Changes in the Risk of Stroke in Dialysis Patients: A Retrospective Analysis over the Last 40 Years
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
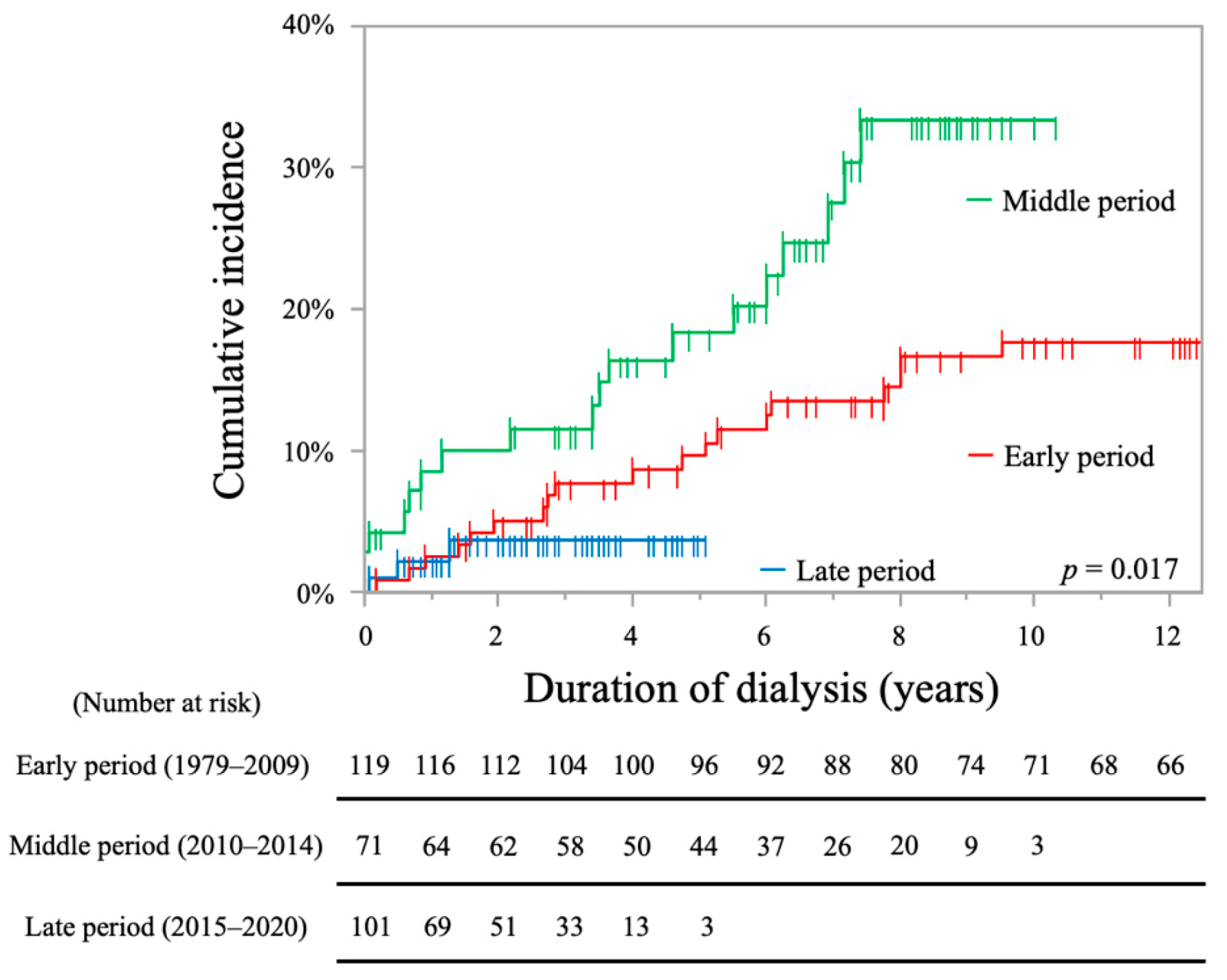
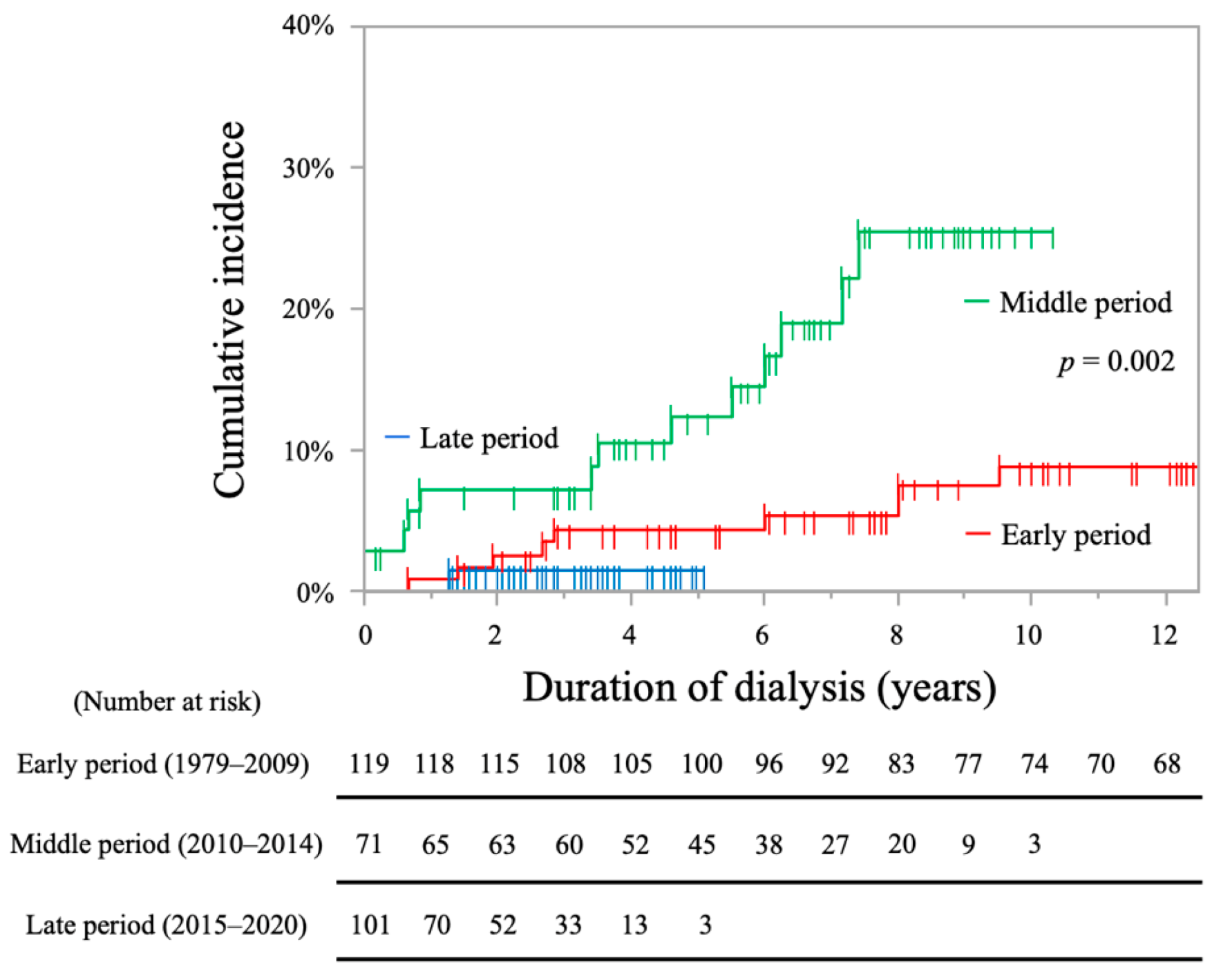
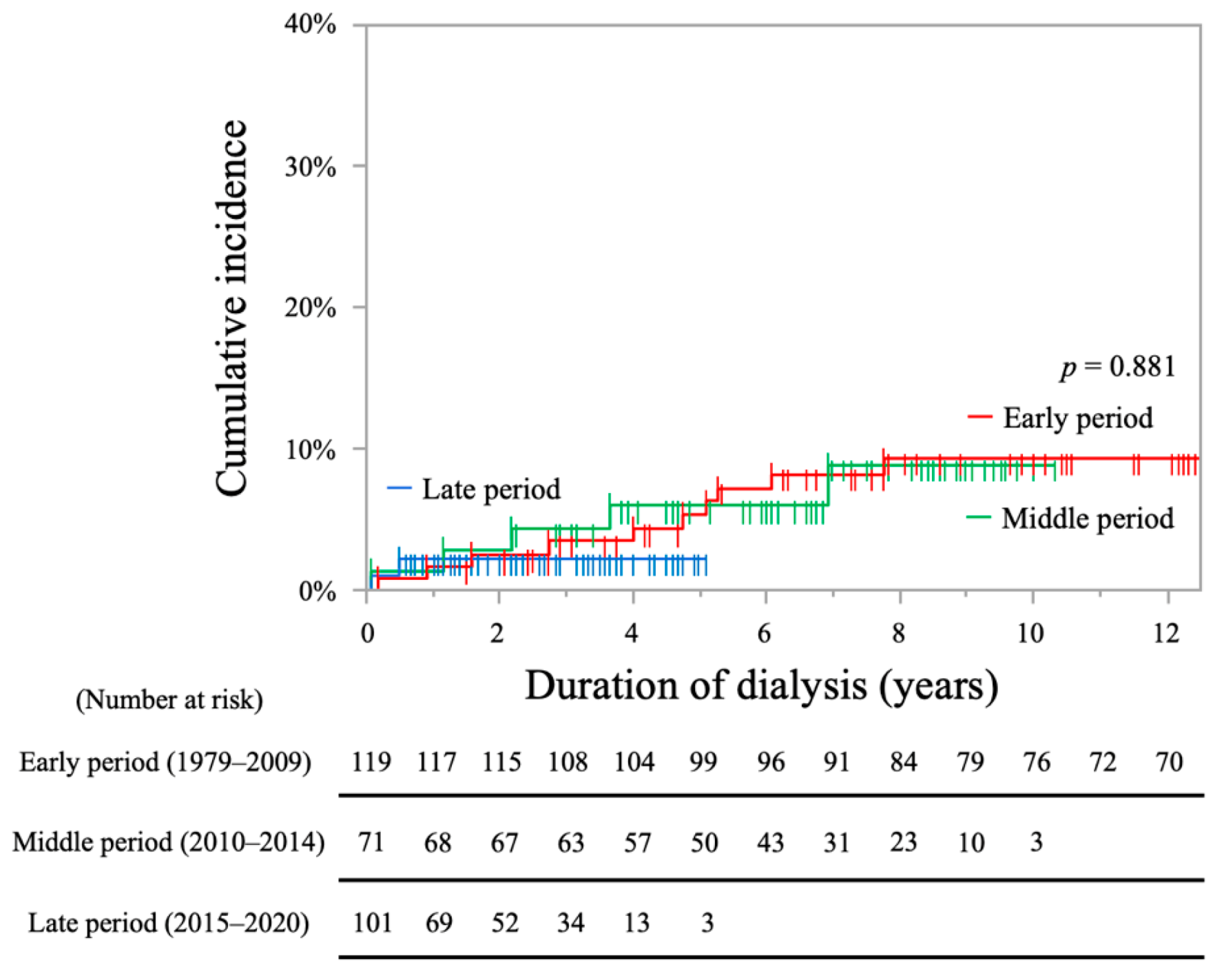
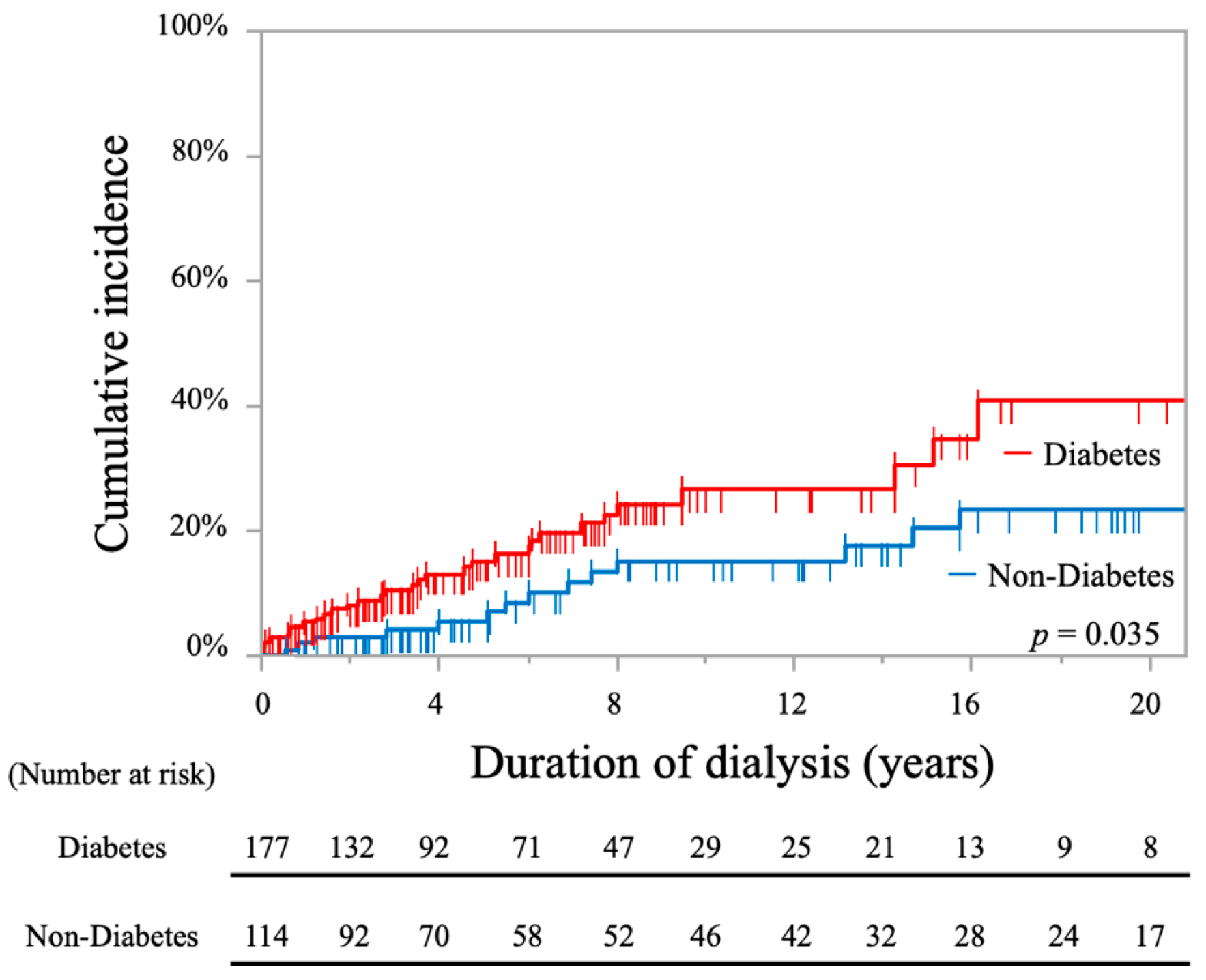
2.2. Analysis of Stroke Incidence
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Population
5.2. HD Procedures and Management
5.3. Diagnosis of Stroke and Renal Diseases
5.4. Biochemical Determinations
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iseki, K.; Kinjo, K.; Kimura, Y.; Osawa, A.; Fukiyama, K. Evidence for high risk of cerebral hemorrhage in chronic dialysis patients. Kidney Int. 1993, 44, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Seliger, S.L.; Gillen, D.L.; Longstreth, W.T.; Kestenbaum, B.; Stehman-Breen, C.O. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003, 64, 603–609. [Google Scholar] [CrossRef]
- Toyoda, K.; Fujii, K.; Fujimi, S.; Kumai, Y.; Tsuchimochi, H.; Ibayashi, S.; Iida, M. Stroke in patients on maintenance hemodialysis: A 22-year single-center study. Am. J. Kidney Dis. 2005, 45, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Ocak, G.; van Stralen, K.J.; Rosendaal, F.R.; Verduijn, M.; Ravani, P.; Palsson, R.; Leivestad, T.; Hoitsma, A.J.; Ferrer-Alamar, M.; Finne, P.; et al. Mortality due to pulmonary embolism, myocardial infarction, and stroke among incident dialysis patients. J. Thromb. Haemost. 2012, 10, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Power, A.; Chan, K.; Singh, S.K.; Taube, D.; Duncan, N. Appraising stroke risk in maintenance hemodialysis patients: A large single-center cohort study. Am. J. Kidney Dis. 2012, 59, 249–257. [Google Scholar] [CrossRef]
- Wang, H.H.; Hung, S.Y.; Sung, J.M.; Hung, K.Y.; Wang, J.D. Risk of stroke in long-term dialysis patients compared with the general population. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2014, 63, 604–611. [Google Scholar] [CrossRef]
- Ozelsancak, R.; Micozkadioglu, H.; Torun, D.; Tekkarismaz, N. Cerebrovascular events in hemodialysis patients; a retrospective observational study. BMC Nephrol. 2019, 20, 466. [Google Scholar] [CrossRef]
- Wetmore, J.B.; Phadnis, M.A.; Ellerbeck, E.F.; Shireman, T.I.; Rigler, S.K.; Mahnken, J.D. Relationship between stroke and mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2015, 10, 80–89. [Google Scholar] [CrossRef]
- Shinya, Y.; Miyawaki, S.; Kumagai, I.; Sugiyama, T.; Takenobu, A.; Saito, N.; Teraoka, A. Risk factors and outcomes of cerebral stroke in end-stage renal disease patients receiving hemodialysis. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2020, 29, 104657. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Bode-Boger, S.; Mallamaci, F.; Benedetto, F.; Tripepi, G.; Malatino, L.; Cataliotti, A.; Bellanuova, I.; Fermo, I.; Frolich, J.; et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet 2001, 358, 2113–2117. [Google Scholar] [CrossRef]
- Seliger, S.L.; Gillen, D.L.; Tirschwell, D.; Wasse, H.; Kestenbaum, B.R.; Stehman-Breen, C.O. Risk factors for incident stroke among patients with end-stage renal disease. J. Am. Soc. Nephrol. JASN 2003, 14, 2623–2631. [Google Scholar] [CrossRef]
- Sanchez-Perales, C.; Vazquez, E.; Garcia-Cortes, M.J.; Borrego, J.; Polaina, M.; Gutierrez, C.P.; Lozano, C.; Liebana, A. Ischaemic stroke in incident dialysis patients. Nephrol. Dial. Transplant. 2010, 25, 3343–3348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Collins, A.J.; Foley, R.N.; Chavers, B.; Gilbertson, D.; Herzog, C.; Johansen, K.; Kasiske, B.; Kutner, N.; Liu, J.; St Peter, W.; et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am. J. Kidney Dis. 2012, 59, A7.e420. [Google Scholar] [CrossRef]
- Power, A. Stroke in dialysis and chronic kidney disease. Blood Purif. 2013, 36, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Goto, S.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report for 2018, JSDT renal data registry: Survey methods, facility data, incidence, prevalence, and mortality. Ren. Replace. Ther. 2020, 6, 41. [Google Scholar] [CrossRef]
- United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2020.
- Burrows, N.R.; Hora, I.; Geiss, L.S.; Gregg, E.W.; Albright, A. Incidence of end-stage renal disease attributed to diabetes among persons with diagnosed diabetes—United States and puerto rico, 2000–2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1165–1170. [Google Scholar] [CrossRef]
- Mineshima, M.; Kawanishi, H.; Ase, T.; Kawasaki, T.; Tomo, T.; Nakamoto, H.; on behalf of the Subcommittee on the Function and Efficacy of Blood Purification Therapy; the Scientific Academic Committee of the Japanese Society for Dialysis Therapy. 2016 update Japanese society for dialysis therapy standard of fluids for hemodialysis and related therapies. Ren. Replace. Ther. 2018, 4, 15. [Google Scholar] [CrossRef]
- Panichi, V.; Rizza, G.M.; Paoletti, S.; Bigazzi, R.; Aloisi, M.; Barsotti, G.; Rindi, P.; Donati, G.; Antonelli, A.; Panicucci, E.; et al. Chronic inflammation and mortality in haemodialysis: Effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol. Dial. Transpl. 2008, 23, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- den Hoedt, C.H.; Bots, M.L.; Grooteman, M.P.; van der Weerd, N.C.; Mazairac, A.H.; Penne, E.L.; Levesque, R.; ter Wee, P.M.; Nubé, M.J.; Blankestijn, P.J.; et al. Online hemodiafiltration reduces systemic inflammation compared to low-flux hemodialysis. Kidney Int. 2014, 86, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Sitter, T.; Bergner, A.; Schiffl, H. Dialysate related cytokine induction and response to recombinant human erythropoietin in haemodialysis patients. Nephrol. Dial. Transpl. 2000, 15, 1207–1211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arizono, K.; Nomura, K.; Motoyama, T.; Matsushita, Y.; Matsuoka, K.; Miyazu, R.; Takeshita, H.; Fukui, H. Use of ultrapure dialysate in reduction of chronic inflammation during hemodialysis. Blood Purif. 2004, 22 (Suppl. 2), 26–29. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Yamagata, K.; Nishi, S.; Hirakata, H.; Hanafusa, N.; Saito, C.; Hattori, M.; Itami, N.; Komatsu, Y.; Kawaguchi, Y.; et al. Japanese society for dialysis therapy clinical guideline for “hemodialysis initiation for maintenance hemodialysis”. Ther. Apher. Dial. 2015, 19 (Suppl. 1), 93–107. [Google Scholar] [CrossRef] [PubMed]
- Eknoyan, G.; Lameire, N.; Eckardt, K.; Kasiske, B.; Wheeler, D.; Levin, A. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]

| All | 1979–2009 | 2010–2014 | 2015–2020 | p Value | |
|---|---|---|---|---|---|
| Number of patients, n | 291 | 119 | 71 | 101 | / |
| Age at initiation of dialysis | |||||
| Mean ± SD | 65.6 ± 14.4 | 59.1 ± 13.9 | 70.4 ± 11.4 | 69.8 ± 14.2 | <0.001 * |
| Range (years) | 21–95 | 21–90 | 43–93 | 33–95 | |
| Follow-up period (years) | |||||
| Mean ± SD | 8.1 ± 7.8 | 14.3 ± 8.5 | 6.3 ± 2.5 | 2.1 ± 1.5 | <0.001 * |
| Range (years) | 0.1–38.3 | 0.3–38.3 | 0.2–10.3 | 0.1–5.1 | |
| Male, n (%) | 187 (64.3%) | 68 (57.1%) | 46 (64.8%) | 73 (72.3%) | 0.065 |
| Underlying diseases, n (%) | |||||
| Hypertension | 283 (97.3%) | 118 (99.2%) | 69 (97.2%) | 96 (95.0%) | 0.178 |
| Diabetes | 177 (60.8%) | 65 (54.6%) | 52 (73.2%) | 60 (59.4%) | 0.037 * |
| Dyslipidemia | 114 (39.2%) | 34 (28.6%) | 28 (39.4%) | 52 (51.5%) | 0.002 * |
| Ischemic heart diseases | 132 (45.4%) | 63 (52.9%) | 38 (53.5%) | 31 (30.7%) | 0.001 * |
| Arteriosclerosis obliterans | 92 (31.6%) | 42 (35.3%) | 28 (39.4%) | 22 (21.8%) | 0.026 * |
| Atrial fibrillation | 31 (10.7%) | 47 (14.3%) | 6 (8.5%) | 8 (7.9%) | 0.252 |
| Smoking, n (%) | 110 (37.8%) | 32 (26.9%) | 26 (36.6%) | 52 (51.5%) | 0.001 * |
| Alcohol, n (%) | 51 (17.5%) | 16 (13.5%) | 12 (16.9%) | 23 (22.8%) | 0.191 |
| Online HDF, n (%) | 69 (23.1%) | 29 (24.4%) | 18 (25.4%) | 22 (21.8%) | 0.842 |
| Antiplatelet therapy, n (%) | 148 (50.9%) | 62 (52.1%) | 40 (56.3%) | 46 (45.5%) | 0.356 |
| Anticoagulant therapy, n (%) | 35 (12.0%) | 21 (17.7%) | 7 (9.9%) | 7 (6.9%) | 0.042 * |
| Primary renal diagnosis of the patients, n (%) | |||||
| Diabetic nephropathy | 116 (39.9%) | 40 (33.6%) | 33 (46.5%) | 43 (42.6%) | 0.001 * |
| Nephrosclerosis | 75 (25.8%) | 22 (18.5%) | 26 (36.6%) | 27 (26.7%) | |
| Glomerulonephritis | 44 (15.1%) | 29 (24.4%) | 6 (8.5%) | 9 (8.9%) | |
| Polycystic kidney disease | 11 (3.8%) | 5 (4.2%) | 2 (2.8%) | 4 (4.0%) | |
| Others | 45 (15.5%) | 23 (19.3%) | 4 (5.6%) | 18 (17.8%) | |
| Laboratory parameters | |||||
| CRP (mg/dL) | |||||
| Mean ± SD | 0.97 ± 3.27 | 0.92 ± 2.93 | 0.59 ± 1.42 | 1.29 ± 4.38 | 0.001 * |
| Range (mg/dL) | 0.00–30.98 | 0.00–26.1 | 0.00–10.1 | 0.00–30.98 | |
| PTH (pg/mL) | |||||
| Mean ± SD | 183 ± 177 | 191 ± 179 | 187 ± 227 | 172 ± 126 | 0.965 |
| Range (pg/mL) | 5–1842 | 6–1260 | 5–1842 | 7–724 | |
| Kt/V | |||||
| Mean ± SD | 1.27 ± 0.33 | 1.30 ± 0.33 | 1.20 ± 0.31 | 1.29 ± 0.33 | 0.061 |
| Range | 0.48–2.21 | 0.48–2.21 | 0.59–1.82 | 0.51–2.16 | |
| URR (%) | |||||
| Mean ± SD | 62.9 ± 10.6 | 63.5 ± 11.3 | 59.6 ± 11.1 | 64.4 ± 8.8 | 0.011 * |
| Range (%) | 26.7–93.9 | 26.7–93.9 | 32.6–77.8 | 36.1–82.5 |
| Period | Patient-Years | All Stroke | Hemorrhagic | Ischemic | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | IR | n (%) | All n (%) | Lacunar n (%) | Atherothrombotic n (%) | Embolism n (%) | ||
| Entire (n = 291) | 2360 | 47 (16.2) | 19.9 | 19 (6.5) | 28 (9.6) | 18 (6.2) | 4 (1.4) | 6 (2.1) |
| Early (n = 119) | 1700 | 26 (21.9) | 15.3 | 12 (10.1) | 14 (11.8) | 11 (9.2) | 2 (1.7) | 1 (0.8) |
| Middle (n = 71) | 450 | 18 (25.4) | 40.0 | 5 (7.0) | 13 (18.3) | 7 (9.9) | 2 (2.8) | 4 (5.6) |
| Late (n = 101) | 210 | 3 (3.0) | 14.3 | 2 (2.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) |
| Bivariate | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age at initiation of HD (ref: 80 years old or over) | ||||
| 20–49 years-old | 0.22 (0.07–0.72) † | 0.012 * | 0.42 (0.10–1.85) † | 0.253 |
| 50–79 years-old | 1.90 (0.91–3.96) † | 0.088 | 0.71 (0.28–1.77) † | 0.464 |
| Hypertension | 1.9 × 108 (0–N/A) | 0.999 | ||
| Diabetes (ref: non-diabetes) | 1.94 (1.04–3.63) | 0.039 * | / | / |
| Dyslipidemia (ref: non-dyslipidemia) | 1.03 (0.56–1.89) | 0.919 | / | / |
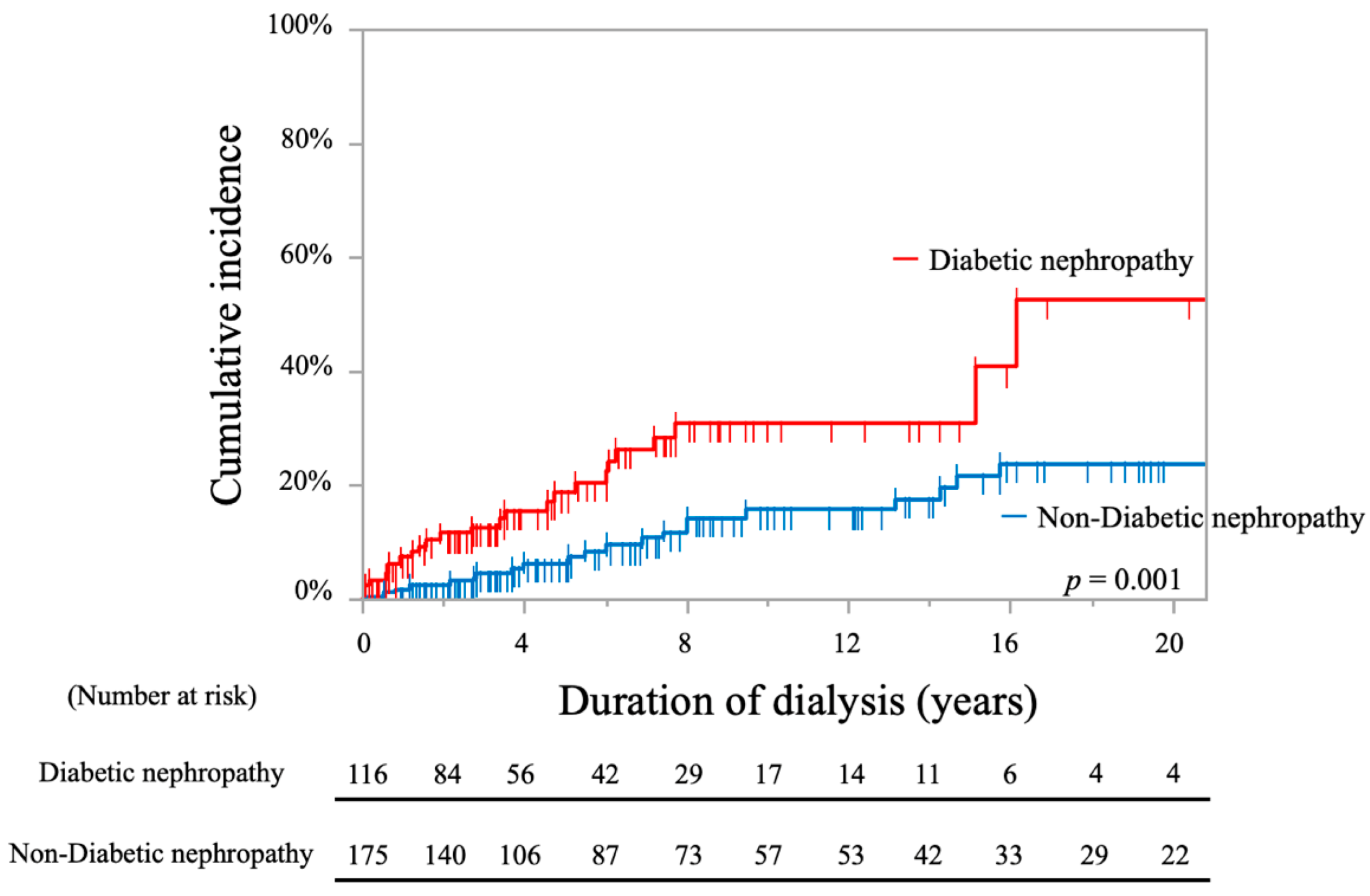
| Diabetic nephropathy (ref: non-diabetic nephropathy) | 2.54 (1.41–4.58) | 0.002 * | 2.24 (1.21–4.12) | 0.001 * |
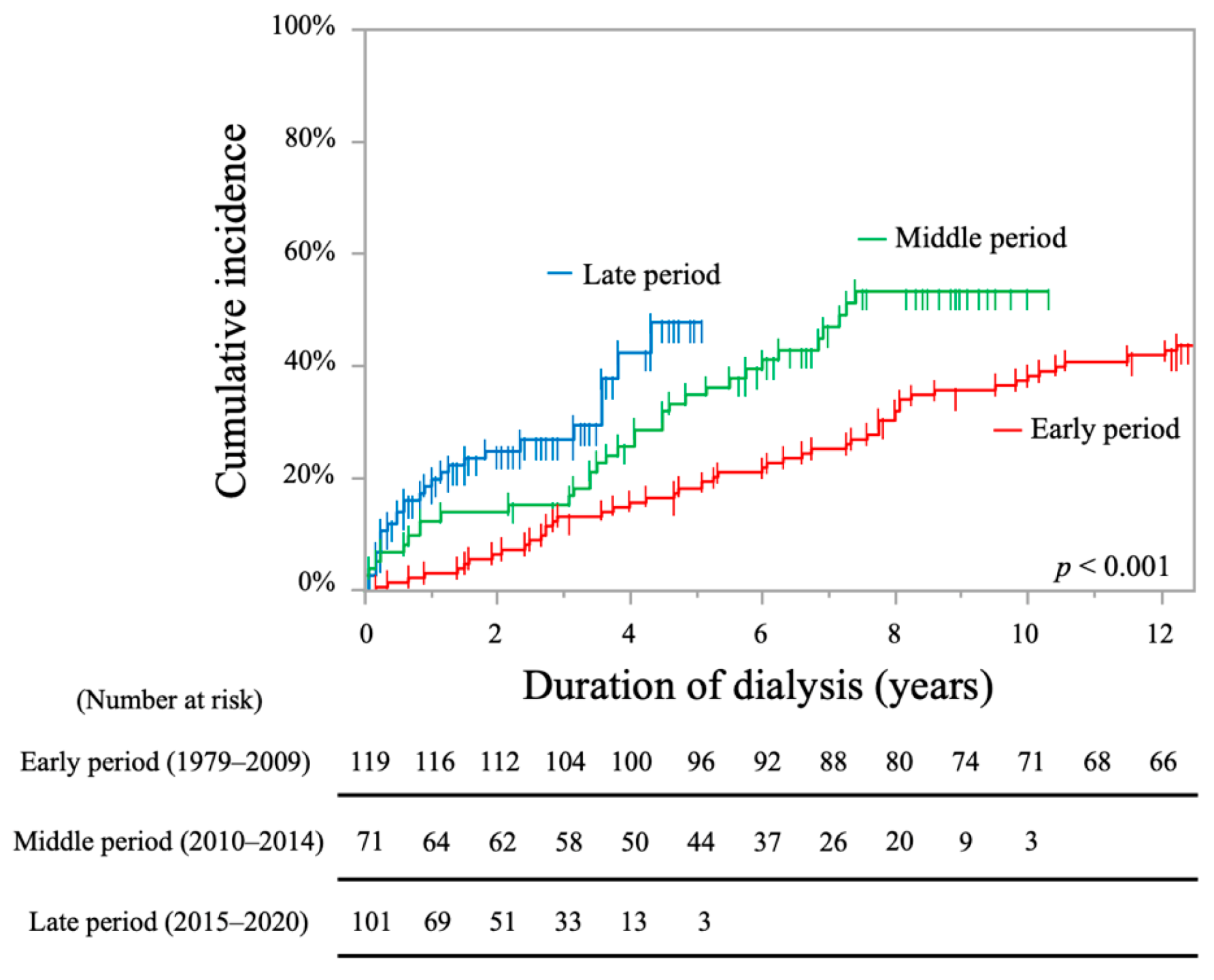
| Period (ref: middle period) | ||||
| Early period | 0.60 (0.31–1.15) ‡ | 0.598 | / | / |
| Late period | 0.42 (0.12–1.43) ‡ | 0.167 | / | / |
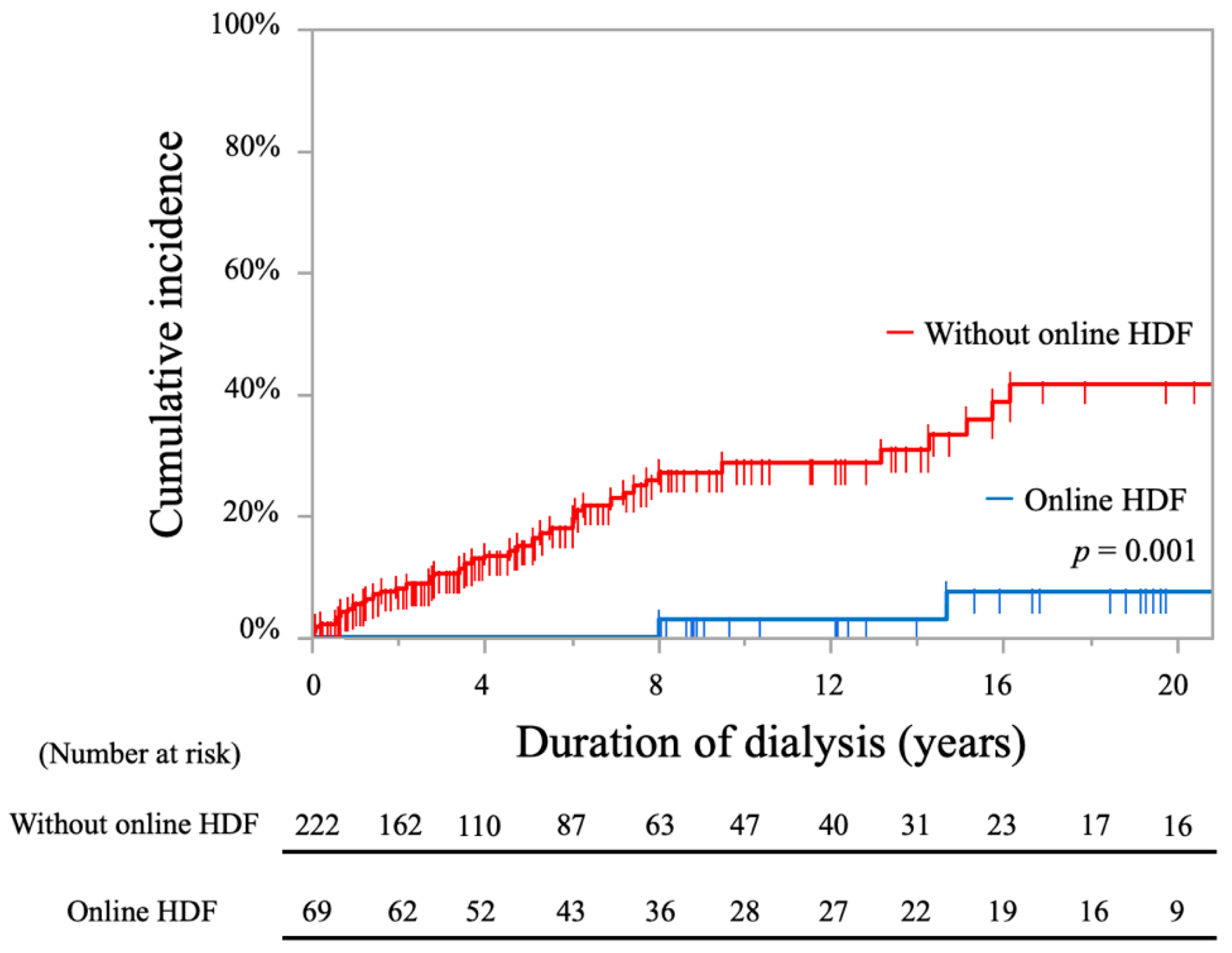
| Online HDF (ref: without online HDF) | 0.09 (0.02–0.39) | <0.001 * | 0.13 (0.03–0.56) | 0.006 * |
| Atrial fibrillation (ref: non-atrial fibrillation) | 1.33 (0.59–2.96) | 0.491 | / | / |
| Laboratory parameters | ||||
| CRP > 0.3 (ref: CRP ≤ 0.3) | 1.62 (0.88–2.98) | 0.122 | / | / |
| PTH > 240 (ref: PTH ≤ 240) | 1.65 (0.89–3.07) | 0.110 | / | / |
| Kt/V < 1.2 (ref: Kt/V ≥ 1.2) | 1.66 (0.93–2.96) | 0.088 | / | / |
| URR < 65 (ref: URR ≥ 65) | 3.47 (1.81–6.68) | <0.001 * | 3.33 (1.73–6.42) | 0.001 * |
| Bivariate | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age at initiation of HD (ref: 80 years old or over) | ||||
| 20–49 years-old | 0.23 (0.05–1.01) † | 0.051 | / | / |
| 50–79 years-old | 2.11 (0.78–5.63) † | 0.137 | / | / |
| Hypertension | 1.9 × 108 (0–N/A) | 0.999 | ||
| Diabetes (ref: non-diabetes) | 1.99 (0.88–4.51) | 0.097 | / | / |
| Dyslipidemia (ref: non-dyslipidemia) | 0.79 (0.35–1.81) | 0.585 | / | / |
| Diabetic nephropathy (ref: non-diabetic nephropathy) | 2.66 (1.23–5.75) | 0.013 * | 2.16 (1.00–4.64) | 0.049 * |
| Period (ref: middle period) | ||||
| Early period | 0.37 (0.15–0.91) ‡ | 0.030 * | 0.31 (0.13–0.75) ‡ | 0.010 * |
| Late period | 0.25 (0.03–1.92) ‡ | 0.248 | 0.16 (0.02–1.29) ‡ | 0.085 |
| Online HDF (ref: without online HDF) | 0.08 (0.01–0.59) | 0.013 * | 0.08 (0.01–0.60) | 0.014 * |
| Atrial fibrillation (ref: non-atrial fibrillation) | 1.71 (0.65–4.51) | 0.279 | / | / |
| Laboratory parameters | ||||
| CRP > 0.3 (ref: CRP ≤ 0.3) | 2.13 (0.99–4.58) | 0.054 | / | / |
| PTH > 240 (ref: PTH ≤ 240) | 1.57 (0.71–3.49) | 0.269 | / | / |
| Kt/V < 1.2 (ref: Kt/V ≥ 1.2) | 2.00 (0.94–4.27) | 0.073 | / | / |
| URR < 65 (ref: URR ≥ 65) | 2.42 (1.09–5.37) | 0.030 * | 2.00 (0.88–4.52) | 0.096 |
| Bivariate | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age at initiation of HD (ref: 80 years old or over) | ||||
| 20–49 years-old | 0.12 (0.05–0.29) † | <0.001 * | 0.13 (0.05–0.35) † | <0.001 * |
| 50–79 years-old | 1.11 (0.76–1.63) † | 0.580 | 0.42 (0.26–0.66) † | 0.001 * |
| Hypertension | 0.42 (0.18–0.96) | 0.039 * | ||
| Diabetes (ref: non-diabetes) | 1.82 (1.26–2.63) | 0.001 * | / | / |
| Dyslipidemia (ref: non-dyslipidemia) | 0.91 (0.63–1.32) | 0.622 | / | / |
| Diabetic nephropathy (ref: non-diabetic nephropathy) | 1.67 (1.17–2.38) | 0.005 * | 1.56 (1.08–2.27) | 0.019 * |
| Period (ref: middle period) | ||||
| Early period | 0.46 (0.31–0.69) ‡ | <0.001 * | 0.69 (0.43–1.10) ‡ | 0.125 |
| Late period | 2.41 (1.48–3.93) ‡ | <0.001 * | 1.84 (1.06–3.21) ‡ | 0.031 * |
| Online HDF (ref: without online HDF) | 0.12 (0.06–0.26) | <0.001 * | 0.20 (0.09–0.44) | <0.001 * |
| Atrial fibrillation (ref: non-atrial fibrillation) | 1.27 (0.78–2.06) | 0.341 | / | / |
| Laboratory parameters | ||||
| CRP > 0.3 (ref: CRP ≤ 0.3) | 2.55 (1.80–3.62) | <0.001 * | 1.93 (1.35–2.76) | 0.001 * |
| PTH > 240 (ref: PTH ≤ 240) | 0.85 (0.55–1.30) | 0.451 | / | / |
| Kt/V < 1.2 (ref: Kt/V ≥ 1.2) | 1.97 (1.39–2.79) | <0.001 * | / | / |
| URR < 65 (ref: URR ≥ 65) | 2.61 (1.80–3.78) | <0.001 * | 2.29 (1.57–3.32) | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aono, T.; Shinya, Y.; Miyawaki, S.; Sugiyama, T.; Kumagai, I.; Takenobu, A.; Shin, M.; Saito, N.; Teraoka, A. Changes in the Risk of Stroke in Dialysis Patients: A Retrospective Analysis over the Last 40 Years. Toxins 2021, 13, 350. https://doi.org/10.3390/toxins13050350
Aono T, Shinya Y, Miyawaki S, Sugiyama T, Kumagai I, Takenobu A, Shin M, Saito N, Teraoka A. Changes in the Risk of Stroke in Dialysis Patients: A Retrospective Analysis over the Last 40 Years. Toxins. 2021; 13(5):350. https://doi.org/10.3390/toxins13050350
Chicago/Turabian StyleAono, Toshiya, Yuki Shinya, Satoru Miyawaki, Takehiro Sugiyama, Isao Kumagai, Atsumi Takenobu, Masahiro Shin, Nobuhito Saito, and Akira Teraoka. 2021. "Changes in the Risk of Stroke in Dialysis Patients: A Retrospective Analysis over the Last 40 Years" Toxins 13, no. 5: 350. https://doi.org/10.3390/toxins13050350
APA StyleAono, T., Shinya, Y., Miyawaki, S., Sugiyama, T., Kumagai, I., Takenobu, A., Shin, M., Saito, N., & Teraoka, A. (2021). Changes in the Risk of Stroke in Dialysis Patients: A Retrospective Analysis over the Last 40 Years. Toxins, 13(5), 350. https://doi.org/10.3390/toxins13050350





