang, F. Analysis of long non-coding RNA profiled following MC-LR-induced hepatotoxicity using high-throughput sequencing. J. Toxicol. Environ. Health. A 2018, 81, 1165–1172. [Google Scholar] [CrossRef]
- Li, T.; Huang, P.; Liang, J.; Fu, W.; Guo, Z.-L.; Xu, L.-H. Microcystin-LR (MCLR) induces a compensation of PP2A activity mediated by α4 protein in HEK293 cells. Int. J. Biol. Sci. 2011, 7, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Arman, T.; Lynch, K.D.; Montonye, M.L.; Goedken, M.; Clarke, J.D. Sub-Chronic Microcystin-LR Liver Toxicity in Preexisting Diet-Induced Nonalcoholic Steatohepatitis in Rats. Toxins 2019, 11, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botha, N.; Gehringer, M.M.; Downing, T.G.; Van De Venter, M.; Shephard, E.G. The role of microcystin-LR in the induction of apoptosis and oxidative stress in CaCo2 cells. Toxicon 2004, 43, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.-X.; Nam Ong, C. Role of oxidative stress and mitochondrial changes in cyanobacteria-induced apoptosis and hepatotoxicity. FEMS Microbiol. Lett. 2003, 220, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.X.; Shen, H.M.; Zhu, H.G.; Ong, C.N. Studies on oxidative damage induced by cyanobacteria extract in primary cultured rat hepatocytes. Environ. Res. 1998, 78, 12–18. [Google Scholar] [CrossRef]
- Ding, W.X.; Shen, H.M.; Ong, C.N. Calpain activation after mitochondrial permeability transition in microcystin-induced cell death in rat hepatocytes. Biochem. Biophys. Res. Commun. 2002, 291, 321–331. [Google Scholar] [CrossRef]
- Nong, Q.; Komatsu, M.; Izumo, K.; Indo, H.P.; Xu, B.; Aoyama, K.; Majima, H.J.; Horiuchi, M.; Morimoto, K.; Takeuchi, T. Involvement of reactive oxygen species in Microcystin-LR-induced cytogenotoxicity. Free Radic. Res. 2007, 41, 1326–1337. [Google Scholar] [CrossRef]
- Žegura, B.; Lah, T.T.; Filipič, M. The role of reactive oxygen species in microcystin-LR-induced DNA damage. Toxicology 2004, 200, 59–68. [Google Scholar]
- Žegura, B.; Volčič, M.; Lah, T.T.; Filipič, M. Different sensitivities of human colon adenocarcinoma (CaCo-2), astrocytoma (IPDDC-A2) and lymphoblastoid (NCNC) cell lines to microcystin-LR induced reactive oxygen species and DNA damage. Toxicon 2008, 52, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Bouaïcha, N.; Maatouk, I. Microcystin-LR and nodularin induce intracellular glutathione alteration, reactive oxygen species production and lipid peroxidation in primary cultured rat hepatocytes. Toxicol. Lett. 2004, 148, 53–63. [Google Scholar] [CrossRef]
- Meng, G.; Liu, J.; Lin, S.; Guo, Z.; Xu, L. Microcystin-LR-Caused ROS generation involved in p38 activation and tau hyperphosphorylation in neuroendocrine (PC12) cells. Environ. Toxicol. 2015, 30, 366–374. [Google Scholar] [CrossRef]
- Puerto, M.; Pichardo, S.; Jos, Á.; Prieto, A.I.; Sevilla, E.; Frías, J.E.; Cameán, A.M. Differential oxidative stress responses to pure Microcystin-LR and Microcystin-containing and non-containing cyanobacterial crude extracts on Caco-2 cells. Toxicon 2010, 55, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Weng, D.; Lu, Y.; Wei, Y.; Liu, Y.; Shen, P. The role of ROS in microcystin-LR-induced hepatocyte apoptosis and liver injury in mice. Toxicology 2007, 232, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Guzman, R.E.; Solter, P.F. Hepatic Oxidative Stress Following Prolonged Sublethal Microcystin LR Exposure. Toxicol. Pathol. 1999, 27, 582–588. [Google Scholar] [CrossRef]
- Wei, Y.; Weng, D.; Li, F.; Zou, X.; Young, D.O.; Ji, J.; Shen, P. Involvement of JNK regulation in oxidative stress-mediated murine liver injury by microcystin-LR. Apoptosis 2008, 13, 1031–1042. [Google Scholar] [CrossRef]
- Moreno, I.; Pichardo, S.; Jos, A.; Gomez-Amores, L.; Mate, A.; Vazquez, C.M.; Camean, A.M. Antioxidant enzyme activity and lipid peroxidation in liver and kidney of rats exposed to microcystin-LR administered intraperitoneally. Toxicon 2005, 45, 395–402. [Google Scholar] [CrossRef]
- Mereish, K.A.; Bunner, D.L.; Ragland, D.R.; Creasia, D.A. Protection against microcystin-LR-induced hepatotoxicity by Silymarin: Biochemistry, histopathology, and lethality. Pharm. Res. 1991, 8, 273–277. [Google Scholar] [CrossRef]
- Hermansky, S.J.; Stohs, S.J.; Eldeen, Z.M.; Roche, V.F.; Mereish, K.A. Evaluation of potential chemoprotectants against microcystin-LR hepatotoxicity in mice. J. Appl. Toxicol. 1991, 11, 65–73. [Google Scholar] [CrossRef]
- Žegura, B.; Zajc, I.; Lah, T.T.; Filipič, M. Patterns of microcystin-LR induced alteration of the expression of genes involved in response to DNA damage and apoptosis. Toxicon 2008, 51, 615–623. [Google Scholar] [CrossRef]
- Menezes, C.; Alverca, E.; Dias, E.; Sam-Bento, F.; Pereira, P. Involvement of endoplasmic reticulum and autophagy in microcystin-LR toxicity in Vero-E6 and HepG2 cell lines. Toxicol. Vitro 2013, 27, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Makita, Y.; Tsutsumi, T.; Nagata, S.; Tashiro, F.; Yoshida, F.; Sekijima, M.; Tamura, S.; Harada, T.; Maita, K.; et al. Immunohistochemical localization of microcystin-LR in the liver of mice: A study on the pathogenesis of microcystin-LR-induced hepatotoxicity. Toxicol. Pathol. 1998, 26, 411–418. [Google Scholar] [CrossRef]
- Wu, J.X.; Huang, H.; Yang, L.; Zhang, X.F.; Zhang, S.S.; Liu, H.H.; Wang, Y.Q.; Yuan, L.; Cheng, X.M.; Zhuang, D.G.; et al. Gastrointestinal toxicity induced by microcystins. World J. Clin. Cases 2018, 6, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, Q.; Cui, J.; Yang, W.; Shi, Q.; Hua, Z.; Ji, J.; Shen, P. Induction of apoptosis in mouse liver by microcystin-LR: A combined transcriptomic, proteomic, and simulation strategy. Mol. Cell. Proteomics 2005, 4, 958–974. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Yuan, C.; Tuo, X.; Zhou, C.; Zhao, Q.; Shen, T. MCLR induces dysregulation of calcium homeostasis and endoplasmic reticulum stress resulting in apoptosis in Sertoli cells. Chemosphere 2021, 263, 127868. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yan, W.; Dang, Y.; Li, J.; Liu, C.; Wang, J. The role of calcineurin signaling in microcystin-LR triggered neuronal toxicity. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falconer, I.R.; Yeung, D.S.K. Cytoskeletal changes in hepatocytes induced by Microcystis toxins and their relation to hyperphosphorylation of cell proteins. Chem. Biol. Interact. 1992, 81, 181–196. [Google Scholar] [CrossRef]
- Ding, W.X.; Shen, H.M.; Ong, C.N. Critical role of reactive oxygen species formation in microcystin-induced cytoskeleton disruption in primary cultured hepatocytes. J. Toxicol. Environ. Health Part A 2001, 64, 507–519. [Google Scholar] [CrossRef]
- Eriksson, J.E.; Paatero, G.I.L.; Meriluoto, J.A.O.; Codd, G.A.; Kass, G.E.N.; Nicotera, P.; Orrenius, S. Rapid microfilament reorganization induced in isolated rat hepatocytes by microcystin-LR, a cyclic peptide toxin. Exp. Cell Res. 1989, 185, 86–100. [Google Scholar] [CrossRef]
- Hooser, S.B.; Easley, V.R.B.; Waite, L.L.; Kuh Lenschmidt, M.S.; Carmichael, W.W.; Haschek, W.M. Actin Filament Alterations in Rat Hepatocytes Induced In Vivo and In Vitro by Microcystin-LR, a Hepatotoxin from the Blue-green Alga, AJicrocystisaeruginosa. Vet. Pathol. 1991, 28, 259–266. [Google Scholar] [CrossRef]
- Milutinović, A.; Živin, M.; Zorc-Pleskovič, R.; Sedmak, B.; Šuput, D. Nephrotoxic effects of chronic administration of microcystins -LR and -YR. Toxicon 2003, 42, 281–288. [Google Scholar] [CrossRef]
- Khan, S.A.; Wickstrom, M.L.; Haschek, W.M.; Schaeffer, D.J.; Ghosh, S.; Beasley, V.R. Microcystin-LR and kinetics of cytoskeletal reorganization in hepatocytes, kidney cells, and fibroblasts. Nat. Toxins 2006, 4, 206–214. [Google Scholar] [CrossRef]
- Zong, W.-S.; Zhang, S.-H.; Wang, Q.; Teng, Y.; Liu, Y.-Z.; Du, Y.-G. Evaluation of the Direct and Indirect Regulation Pathways of Glutathione Target to the Hepatotoxicity of Microcystin-LR. BioMed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.P.; Davis, M.A.; Ryan, T.P.; Searfoss, G.H.; Hooser, S.B. Hepatic Gene Expression Changes in Mice Associated with Prolonged Sublethal Microcystin Exposure. Toxicol. Pathol. 2007, 35, 594–605. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Zhou, W.; Qiao, Q.; Liang, H.; Li, G.; Wang, J.; Cai, F. The Interactive Effects of Cytoskeleton Disruption and Mitochondria Dysfunction Lead to Reproductive Toxicity Induced by Microcystin-LR. PLoS ONE 2013, 8, e53949. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, W.W. The toxins of cyanobacteria. Sci. Am. 1994, 270, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Fleming, L.; Rivero, C.; Burns, J.; Williams, C.; Bean, J.; Shea, K.; Stinn, J. Blue green algal (cyanobacterial) toxins, surface drinking water, and liver cancer in Florida. Harmful Algae 2002, 1, 157–168. [Google Scholar] [CrossRef]
- Azevedo, S.M.F.O.; Carmichael, W.W.; Jochimsen, E.M.; Rinehart, K.L.; Lau, S.; Shaw, G.R.; Eaglesham, G.K. Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology 2002, 181–182, 441–446. [Google Scholar] [CrossRef]
- Gu, S.; Yan, M.; Wang, C.; Meng, X.; Xiang, Z.; Qiu, Y.; Han, X. Microcystin-leucine-arginine induces liver fibrosis by activating the Hedgehog pathway in hepatic stellate cells. Biochem. Biophys. Res. Commun. 2020, 533, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xue, Q.; Su, X.; Xie, L.; Yan, Y.; Steinman, A.D. Microcystin-LR induced thyroid dysfunction and metabolic disorders in mice. Toxicology 2015, 328, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, K.; Zhang, S.; Liu, J.; Xu, C.; Xu, L.; Huang, P.; Guo, Z. Microcystin-LR disrupts insulin signaling by hyperphosphorylating insulin receptor substrate 1 and glycogen synthase. Environ. Toxicol. 2017, 33, 16–22. [Google Scholar] [CrossRef]
- Su, R.C.; Lad, A.; Breidenbach, J.D.; Kleinhenz, A.L.; Modyanov, N.; Malhotra, D.; Haller, S.T.; Kennedy, D.J. Assessment of diagnostic biomarkers of liver injury in the setting of microcystin-LR (MC-LR) hepatotoxicity. Chemosphere 2020, 257, 127111. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.; Kondo, F.; Terao, K.; Harada, K. Neoplastic nodular formation in mouse liver induced by repeated intraperitoneal injections of microcystin-LR. Toxicon 1997, 35, 1453–1457. [Google Scholar] [CrossRef]
- Fujiki, H.; Suganuma, M. Tumor promoters--microcystin-LR, nodularin and TNF-α and human cancer development. Anticancer Agents Med. Chem. 2011, 11, 4–18. [Google Scholar] [CrossRef]
- Lei, F.; Lei, X.; Li, R.; Tan, H. Microcystin-LR in peripheral circulation worsens the prognosis partly through oxidative stress in patients with hepatocellular carcinoma. Clin. Exp. Med. 2019, 19, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yi, X.; Liu, W.; Zhang, C.; Massey, I.Y.; Yang, F.; Tian, L. A Review of Nephrotoxicity of Microcystins. Toxins 2020, 12, 693. [Google Scholar] [CrossRef]
- Yi, X.; Xu, S.; Huang, F.; Wen, C.; Zheng, S.; Feng, H.; Guo, J.; Chen, J.; Feng, X.; Yang, F. Effects of Chronic Exposure to Microcystin-LR on Kidney in Mice. Int. J. Environ. Res. Public Health 2019, 16, 5030. [Google Scholar] [CrossRef] [Green Version]
- Milutinović, A.; Sedmak, B.; Horvat-Znidarsic, I.; Suput, D. Renal injuries induced by chronic intoxication with microcystins. Cell. Mol. Biol. Lett. 2002, 7, 139–141. [Google Scholar]
- Nobre, A.C.L.; Jorge, M.C.M.; Menezes, D.B.; Fonteles, M.C.; Monteiro, H.S.A. Effects of microcystin-LR in isolated perfused rat kidney. Braz. J. Med. Biol. Res. 1999, 32, 985–988. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Liu, W.; Zeng, H.; Pu, C.; Zhang, R.; Qiu, Z.; Chen, J.-A.; Wang, L.; Tan, Y.; Zheng, C.; et al. Determination of Environmental Exposure to Microcystin and Aflatoxin as a Risk for Renal Function Based on 5493 Rural People in Southwest China. Environ. Sci. Technol. 2016, 50, 5346–5356. [Google Scholar] [CrossRef]
- Tamele, I.J.; Vasconcelos, V. Microcystin incidence in the drinking water of mozambique: Challenges for public health protection. Toxins 2020, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; Chorus, I.; Welker, M. (Eds.) World Health Organization: Geneva, Switzerland, 2021; ISBN 9781626239777. [Google Scholar]
- Kubickova, B.; Babica, P.; Hilscherová, K.; Šindlerová, L. Effects of cyanobacterial toxins on the human gastrointestinal tract and the mucosal innate immune system. Environ. Sci. Eur. 2019, 31, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Xu, X.; Yu, B.; Yu, G. Characterization of in vitro effects of microcystin-LR on intestinal epithelial cells. Environ. Toxicol. 2017, 32, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.; Kondo, F.; Harada, K. Hepatic Necrosis in Aged Mice by Oral Administration of Microcystin-LR. Toxicon 1997, 35, 231–239. [Google Scholar] [CrossRef]
- Chen, J.; Xie, P.; Lin, J.; He, J.; Zeng, C.; Chen, J. Effects of microcystin-LR on gut microflora in different gut regions of mice. J. Toxicol. Sci. 2015, 40, 485–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilotto, L.S.; Douglas, R.M.; Burch, M.D.; Cameron, S.; Beers, M.; Rouch, G.J.; Robinson, P.; Kirk, M.; Cowie, C.T.; Hardiman, S.; et al. Health effects of exposure to cyanobacteria (blue-green algae) during recreational water-related activities. Aust. N. Z. J. Public Health 1997, 21, 562–566. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Yu, H.; Chen, K. Relationship between microcystin in drinking water and colorectal cancer. Biomed. Environ. Sci. 2002, 15, 166–171. [Google Scholar]
- Wang, J.; Lin, F.; Cai, F.; Yan, W.; Zhou, Q.; Xie, L. Microcystin-LR Inhibited Hippocampal Long-Term Potential via Regulation of the Glycogen Synthase Kinase-3β Pathway. Chemosphere 2013, 93, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Maidana, M.; Carlis, V.; Galhardi, F.G.; Yunes, J.S.; Geracitano, L.A.; Monserrat, J.M.; Barros, D.M. Effects of microcystins over short- and long-term memory and oxidative stress generation in hippocampus of rats. Chem. Biol. Interact. 2006, 159, 223–234. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Zhu, J.; Ding, J.; Chen, Y.; Han, X. Blood-brain barrier disruption and inflammation reaction in mice after chronic exposure to Microcystin-LR. Sci. Total Environ. 2019, 689, 662–678. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Cai, F.; Yan, W.; Li, C.; Wang, J. A Proteomic Analysis of MCLR-induced Neurotoxicity: Implications for Alzheimer’s Disease. Toxicol. Sci. 2012, 127, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Gu, S.; Yin, X.; Yuan, M.; Xiang, Z.; Li, Z.; Cao, H.; Meng, X.; Hu, K.; Han, X. The toxic effects of microcystin-LR on mouse lungs and alveolar type II epithelial cells. Toxicon 2016, 115, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xu, L.; Zhou, W.; Zhao, Q.; Wang, Y. Chronic exposure to microcystin-LR affected mitochondrial DNA maintenance and caused pathological changes of lung tissue in mice. Environ. Pollut. 2016, 210, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Šuput, D.; Zorc-Pleskovič, R.; Petrovič, D.; Milutinović, A. Cardiotoxic injury caused by chronic administration of microcystin-YR. Folia Biol. 2010, 56, 14–18. [Google Scholar]
- Qiu, T.; Xie, P.; Liu, Y.; Li, G.; Xiong, Q.; Hao, L.; Li, H. The profound effects of microcystin on cardiac antioxidant enzymes, mitochondrial function and cardiac toxicity in rat. Toxicology 2009, 257, 86–94. [Google Scholar] [CrossRef]
- Zhang, S.; Du, X.; Liu, H.; Losiewic, M.D.; Chen, X.; Ma, Y.; Wang, R.; Tian, Z.; Shi, L.; Guo, H.; et al. The latest advances in the reproductive toxicity of microcystin-LR. Environ. Res. 2021, 192, 110254. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- He, J.; Li, G.; Chen, J.; Lin, J.; Zeng, C.; Chen, J.; Deng, J.; Xie, P. Prolonged exposure to low-dose microcystin induces nonalcoholic steatohepatitis in mice: A systems toxicology study. Arch. Toxicol. 2017, 91, 465–480. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, Y.; Xie, L.; Wang, L.; He, Y.; Wan, X.; Xue, Q. Long-term environmental exposure to microcystins increases the risk of nonalcoholic fatty liver disease in humans: A combined fisher-based investigation and murine model study. Environ. Int. 2020, 138, 105648. [Google Scholar] [CrossRef]
- Lad, A.; Su, R.C.; Breidenbach, J.D.; Stemmer, P.M.; Carruthers, N.J.; Sanchez, N.K.; Khalaf, F.K.; Zhang, S.; Kleinhenz, A.L.; Dube, P.; et al. Chronic Low Dose Oral Exposure to Microcystin-LR Exacerbates Hepatic Injury in a Murine Model of Non-Alcoholic Fatty Liver Disease. Toxins 2019, 11, 486. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Lee, J.; Liang, S.; Shum, C.K. Cyanobacteria blooms and non-alcoholic liver disease: Evidence from a county level ecological study in the United States. Environ. Health 2015, 14, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Wang, L.; Yang, X.; Zeng, H.; Zhang, R.; Pu, C.; Zheng, C.; Tan, Y.; Luo, Y.; Feng, X.; et al. Environmental Microcystin Exposure Increases Liver Injury Risk Induced by Hepatitis B Virus Combined with Aflatoxin: A Cross-Sectional Study in Southwest China. Environ. Sci. Technol. 2017, 51, 6367–6378. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, L.; Zheng, C.; Liu, L.; Wang, J.; Li, D.; Tan, Y.; Zhao, X.; He, L.; Shu, W. Microcystin-LR increases genotoxicity induced by aflatoxin B1 through oxidative stress and DNA base excision repair genes in human hepatic cell lines. Environ. Pollut. 2018, 233, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, L.; Zeng, H.; Fu, W.; Wang, J.; Tan, Y.; Zheng, C.; Qiu, Z.; Luo, J.; Lv, C.; et al. Low-dose microcystin-LR antagonizes aflatoxin B1 induced hepatocarcinogenesis through decreasing cytochrome P450 1A2 expression and aflatoxin B1-DNA adduct generation. Chemosphere 2020, 248, 126036. [Google Scholar] [CrossRef]
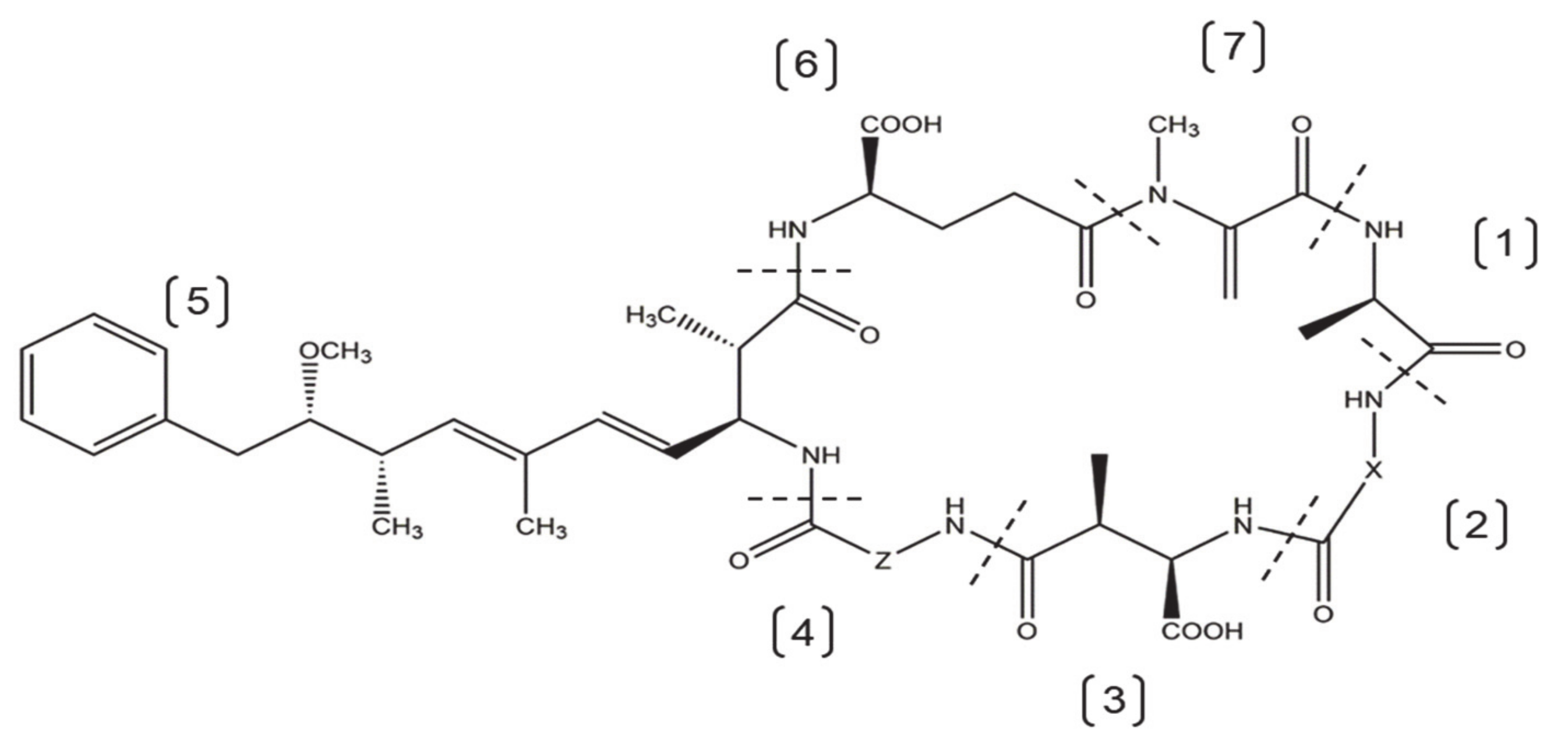


| Toxin | Producing Genera | Primary Toxicity | Mode of Action | Toxic Effects |
|---|---|---|---|---|
| Microcystin | Microcystis, Anabaena, Nostoc, Planktothrix, Hapalosiphon, Phormidium | Hepatotoxicity | Inhibition of protein phosphatases | Liver and kidney damage, gastroenteritis, tumor promotion, reduced DNA repair, and reproductive toxicity |
| Nodularins | Nodularia | Hepatotoxicity | Inhibition of protein phosphatases | Liver and kidney damage, gastroenteritis, tumor promotion, reduced DNA repair, and reproductive toxicity, carcinogenic |
| Cylindrospermopsins | Cylindrospermopsis, Anabaena, Raphidiopsis, Aphanizomenon, Chrysosporum, Umezakia | Hepatotoxicity | Inhibition of protein phosphatases | Liver, kidney, spleen, lungs and intestinal damage, genotoxicity |
| Anatoxin-a | Anabaena, Aphanizomenon, Cuspidothrix, Dolichospermum, Oscillatoria, Phormidium | Neurotoxicity | Nicotinic acetylcholine receptor agonists | Muscular paralysis, respiratory failure |
| Anatoxin-a(s) | Dolichospermum, Anabaena | Neurotoxicity | Inhibition of acetylcholinesterase | Muscular weakness, dyspnea, convulsions |
| Saxitoxins | Aphanizomenon, Cuspidothrix, Cylindrospermopsis, Dolichospermum | Neurotoxicity | Blocking of sodium channels | Convulsions, paralysis, respiratory failure |
| BMAA (β-Methylamino-L-alanine) | Microcystis, Nostoc, Anabaena, Aphanizomenon, Nodularia | Neurotoxicity | Excessive stimulation of glutamate receptors in neurons | Neurodegenerative syndrome |
| Aplysiatoxin | Lyngbya, Schizothrix, Oscillatoria | Dermatoxicity | Activation of protein kinase C | Tumor promotion, skin irritation, asthma |
| Lyngbyatoxins | Lyngbya, Schizothrix, Oscillatoria | Dermatoxicity | Activation of protein kinase C | Tumor promotion, skin and eye irritation, respiratory problems |
| Lipopolysaccharide | All cyanobacteria | Dermatoxicity | Activation of toll-like receptors | Skin and eye irritation, fever, gastrointestinal upset |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arman, T.; Clarke, J.D. Microcystin Toxicokinetics, Molecular Toxicology, and Pathophysiology in Preclinical Rodent Models and Humans. Toxins 2021, 13, 537. https://doi.org/10.3390/toxins13080537
Arman T, Clarke JD. Microcystin Toxicokinetics, Molecular Toxicology, and Pathophysiology in Preclinical Rodent Models and Humans. Toxins. 2021; 13(8):537. https://doi.org/10.3390/toxins13080537
Chicago/Turabian StyleArman, Tarana, and John D. Clarke. 2021. "Microcystin Toxicokinetics, Molecular Toxicology, and Pathophysiology in Preclinical Rodent Models and Humans" Toxins 13, no. 8: 537. https://doi.org/10.3390/toxins13080537
APA StyleArman, T., & Clarke, J. D. (2021). Microcystin Toxicokinetics, Molecular Toxicology, and Pathophysiology in Preclinical Rodent Models and Humans. Toxins, 13(8), 537. https://doi.org/10.3390/toxins13080537





