Dietary Supplements Based on Red Yeast Rice—A Source of Citrinin?
Abstract
:1. Introduction
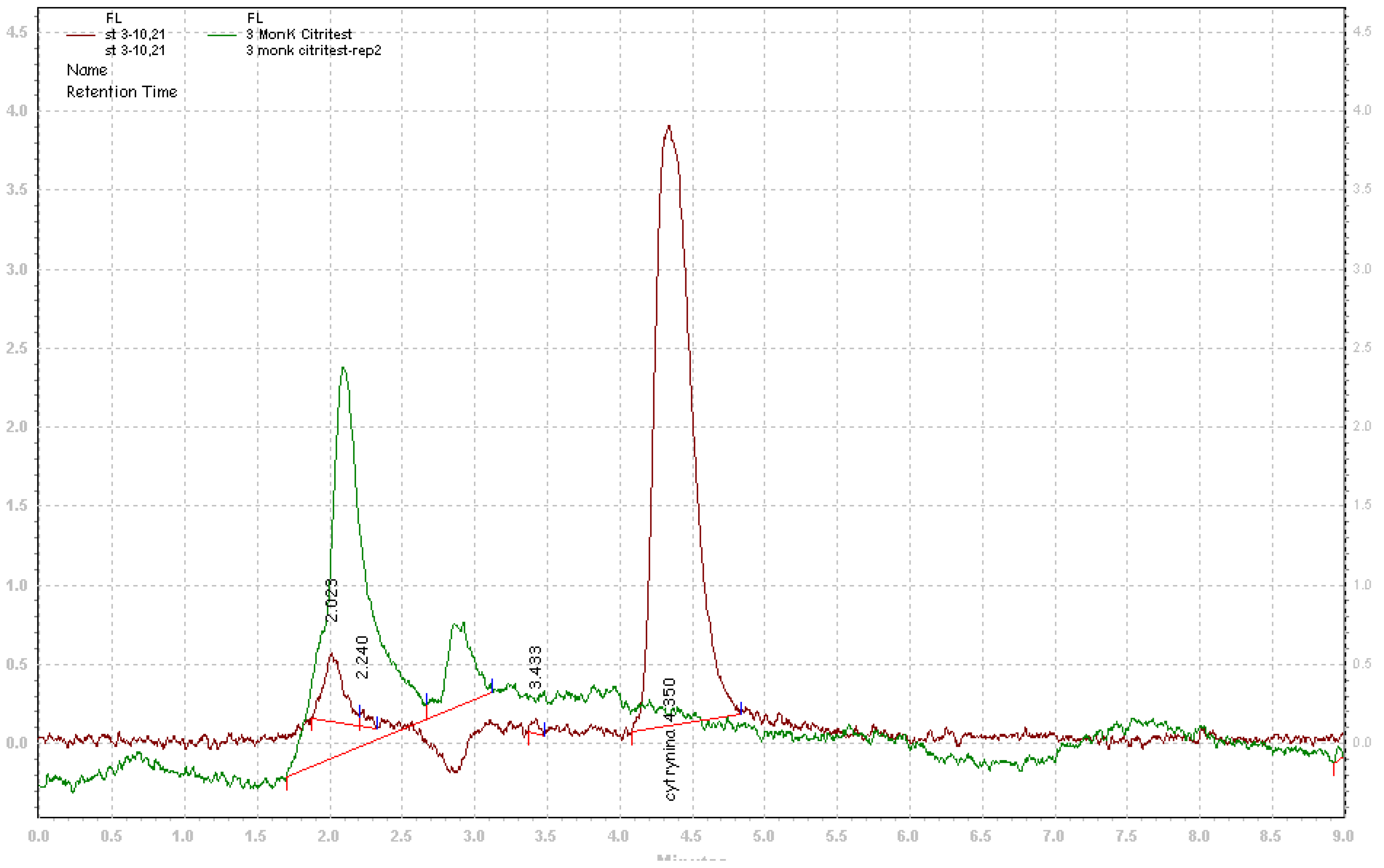
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Extraction of CIT
4.2.1. Preparation of 0.01 M Phosphoric Acid
4.2.2. Sample Extraction
4.3. Chromatographic Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Moreau, C. Moulds, toxins and food. John Wiley and Sons, New York-Toronto. Biochem. Educ. 1979, 7. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Pereira, A.M.P.T.; Pena, A.; Lino, C.M. Citrinin in Foods and Supplements: A Review of Occurrence and Analytical Methodologies. Foods 2021, 10, 14. [Google Scholar] [CrossRef]
- Blane, P.J.; Loret, M.O.; Goma, G. Production of citrinin by various species of Monoascus. Biotechnol. Lett. 1995, 17, 291–294. [Google Scholar] [CrossRef]
- Manabe, M. Fermented foods and mycotoxins. Mycotoxins 2001, 51, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Lu, C.; Mu, X.; Chen, J.; Chen, Y.; Gu, Y.; Wu, Y.; Sheng, F.; Wu, M. A study on the production of citrinin by Monascus spp. Arch. Lebensmittelhyg. 1999, 50, 88–91. [Google Scholar]
- Kitabatake, N.; Ben Trivedi, A.; Doi, E. Thermal decomposition and detoxification of citrinin under various moisture conditions. J. Agric. Food Chem. 1991, 39, 2240–2244. [Google Scholar] [CrossRef]
- Xu, B.-J.; Jia, X.-Q.; Gu, L.-J.; Sung, C.-K. Review on the qualitative and quantitative analysis of the mycotoxin citrinin. Food Control 2006, 17, 271–285. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Q.; Zhang, X.; Zhang, H.; Huang, Q.; Li, D.; Yao, J. Comparison of extraction methods for analysis of citrinin in red fermented rice. Food Chem. 2014, 157, 408–412. [Google Scholar] [CrossRef]
- Arai, M.; Hibino, T. Tumorigenicity of citrinin in male F344 rats. Cancer Lett. 1983, 17, 281–287. [Google Scholar] [CrossRef]
- Sándor, G.; Busch, A.; Watzke, H.; Reek, J.; Ványi, A. Subacute toxicity testing of ochratoxin A and citrinin in swine. Acta Veter. Hung. 1991, 39, 149–160. [Google Scholar]
- International Agency for Research on Cancer (IARC). Some Naturally Occurring and Synthetic Food Components, Furocoumarins and Ultraviolet Radiation. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 1986; Volume 40, p. 67. [Google Scholar]
- Kanisawa, M. Synergistic effect of citrinin on hepatorenal carcinogenesis of ochratoxin A in mice. Dev. Food Sci. 1984, 7, 245–254. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific opinion on the risks for public and animal health related to the presence of Citrinin in food and feed. EFSA panel on contaminants in the food chain (CONTAM). EFSA J. 2012, 10. [Google Scholar] [CrossRef]
- Flajs, D.; Peraica, M. Toxicological Properties of Citrinin. Arh. Hig. Rada Toksikol. 2009, 60, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Nelson, T.S.; Kirby, L.K.; Beasley, J.N.; Johnson, Z.B.; Ciegler, A. The Effect of Drying Method and Storage Time on Citrinin Activity in Corn. Poult. Sci. 1985, 64, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.M.; van Walbeek, W.; Kennedy, B.; Anyeti, B. Mycotoxins (ochratoxin A, citrinin and sterigmatocystin) and toxigenic fungi in grains and other agricultural products. J. Agric. Food Chem. 1972, 20, 1103–1109. [Google Scholar] [CrossRef]
- Tanaka, K.; Sago, Y.; Zheng, Y.; Nakagawa, H.; Kushiro, M. Mycotoxins in rice. Int. J. Food Microbiol. 2007, 119, 59–66. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Ruprich, J. Producres and important dietary sources od ochratoxin A and citrinin. Toxins 2013, 5, 1574–1586. [Google Scholar] [CrossRef] [Green Version]
- Commission Regulation (EU). No 212/2014 of 6 March 2014 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of the Contaminant Citrinin in Food Supplements Based on Rice Fermented with Red Yeast Monascus Purpureus. Available online: https://op.europa.eu/en/publication-detail/-/publication/26b34f77-a5d1-11e3-8438-01aa75ed71a1 (accessed on 9 June 2021).
- European Commission. European Commission Regulation (EC) No 1881/2006 of 19 December 2006. Setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Erdogrull, O.; Azirak, S. Review of the studies on the red yeast rice (Monascus purpureus). Turk. Electron. J. Biotechnol. 2004, 2, 37–49. [Google Scholar]
- Pan, T.M.; Hsu, W.H. Monascus-Fermented Products. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 815–825. [Google Scholar]
- Burke, F.M. Red Yeast Rice for the Treatment of Dyslipidemia. Curr. Atheroscler. Rep. 2015, 17. [Google Scholar] [CrossRef]
- Wen, Q.; Cao, X.; Chen, Z.; Xiong, Z.; Liu, J.; Cheng, Z.; Zheng, Z.; Long, C.; Zheng, B.; Huang, Z. An overview of Monascus fermentation processes for monacolin K production. Open Chem. 2020, 18, 10–21. [Google Scholar] [CrossRef]
- Heber, D.; Yip, I.; Ashley, J.M.; Elashoff, D.A.; Elashoff, R.M.; Go, V.L.W. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am. J. Clin. Nutr. 1999, 69, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Functional food red yeast rice (RYR) for metabolic syndrome amelioration: A review on pros and cons. World J. Microbiol. Biotechnol. 2016, 32. [Google Scholar] [CrossRef]
- Zhang, Z.; Ali, Z.; Khan, S.I.; Khan, I.A. Cytotoxic monacolins from red yeast rice, a Chinese medicine and food. Food Chem. 2016, 202, 262–268. [Google Scholar] [CrossRef]
- Lu, Z.; Kou, W.; Du, B.; Wu, Y.; Zhao, S.; Brusco, O.A.; Morgan, J.M.; Capuzzi, D.M.; Chinese Coronary Secondary Prevention Study Group; Li, S. Effect of Xuezhikang, an Extract from Red Yeast Chinese Rice, on Coronary Events in a Chinese Population with Previous Myocardial Infarction. Am. J. Cardiol. 2008, 10, 1689–1693. [Google Scholar] [CrossRef]
- Cicero, A.F.; Morbini, M.; Parini, A.; Urso, R.; Rosticci, M.; Grandi, E.; Borghi, C. Effect of red yeast rice combined with antioxidants on lipid pattern, hs-CRP level, and endothelial function in moderately hypercholesterolemic subjects. Ther. Clin. Risk Manag. 2016, 12, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.H.W.; Gerdes, V.E.A. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jiang, L.; Jia, Z.; Xin, W.; Yang, S.; Yang, Q.; Wang, L. A Meta-Analysis of Red Yeast Rice: An Effective and Relatively Safe Alternative Approach for Dyslipidemia. PLoS ONE 2014, 9, e98611. [Google Scholar] [CrossRef]
- Ricky, W.K.; Bakr, R. Chinese red yeast rice (Monascus pupureus fermented-rice) promotes bone formation. Chin. Med. 2008, 3. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, C.; Bresson, J.L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Pagona, L.; Lovik, M.; Marchelli, R.; Martin, A.; et al. Scientific opinion on the substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL cholesterol concentrations (ID 1648, 1700) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2304–2320. [Google Scholar]
- Liao, C.D.; Chen, Y.C.; Lin, H.Y.; Chiueh, L.C.; Shih, D.Y.C. Incidence of citrinin in red yeast rice and various commercial Monascus products in Taiwan from 2009 to 2012. Food Control 2014, 38, 178–183. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.-C.; Yang, M.-H.; Ou-Yang, Z. Natural occurrence of citrinin in widely consumed traditional Chinese food red yeast rice, medicinal plants and their related products. Food Chem. 2012, 132, 1040–1045. [Google Scholar] [CrossRef]
- Liu, R.; Xu, B. Optimization of Extraction Conditions of Citrinin from Red Yeast Rice by Orthogonal Design and Quantification of Citrinin by High-Performance Liquid Chromatography. Food Anal. Methods 2013, 6, 677–682. [Google Scholar] [CrossRef]
- Heber, D.; Lembertas, A.; Lu, Q.-Y.; Bowerman, S.; Go, V.L.W. An Analysis of Nine Proprietary Chinese Red Yeast Rice Dietary Supplements: Implications of Variability in Chemical Profile and Contents. J. Altern. Complement. Med. 2004, 7, 133–139. [Google Scholar] [CrossRef]
- Liu, B.-H.; Wu, T.-S.; Su, M.-C.; Chung, C.P.; Yu, F.-Y. Evaluation of Citrinin Occurrence and Cytotoxicity inMonascusFermentation Products. J. Agric. Food Chem. 2005, 53, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, N.L.; Abdullah, N. A preliminary survey on the occurrence of mycotoxigenic fungi and mycotoxins contaminating red rice at consumer level in Selangor, Malaysia. Mycotoxin Res. 2013, 29, 89–96. [Google Scholar] [CrossRef]
- Marley, E.; Brown, P.; Leeman, D.; Donnelly, C. Analysis of Citrinin in Cereals, Red Yeast Rice Dietary Supplement, and Animal Feed by Immunoaffinity Column Cleanup and LC with Fluorescence Detection. J. AOAC Int. 2016, 99, 1025–1031. [Google Scholar] [CrossRef]
- Mornar, A.; Sertić, M.; Nigović, B. Developmnet of a rapid LC/DAD/FLD/MSn method for the simutaneous determination of monacolins and citrinin n red fermented rice produkct. J. Agric. Food Chem. 2013, 61, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Xu, J.; Wang, X.; Qi, P.; Wei, W.; Chen, X.; Li, R.; Zhou, Y. Citrinin Determination in Red Fermented Rice Products by Optimized Extraction Method Coupled to Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS). J. Food Sci. 2015, 80, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Sáncheza, P.L.; De Nijsa, M.; Spanjerb, M.; Pietric, A.; Bertuzzic, T.; Starski, A.; Postupolski, J.; Castellari, M.; Hortós, M. Generation of occurrence data on citrinin in food. EFSA J. Support. Publ. 2017. [Google Scholar] [CrossRef] [Green Version]
- Tangni, E.K.; Van Hove, F.; Huybrechts, B.; Masquelier, J.; Vandermeiren, K.; Van Hoeck, E. Citrinin Determination in Food and Food Supplements by LC-MS/MS: Development and Use of Reference Materials in an International Collaborative Study. Toxins 2021, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU). No. 519/2014 of 16 May 2014 amending Regulation (EC) No 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T-2, HT-2 toxin and citrinin and screening methods of analysis. Available online: https://op.europa.eu/en/publication-detail/-/publication/5a9dcba3-dd8b-11e3-8cd4-01aa75ed71a1/language-en (accessed on 6 June 2021).
| Recovery (%) ± RSD (%), n = 3 | LOD (ng/g) | LOQ (ng/g) | |
|---|---|---|---|
| CIT | 16.0 | 26.0 | |
| Spiking level—50 ng/g | 89.6 ± 3.2 | ||
| Spiking level—200 ng/g | 88.4 ± 3.9 | ||
| Spiking level—500 ng/g | 85.3 ± 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Twarużek, M.; Ałtyn, I.; Kosicki, R. Dietary Supplements Based on Red Yeast Rice—A Source of Citrinin? Toxins 2021, 13, 497. https://doi.org/10.3390/toxins13070497
Twarużek M, Ałtyn I, Kosicki R. Dietary Supplements Based on Red Yeast Rice—A Source of Citrinin? Toxins. 2021; 13(7):497. https://doi.org/10.3390/toxins13070497
Chicago/Turabian StyleTwarużek, Magdalena, Iwona Ałtyn, and Robert Kosicki. 2021. "Dietary Supplements Based on Red Yeast Rice—A Source of Citrinin?" Toxins 13, no. 7: 497. https://doi.org/10.3390/toxins13070497
APA StyleTwarużek, M., Ałtyn, I., & Kosicki, R. (2021). Dietary Supplements Based on Red Yeast Rice—A Source of Citrinin? Toxins, 13(7), 497. https://doi.org/10.3390/toxins13070497





