Baseline Susceptibility and Laboratory Selection of Resistance to Bt Cry1Ab Protein of Chinese Populations of Yellow Peach Moth, Conogethes punctiferalis (Guenée)
Abstract
1. Introduction
2. Results
2.1. Baseline Susceptibility of YPM to Bt Cry1Ab Protein
2.2. YPM Larval Growth Inhibition to Bt Cry1Ab Protein
2.3. Development of Diagnostic Concentration
2.4. Laboratory Selection
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Insects Collection
5.2. Laboratory Rearing of Field-Collected YPM
5.3. Baseline Susceptibility Bioassay
5.4. Establishment of Diagnostic Concentrations of Bt Cry1Ab Protein
5.5. Laboratory Selection
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aronson, A.I.; Beckman, W.; Dunn, P. Bacillus thuringiensis and related insect pathogens. Microbiol. Rev. 1986, 50, 1–24. [Google Scholar] [CrossRef]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. A structure-based nomenclature for Bacillus thuringiensis and other bacteria-derived pesticidal proteins. J. Invertebr. Pathol. 2020, 10, 107438. [Google Scholar] [CrossRef] [PubMed]
- Ferry, N.; Edwards, M.G.; Gatehouse, J.; Capell, T.; Christou, P.; Gatehouse, A.M.R. Transgenic plants for insect pest control: A forward looking scientific perspective. Transgenic Res. 2006, 15, 13–19. [Google Scholar] [CrossRef]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef] [PubMed]
- van Frankenhuyzen, K. Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 2009, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2018: Biotech Crops Continue to Help Meet the Challenges of Increased Population and Climate Change; Brief No. 54; ISAAA: Ithaca, NY, USA, 2018. [Google Scholar]
- Dively, G.P.; Venugopal, P.D.; Bean, D.; Whalen, J.; Holmstrom, K.; Kuhar, T.P.; Doughty, H.B.; Patton, T.; Cissel, W.; Hutchison, W.D. Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc. Natl. Acad. Sci. USA 2018, 115, 3320–3325. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, W.D.; Burkness, E.C.; Mitchell, P.D.; Moon, R.D.; Leslie, T.W.; Fleischer, S.J.; Abrahamson, M.; Hamilton, K.L.; Steffey, K.L.; Gray, M.E.; et al. Areawide suppression of european corn borer with Bt maize reaps savings to non-Bt maize growers. Science 2010, 330, 222–225. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, K.; Jiang, Y.; Guo, Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nat. Cell Biol. 2012, 487, 362–365. [Google Scholar] [CrossRef]
- Pardo-López, L.; Soberón, M.; Bravo, A. Bacillus thuringiensisinsecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef]
- Brookes, G.; Barfoot, P. Global Impact of Biotech Crops: Socio-Economic and Environmental Effects in the First Ten Years of Commercial Use. AgBioForum 2006, 9, 139–151. Available online: https://ssrn.com/abstract=964982 (accessed on 19 January 2021).
- Crespo, A.L.; Spencer, T.A.; Alves, A.P.; Hellmich, R.L.; Blankenship, E.E.; Magalhães, L.C.; Siegfried, B.D. On-plant survival and inheritance of resistance to Cry1Ab toxin from Bacillus thuringiensis in a field-derived strain of European corn borer, Ostrinia nubilalis. Pest Manag. Sci. 2009, 65, 1071–1081. [Google Scholar] [CrossRef]
- Dhurua, S.; Gujar, G.T. Field-evolved resistance to Bt toxin Cry1Ac in the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), from India. Pest Manag. Sci. 2011, 67, 898–903. [Google Scholar] [CrossRef]
- He, K.; Wang, Z.; Zhou, D.; Wen, L.; Song, Y.; Yao, Z. Evaluation of transgenic Bt corn for resistance to the asian corn borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 2003, 96, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, Z.; Bravo, A.; Soberón, M.; He, K. Genetic basis of Cry1F-resistance in a laboratory selected asian corn borer strain and its cross-resistance to other Bacillus thuringiensis toxins. PLoS ONE 2016, 11, e0161189. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Gassmann, A.J.; Crowder, D.W.; Carriére, Y. Insect resistance to Bt crops: Evidence versus theory. Nat. Biotechnol. 2008, 26, 199–202. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Huang, F.; Ghimire, M.N.; Leonard, B.R.; Siegfried, B.D.; Rangasamy, M.; Yang, Y.; Wu, Y.; Gahan, L.J.; Heckel, D.G.; et al. Efficacy of genetically modified Bt toxins against insects with different genetic mechanisms of resistance. Nat. Biotechnol. 2011, 29, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.Z.; Quan, Y.; Wang, Z.; Bravo, A.; Soberón, M.; He, K. Characterization of the Cry1Ah resistance in Asian corn Borer and its cross-resistance to other Bacillus thuringiensis toxins. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Monnerat, R.; Martins, E.; Macedo, C.; Queiroz, P.; Praça, L.; Soares, C.M.; Moreira, H.; Grisi, I.; Silva, J.; Soberon, M.; et al. Evidence of field-evolved resistance of Spodoptera frugiperda to Bt corn expressing Cry1F in Brazil that is still sensitive to modified Bt toxins. PLoS ONE 2015, 10, e0119544. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, H.M.T.; Wearing, C.H. The safe and rational deployment ofBacillus thuringiensisgenes in crop plants: Conclusions and recommendations of OECD workshop on ecological implications of transgenic crops containing Bt toxin genes. Biocontrol Sci. Technol. 1994, 4, 399–404. [Google Scholar] [CrossRef]
- Dennehy, T.J. Decision-making for managing pest resistance to pesticides. In Combating Resistance to Xenobiotics: Biological and Chemical Approaches; Ford, M.G., Holloman, D.W., Khambay, B.P.S., Sawicki, R.M., Eds.; Ellis Horwood: Chichester, UK, 1987; pp. 118–126. [Google Scholar]
- Wu, Y. Detection and mechanisms of resistance evolved in insects to Cry toxins from Bacillus thuringiensis. Adv. Insect. Physiol. 2014, 47, 297–342. [Google Scholar] [CrossRef]
- Marçon, P.C.R.G.; Siegfried, B.D.; Spencer, T.; Hutchison, W.D. Development of diagnostic concentrations for monitoring Bacillus thuringiensis resistance in European corn borer (Lepidoptera: Crambidae). J. Econ. Entomol. 2000, 93, 925–930. [Google Scholar] [CrossRef]
- Roush, R.T. Resistance detection and documentation: The relative roles of pesticidal and biochemical assays. In Pesticide Resistance in Arthropods; Springer: Boston, MA, USA, 1990; pp. 4–38. [Google Scholar]
- He, K.; Wang, Z.; Wen, L.; Bai, S.; Ma, X.; Yao, Z. Determination of baseline susceptibility to Cry1Ab protein for Asian corn borer (Lep., Crambidae). J. Appl. Entomol. 2005, 129, 407–412. [Google Scholar] [CrossRef]
- Li, S.K.; Wang, C.T. Potential and Ways to High Yield in Maize; Science Press: Beijing, China, 2010. [Google Scholar]
- Cock, M.; Tang, R.; Liu, Z.; Wan, H.; McGillivray, L.; Thomas, S.; Cameron, K.; Zhang, F. The main agricultural insect and disease pests of China and implications for the use of remote sensing for their management. CAB Rev. 2016, 11, 14. [Google Scholar] [CrossRef]
- Jing, D.; Guo, J.; Jiang, Y.; Zhao, J.; Sethi, A.; He, K.; Wang, Z. Initial detections and spread of invasive Spodoptera frugiperda in China and comparisons with other noctuid larvae in cornfields using molecular techniques. Insect Sci. 2020, 27, 780–790. [Google Scholar] [CrossRef]
- Wang, Z.; He, K.; Wen, L.; Zhang, G.; Zheng, L. Spatial-temporal distributions and cotton bollworm eggs on summer corn seeded at different times in North China. Sci. Agric. Sin. 2001, 34, 153–156. [Google Scholar]
- Wang, Z.Y.; Wang, X.M. Current status and management strategies for corn pests ans diseases in China. Plant Prot. 2019, 45, 1–11. [Google Scholar]
- Wei, H. A key insect pest of sunflower-yellow peach moth. Chin. Bull. Entomol. 1956, 2, 78. [Google Scholar]
- Wu, J.; Zhu, Y.; Zhu, G.; Zhu, C. Occurrence and control of yellow peach moth on chestnut. Hebei Fruits 1999, 4, 17–18. [Google Scholar]
- Wang, Y.H.; Pan, X.X.; Cheng, K.L.; Huang, F.; Wang, Z.M.; Liu, M.X.; Liu, X.Y.; Zhang, C.W. Community composition and distribution of insect pests on sorghum panicles in Sichuan. J. Southwest Agric. Univ. 1991, 13, 569–574. [Google Scholar]
- CABI. Conogethes punctiferalis datasheet. In Crop Protection Compendium; CAB International: Wallingford, UK, 2011. [Google Scholar]
- Lu, J.Q.; Zhenying, W.; Kanglai, H.; Yong, L. Research history, progresses and prospects in the yellow peach moth, Conogethes punctiferalis. Plant Prot. 2010, 36, 31–38. [Google Scholar]
- Wang, Z.Y.; He, K.L.; Shi, J.; Ma, S.Y. Analysis of the heavily occurrence trend of the yellow peach borer in corn and management strategy for the insect pest. Plant Prot. 2006, 32, 67–69. [Google Scholar]
- Yang, S.; Shi, J.; Zhang, H.; Guo, N.; Li, P.; Wang, Z. Impact of durian fruit borer Conogethes punctiferalis on yield loss of summer corn by injuring corn ears. J. Plant Prot. 2015, 42, 991–996. [Google Scholar]
- Sekiguchi, K. Morphology, biology and control of the yellow peach moth Dichocrocis punctiferalis Guenee (Lepidoptera, Pyraustidae). Bull. Ibaraki Ken Hortic. Exp. Stn. 1974, 1–90. [Google Scholar]
- Marulasiddesha, K.; Sankar, M.; Gouda, G.R. Short communication. Screening of sorghum genotypes for resistance to damage caused by the stem borer Chilo partellus (Swinhoe). Span. J. Agric. Res. 2007, 5, 79. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Alcantara, E.; Estrada, A.; Alpuerto, V.; Head, G. Monitoring Cry1Ab susceptibility in Asian corn borer (Lepidoptera: Crambidae) on Bt corn in the Philippines. Crop Prot. 2011, 30, 554–559. [Google Scholar] [CrossRef]
- Le, D.K.; Le, Q.K.; Tran, T.T.H.; Nguyen, D.V.; Dao, T.H.; Nguyen, T.T.; Truong, X.L.; Nguyen, Q.C.; Pham, H.P.; Phan, T.T.T.; et al. Baseline susceptibility of Asian corn borer (Ostrinia furnacalis (Guenée)) populations in Vietnam to Cry1Ab insecticidal protein. J. Asia-Pac. Entomol. 2019, 22, 493–498. [Google Scholar] [CrossRef]
- Marçon, P.C.R.G.; Young, L.J.; Steffey, K.L.; Siegfried, B.D. Baseline susceptibility of European corn borer (Lepidoptera: Crambidae) to Bacillus thuringiensis toxins. J. Econ. Entomol. 1999, 92, 279–285. [Google Scholar] [CrossRef]
- Shwe, S.M.; Wang, Y.; Gao, Z.; Li, X.; Liu, S.; Bai, S.; Zhang, T.; He, K.; Wang, Z. Toxicity of Cry1-Class, Cry2Aa, and Vip3Aa19 Bt proteins and their interactions against yellow peach Moth, Conogethes punctiferalis (Guenée) (Lepidoptera: Crambidae). J. Invertebr. Pathol. 2021, 178, 107507. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wang, Z.; He, K. Genetic diversity of Conogethes punctiferalis (Guenée)(Lepidoptera: Crambidae) populations from different geographic regions in China. Acta Entomol. Sin. 2010, 53, 1022–1029. [Google Scholar]
- Boom, C.E.M.; Beek, T.A.; Dicke, M. Differences among plant species in acceptance by the spider mite Tetranychus urticae Koch. J. Appl. Entomol. 2003, 127, 177–183. [Google Scholar] [CrossRef]
- Siegfried, B.D.; Spencer, T.; Crespo, A.L.; Storer, N.P.; Head, G.P.; Owens, E.D.; Guyer, D. Ten years of Bt resistance monitoring in the European corn borer: What we know, what we don’t know, and what we can do better. Am. Entomol. 2007, 53, 208–214. [Google Scholar] [CrossRef]
- Li, J.; Coates, B.S.; Kim, K.S.; Bourguet, D.; Ponsard, S.; He, K.; Wang, Z. The genetic structure of Asian corn borer, Ostrinia furnacalis, populations in China: Haplotype variance in northern populations and potential impact on management of resistance to transgenic maize. J. Hered. 2014, 105, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Leite, N.A.; Pereira, R.M.; Durigan, M.R.; Amado, D.; Fatoretto, J.; Medeiros, F.C.L.; Omoto, C. Susceptibility of Brazilian Populations of Helicoverpa armigera and Helicoverpa zea (Lepidoptera: Noctuidae) to Vip3Aa20. J. Econ. Entomol. 2017, 111, 399–404. [Google Scholar] [CrossRef] [PubMed]
- González-Núñez, M.; Ortego, F.; Castañera, P. Susceptibility of Spanish populations of the corn borers Sesamia nonagrioides(Lepidoptera: Noctuidae) andOstrinia nubilalis(Lepidoptera: Crambidae) to a Bacillus thuringiensis Endotoxin. J. Econ. Entomol. 2000, 93, 459–463. [Google Scholar] [CrossRef]
- Stone, T.B.; Sims, S.R. Geographic susceptibility of Heliothis virescens and Helicoverpa zea (Lepidoptera: Noctuidae) to Bacillus thuringiensis. J. Econ. Entomol. 1993, 86, 989–994. [Google Scholar] [CrossRef]
- Wu, K.; Guo, Y.; Lv, N. Geographic variation in susceptibility of Helicoverpa armigera (Lepidoptera: Noctuidae) to Bacillus thuringiensis insecticidal protein in China. J. Econ. Entomol. 1999, 92, 273–278. [Google Scholar] [CrossRef]
- Sims, S.B.; Greenplate, J.T.; Stone, T.B.; Caprio, M.A.; Gould, F.L. Monitoring strategies for early detection of lepidoptera resistance to Bacillus thuringiensis insecticidal proteins. In Molecular Genetics and Evolution of Pesticide Resistance; ACS Publications: Washington, DC, USA, 1996; pp. 229–242. [Google Scholar]
- Gould, F.; Martínez-Ramírez, A.; Anderson, A.; Ferré, J.; Silva, F.J.; Moar, W.J. Broad-spectrum resistance to Bacillus thuringiensis toxins in Heliothis virescens. Proc. Natl. Acad. Sci. USA 1992, 89, 7986–7990. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Mandaokar, A.; Kumar, P.; Sharma, R. Efficacy of lepidopteran specific δ-endotoxins of Bacillus thuringiensis against Helicoverpa armigera. J. Invertebr. Pathol. 1998, 72, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Gujar, G.; Kumari, A.; Kalia, V.; Chandrashekar, K. Spatial and Temporal Variation in Susceptibility of the American Bollworm, Helicoverpa armigera (Hübner) to Bacillus thuringiensis var. kurstaki in India. Curr. Sci. 2000, 78, 995–1001. Available online: http://www.jstor.org/stable/24103737 (accessed on 7 December 2020).
- Kranthi, K.; Kranthi, S.; Ali, S.; Banerjee, S. Resistance to CrylAc δ-Endotoxin of Bacillus thuringiensis’ in a Laboratory Selected Strain of Helicoverpa armigera (Hubner). Curr. Sci. 2000, 78, 1001–1004. Available online: https://www.jstor.org/stable/24103738 (accessed on 16 February 2021).
- Roush, R.T.; Miller, G.L. Considerations for design of insecticide resistance monitoring programs. J. Econ. Entomol. 1986, 79, 293–298. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Ji, T.; Tian, C.; Zhao, X.; Feng, H. Baseline susceptibility and resistance allele frequency in Ostrinia furnacalis related to Cry1 toxins in the Huanghuaihai summer corn region of China. Pest Manag. Sci. 2020, 76, 4311–4317. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Z.; Zhang, J.; He, K.; Ferry, N.; Gatehouse, A.M.R. Cross-resistance of Cry1Ab-selected Asian corn borer to other Cry toxins. J. Appl. Entomol. 2010, 134, 429–438. [Google Scholar] [CrossRef]
- Han, H.; Li, G.; Wang, Z.; Zhang, J.; He, K. Cross-resistance of Cry1 Ac-selected Asian corn borer to other Bt toxins. Acta Phytophyl. Sin. 2009, 36, 329–334. [Google Scholar] [CrossRef]
- He, M.; He, K.; Wang, Z.; Wang, X.; Li, Q. Selection for Cry1Ie resistance and cross-resistance of the selected strain to other Cry toxins in the Asian corn borer, Ostrinia furnacalis (Lepidoptera: Crambidae). Acta Entomol. Sin. 2013, 56, 1135–1142. [Google Scholar]
- González-Cabrera, J.; García, M.; Hernández-Crespo, P.; Farinós, G.P.; Ortego, F.; Castañera, P. Resistance to Bt maize in Mythimna unipuncta (Lepidoptera: Noctuidae) is mediated by alteration in Cry1Ab protein activation. Insect Biochem. Mol. Biol. 2013, 43, 635–643. [Google Scholar] [CrossRef]
- Omoto, C.; Bernardi, O.; Salmeron, E.; Sorgatto, R.J.; Dourado, P.M.; Crivellari, A.; Carvalho, R.A.; Willse, A.; Martinelli, S.; Head, G.P. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manag. Sci. 2016, 72, 1727–1736. [Google Scholar] [CrossRef]
- Jing, D.; Zhang, T.; Bai, S.; He, K.; Prabu, S.; Wang, Z. Artificial diet development for mass rearing and its effect on the reproduction of yellow peach moth, Conogethes punctiferalis (Guenée). Entomol. Res. 2021, 51, 127–132. [Google Scholar] [CrossRef]
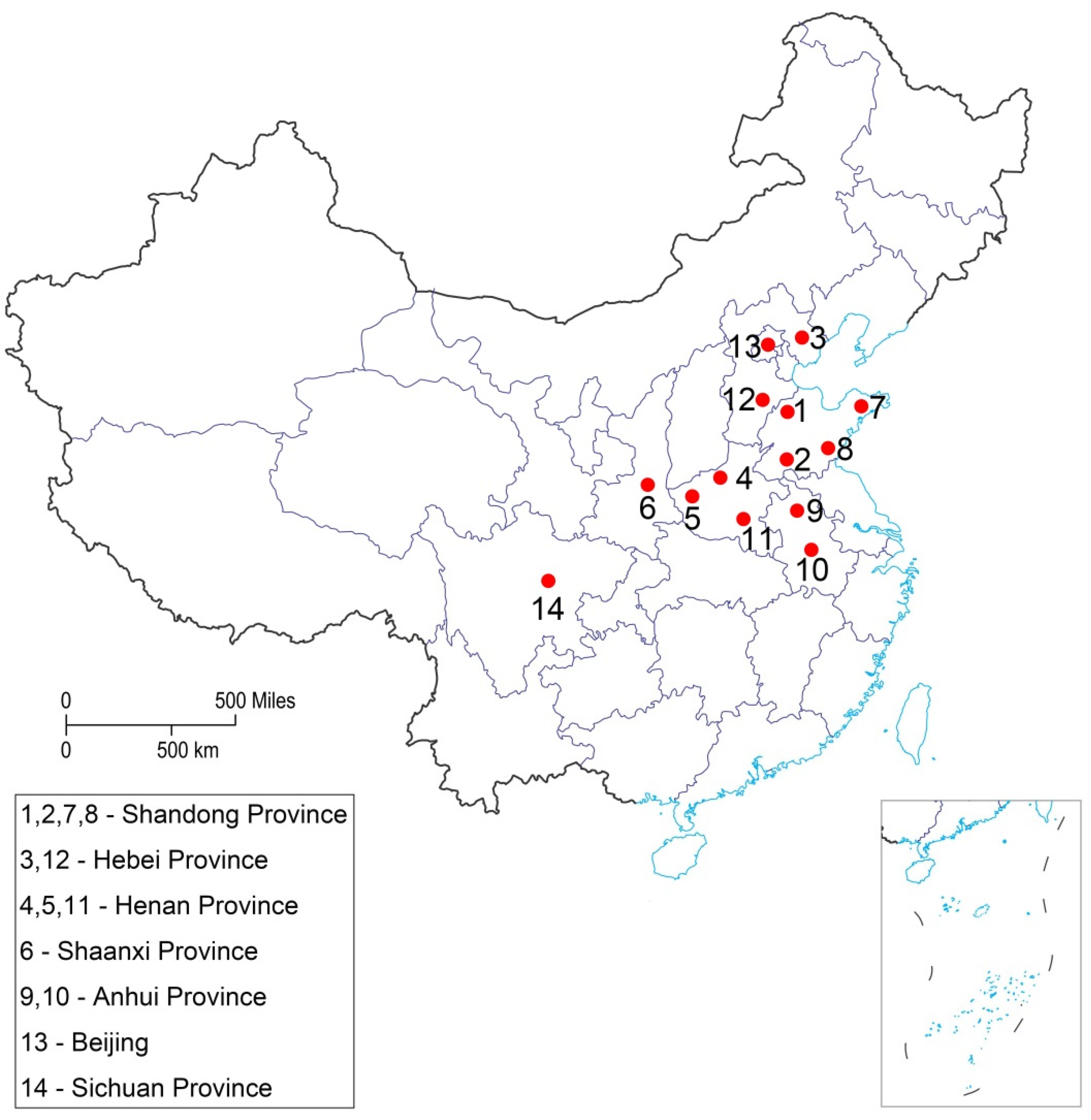
| Province | Location | Coordinates | Collected Month | Number of Collected Larvae |
|---|---|---|---|---|
| Shandong | Dezhou (1) | 37°26′10″ N; 116°21′32″ E | August 2019 | 250 |
| Shandong | Jining (2) | 35°24′54″ N; 116°35′14″ E | August 2019 | 350 |
| Hebei | Tangshan (3) | 39°37′52″ N; 118°10′48″ E | August 2019 | 350 |
| Henan | Luoyang (4) | 34°37′11″ N; 112°27′14″ E | September 2019 | 300 |
| Henan | Jiyuan (5) | 35°04′01″ N; 112°36′07″ E | September 2019 | 200 |
| Shaanxi | Xi’an (6) | 35°04′01″ N; 112°36′07″ E | September 2019 | 300 |
| Shandong | Yantai (7) | 37°28′35″ N; 121°26′26″ E | September 2020 | 340 |
| Shandong | Linyi (8) | 35°6′16.82″ N; 118°21′23.21″ E | September 2020 | 270 |
| Anhui | Suzhou (9) | 33°38′46.89″ N; 116°57′51.69″ E | September 2020 | 230 |
| Anhui | Hefei (10) | 31°51′50″ N; 117°16′50″ E | September 2020 | 220 |
| Henan | Luohe (11) | 33°34′53.09″ N; 114°0′59.54″ E | September 2020 | 320 |
| Hebei | Handan (12) | 36°37′32.37″ N; 114°32′20.26″ E | September 2020 | 330 |
| Beijing | Shunyi (13) | 40°7′49.25″ N; 116°39′16.74″ E | September 2020 | 340 |
| Sichuan | Chengdu (14) | 30°39′25.2000″ N; 104°3′57.6072″ E | October 2020 | 65 |
| Year | Province | Location | g | n | Slope ± SE | LC50 (95% FL) ng/cm2 * | LC99 (95% FL) ng/cm2 * | x2 |
|---|---|---|---|---|---|---|---|---|
| 2019 | Shandong | Dezhou (1) | F1 | 576 | 0.98 ±.071 | 1.48 (1.05–2.10) bc | 350.61 (160.66–967.20) b | 7.80 |
| Shandong | Jining (2) | F1 | 576 | 1.32 ± 0.10 | 1.04 (0.78–1.40) bc | 59.42 (32.67–129.88) ab | 8.32 | |
| Hebei | Tangshan (3) | F1 | 576 | 1.12 ± 0.12 | 2.38 (1.52–3.49) c | 282.32 (125.59–951.33) b | 8.38 | |
| Henan | Luoyang (4) | F1 | 576 | 0.93 ± 0.07 | 0.55 (0.39–0.79) ab | 178.28 (79.98–506.09) ab | 8.96 | |
| Henan | Jiyuan (5) | F2 | 576 | 1.03 ± 0.07 | 0.65 (0.47–0.91) ab | 117.92 (56.96–302.38) ab | 9.44 | |
| Shaanxi | Xi’an (6) | F1 | 576 | 1.06 ± 0.08 | 0.88 (0.64–1.22) b | 136.95 (67.55–340.90) ab | 9.48 | |
| 2020 | Shandong | Yantai (7) | F1 | 576 | 1.16 ± 0.14 | 1.76 (1.05–2.64) bc | 181.37 (80.55–654.10) ab | 9.46 |
| Shandong | Linyi (8) | F1 | 576 | 1.02 ± 0.07 | 1.72 (1.23–2.42) c | 321.31 (150.70–860.99) b | 14.37 | |
| Hebei | Handan (9) | F1 | 576 | 1.19 ± 0.12 | 2.19 (1.50–3.10) c | 199.27 (96.07–563.99) ab | 9.83 | |
| Henan | Luohe (10) | F1 | 576 | 0.97 ± 0.07 | 0.35 (0.25–0.48) a | 87.94 (40.98–237.27) ab | 7.40 | |
| Anhui | Suzhou (11) | F1 | 576 | 1.83 ± 0.28 | 2.14 (1.38–2.96) c | 40.13 (21.97–114.96) a | 6.06 | |
| Anhui | Hefei (12) | F1 | 576 | 1.16 ± 0.09 | 1.60 (1.18–2.20) bc | 163.83 (83.12–397.19) ab | 11.74 | |
| Beijing | Shunyi (13) | F1 | 576 | 0.93 ± 0.07 | 1.32 (0.93–1.88) bc | 409.65 (182.33–1171.81) b | 7.03 | |
| Sichuan | Chengdu (14) | F3 | 576 | 1.20 ± 0.13 | 0.97 (0.61–1.42) bc | 84.63 (41.05–247.33) ab | 5.56 | |
| Population pooled from 2019 collection | F2 | 576 | 1.39 ± 0.11 | 0.92 (0.70–1.22) b | 43.33 (24.28–92.73) a | 9.50 | ||
| Laboratory strain | F19 | 576 | 0.93 ± 0.07 | 1.08 (0.76–1.55) bc | 349.77 (154.29–1012.84) b | 10.46 | ||
| Cry1Ab (ng/cm2) | Larval Growth Inhibition (%) | Mean ± SEM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dezhou | Jining | Yantai | Linyi | Tang-shan | Handan | Luo-yang | Jiyuan | Luohe | Xi’an | Suzhou | Hefei | Shunyi | Cheng-du | ||
| 0.004 | 51.17 | 52.26 | 51.52 | 52.41 | 54.02 | 54.35 | 54.52 | 58.89 | 54.49 | 57.91 | 52.29 | 52.21 | 51.69 | 55.94 | 53.83 ± 0.64 |
| 0.02 | 57.00 | 61.45 | 57.86 | 58.47 | 63.44 | 61.97 | 61.13 | 62.19 | 61.60 | 59.85 | 55.92 | 54.47 | 57.90 | 59.86 | 59.51 ± 0.71 |
| 0.11 | 61.68 | 66.23 | 61.85 | 63.23 | 69.73 | 66.47 | 70.67 | 70.59 | 70.45 | 70.67 | 63.12 | 63.60 | 60.46 | 62.96 | 65.84 ± 1.04 |
| 0.55 | 76.10 | 70.00 | 76.96 | 75.70 | 74.11 | 73.79 | 74.59 | 72.62 | 74.90 | 71.23 | 75.61 | 75.66 | 79.10 | 77.42 | 74.84 ± 0.65 |
| 2.74 | 85.01 | 90.83 | 86.21 | 85.12 | 93.89 | 94.05 | 88.98 | 88.79 | 92.85 | 92.19 | 83.35 | 83.43 | 86.21 | 91.36 | 88.73 ± 1.03 |
| 13.68 | 99.78 | 99.98 | 99.79 | 99.76 | 99.84 | 99.92 | 99.91 | 99.67 | 99.91 | 99.85 | 99.78 | 99.80 | 99.70 | 99.83 | 99.82 ± 0.02 |
| 68.42 | 99.99 | 100.00 | 100.00 | 100.00 | 99.99 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 99.99 | 100.00 | 100.00 ± 0.00 |
| Location | n | Mortality % | ||
|---|---|---|---|---|
| 24.28 ng/cm2 * | 43.33 ng/cm2 * | 92.73 ng/cm2 * | ||
| Yantai | 240 | 80.83a | 93.33a | 99.17a |
| Linyi | 240 | 81.67a | 94.58a | 99.58a |
| Handan | 240 | 82.50a | 95.00a | 99.58a |
| Luohe | 240 | 82.92a | 95.42a | 100.00a |
| Suzhou | 240 | 81.25a | 94.17a | 99.17a |
| Hefei | 240 | 81.25a | 93.33a | 100.00a |
| Shunyi | 240 | 82.08a | 95.42a | 100.00a |
| Chengdu | 240 | 81.67a | 95.00a | 100.00a |
| Generation | Concentration ng/cm2 | LC50 (95% FL) ng/cm2 | LC90 (95% FL) ng/cm2 | Slope ± SE | RR | x2 |
|---|---|---|---|---|---|---|
| Cry1AbS | 1.08 (0.76–1.55) | 26.07 (15.17–51.59) | 0. 93 ± 0.07 | 10.46 | ||
| F1 | 1.08 | 1.14 (0.82–1.59) | 19.58 (12.07–35.89) | 1.04 ± 0.08 | 1.06 | 7.29 |
| F2 | 1.08 | 1.78 (1.25–2.58) | 45.67 (25.99–93.42) | 0.91 ± 0.07 | 1.65 | 4.50 |
| F3 | 1.08 | 2.44 (1.61–3.55) | 36.51 (22.04–72.45) | 1.09 ± 0.11 | 2.26 | 9.90 |
| F4 | 1.08 | 3.07 (2.233–4.08) | 19.53 (13.42–32.83) | 1.59 ± 0.17 | 2.84 | 7.96 |
| F5 | 1.08 | 3.30 (2.37–4.66) | 56.36 (33.70–108.74) | 1.04 ± 0.08 | 3.06 | 9.76 |
| F6 | 2.05 | 3.57 (2.58–4.87) | 32.64 (21.34–57.60) | 1.33 ± 0.13 | 3.30 | 6.97 |
| F7 | 2.05 | 4.11 (2.94–5.68) | 44.10 (27.95–81.19) | 1.24 ± 0.12 | 3.81 | 6.51 |
| F8 | 2.05 | 5.03 (3.42–7.35) | 87.71 (49.78–191.88) | 1.03 ± 0.10 | 4.67 | 7.05 |
| F9 | 3.12 | 5.14 (3.57–7.61) | 121.85 (66.25–269.96) | 0.93 ± 0.07 | 4.76 | 7.62 |
| F10 | 3.12 | 5.42 (3.80–7.97) | 112.62 (62.34–243.97) | 0.97 ± 0.08 | 5.02 | 9.01 |
| F11 | 4.32 | 5.85 (3.94–8.59) | 100.71 (56.21–230.14) | 1.04 ± 0.11 | 5.42 | 5.91 |
| F12 | 4.32 | 6.02 (4.17–9.01) | 147.30 (78.36–338.77) | 0.92 ± 0.08 | 5.57 | 5.94 |
| F13 | 6.16 | 6.56 (4.04–11.59) | 581.99 (224.53–2131.07) | 0.66 ± 0.06 | 6.08 | 13.19 |
| F14 | 6.16 | 7.67 (5.22–11.78) | 210.74 (106.67–523.77) | 0.89 ± 0.08 | 7.10 | 5.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shwe, S.M.; Prabu, S.; Chen, Y.; Li, Q.; Jing, D.; Bai, S.; He, K.; Wang, Z. Baseline Susceptibility and Laboratory Selection of Resistance to Bt Cry1Ab Protein of Chinese Populations of Yellow Peach Moth, Conogethes punctiferalis (Guenée). Toxins 2021, 13, 335. https://doi.org/10.3390/toxins13050335
Shwe SM, Prabu S, Chen Y, Li Q, Jing D, Bai S, He K, Wang Z. Baseline Susceptibility and Laboratory Selection of Resistance to Bt Cry1Ab Protein of Chinese Populations of Yellow Peach Moth, Conogethes punctiferalis (Guenée). Toxins. 2021; 13(5):335. https://doi.org/10.3390/toxins13050335
Chicago/Turabian StyleShwe, Su Mon, Sivaprasath Prabu, Yu Chen, Qincheng Li, Dapeng Jing, Shuxiong Bai, Kanglai He, and Zhenying Wang. 2021. "Baseline Susceptibility and Laboratory Selection of Resistance to Bt Cry1Ab Protein of Chinese Populations of Yellow Peach Moth, Conogethes punctiferalis (Guenée)" Toxins 13, no. 5: 335. https://doi.org/10.3390/toxins13050335
APA StyleShwe, S. M., Prabu, S., Chen, Y., Li, Q., Jing, D., Bai, S., He, K., & Wang, Z. (2021). Baseline Susceptibility and Laboratory Selection of Resistance to Bt Cry1Ab Protein of Chinese Populations of Yellow Peach Moth, Conogethes punctiferalis (Guenée). Toxins, 13(5), 335. https://doi.org/10.3390/toxins13050335






