Tetrodotoxin for Chemotherapy-Induced Neuropathic Pain: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Dose Finding Trial
Abstract
1. Introduction
2. Results
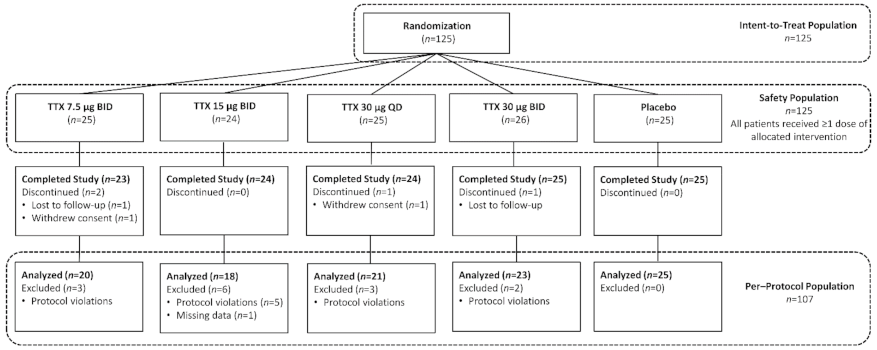
2.1. Patient Demographics
2.2. Planned Outcome Measures
2.2.1. Numeric Pain Rating Scale (NPRS) Score
2.2.2. SF-36, EORTC CIPN20, and PGIC
2.2.3. Time to Peak Pain Relief and Use of Rescue Medication
2.3. Exploratory Outcome Measures
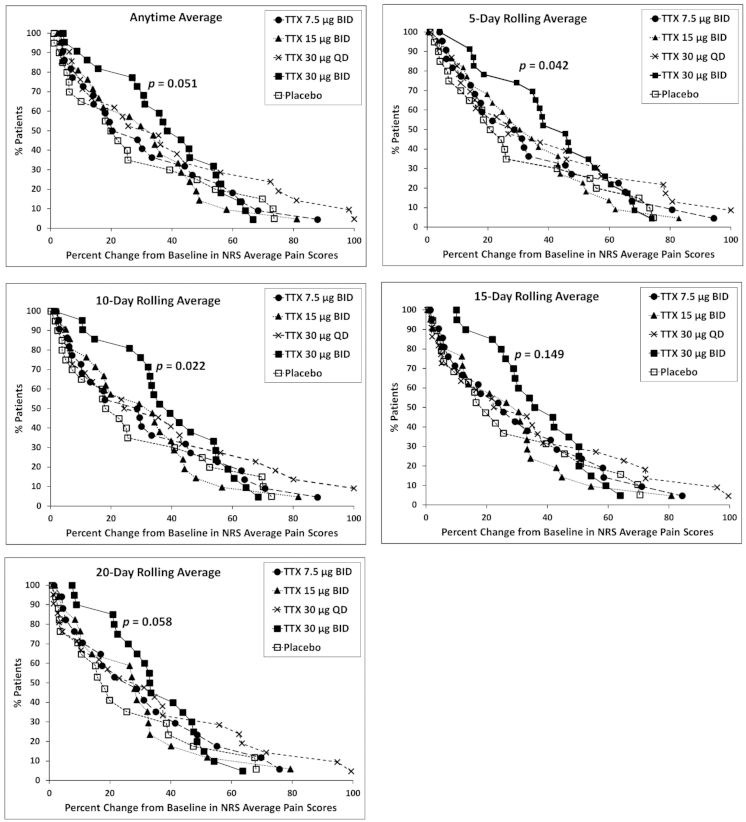
2.3.1. Responder Analysis
2.3.2. High Placebo Responders
2.4. Safety and Tolerability
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Standard Protocol Approvals, Registrations, and Patient Consents
5.2. Participants
5.3. Study Design
5.4. Randomization and Blinding
5.5. Outcome Measures
5.6. Safety Monitoring
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banach, M.; Juranek, J.K.; Zygulska, A.L. Chemotherapy-Induced Neuropathies-a Growing Problem for Patients and Health Care Providers. Brain Behav. 2017, 7, e00558. [Google Scholar] [CrossRef]
- Aromolaran, K.A.; Goldstein, P.A. Ion Channels and Neuronal Hyperexcitability in Chemotherapy-Induced Peripheral Neuropathy: Cause and Effect? Mol. Pain 2017, 13. [Google Scholar] [CrossRef]
- Smith, E.M.L.; Cohen, J.A.; Pett, M.A.; Beck, S.L. The Reliability and Validity of a Modified Total Neuropathy Score-Reduced and Neuropathic Pain Severity Items When Used to Measure Chemotherapy-Induced Peripheral Neuropathy in Patients Receiving Taxanes and Platinums. Cancer Nurs. 2010, 33, 173–183. [Google Scholar] [CrossRef]
- Kautio, A.L.; Haanpää, M.; Kautiainen, H.; Kalso, E.; Saarto, T. Burden of Chemotherapy-Induced Neuropathy-a Cross-Sectional Study. Support. Care Cancer 2011, 19, 1991–1996. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Reeves, B.N.; Dakhil, S.R.; Sloan, J.A.; Wolf, S.L.; Burger, K.N.; Kamal, A.; Le-Lindqwister, N.A.; Soori, G.S.; Jaslowski, A.J.; et al. Natural History of Paclitaxel-Associated Acute Pain Syndrome: Prospective Cohort Study NCCTG N08C1. J. Clin. Oncol. 2011, 29, 1472–1478. [Google Scholar] [CrossRef]
- Smith, E.M.L.; Bakitas, M.A.; Homel, P.; Piehl, M.; Kingman, L.; Fadul, C.E.; Bookbinder, M. Preliminary Assessment of a Neuropathic Pain Treatment and Referral Algorithm for Patients with Cancer. J. Pain Symptom Manag. 2011, 42, 822–838. [Google Scholar] [CrossRef] [PubMed]
- Shimozuma, K.; Ohashi, Y.; Takeuchi, A.; Aranishi, T.; Morita, S.; Kuroi, K.; Ohsumi, S.; Makino, H.; Katsumata, N.; Kuranami, M.; et al. Taxane-Induced Peripheral Neuropathy and Health-Related Quality of Life in Postoperative Breast Cancer Patients Undergoing Adjuvant Chemotherapy: N-SAS BC 02, a Randomized Clinical Trial. Support. Care Cancer 2012, 20, 3355–3364. [Google Scholar] [CrossRef] [PubMed]
- Tofthagen, C. Surviving Chemotherapy for Colon Cancer and Living with the Consequences. J. Palliat. Med. 2010, 13, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Einzig, A.I.; Wiernik, P.H.; Wadler, S.; Kaplan, J.; Benson, L.T.; Tentoramano, L.; Tan, V. Phase I Study of Paclitaxel (Taxol) and Granulocyte Colony Stimulating Factor (G-CSF) in Patients with Unresectable Malignancy. Investig. New Drugs 1998, 16, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef]
- Smith, E.M.L.; Pang, H.; Cirrincione, C.; Fleishman, S.; Paskett, E.D.; Ahles, T.; Bressler, L.R.; Fadul, C.E.; Knox, C.; Le-Lindqwister, N.; et al. Effect of Duloxetine on Pain, Function, and Quality of Life among Patients with Chemotherapy-Induced Painful Peripheral Neuropathy: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2013, 309, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Windebank, A.J. Chemotherapeutic Neuropathy. Curr. Opin. Neurol. 1999, 12, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.H.; Vyshnevska, A.; Hartke, T.V.; De Col, R.; Mankowski, J.L.; Turnquist, B.; Bosmans, F.; Reeh, P.W.; Schmelz, M.; Carr, R.W.; et al. Sodium Channel Nav1.8 Underlies TTX-Resistant Axonal Action Potential Conduction in Somatosensory C-Fibers of Distal Cutaneous Nerves. J. Neurosci. 2017, 37, 5204–5214. [Google Scholar] [CrossRef]
- Bagal, S.K.; Marron, B.E.; Owen, R.M.; Storer, R.I.; Swain, N.A. Voltage Gated Sodium Channels as Drug Discovery Targets. Channels 2015, 9, 360–366. [Google Scholar] [CrossRef]
- Cummins, T.R.; Sheets, P.L.; Waxman, S.G. The Roles of Sodium Channels in Nociception: Implications for Mechanisms of Pain. Pain 2007, 131, 243–257. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Cavaletti, G.; Antonacopoulou, A.; Genazzani, A.A.; Briani, C.; Bruna, J.; Terrazzino, S.; Velasco, R.; Alberti, P.; Campagnolo, M.; et al. Voltage-Gated Sodium Channel Polymorphisms Play a Pivotal Role in the Development of Oxaliplatin-Induced Peripheral Neurotoxicity: Results from a Prospective Multicenter Study. Cancer 2013, 119, 3570–3577. [Google Scholar] [CrossRef] [PubMed]
- Sittl, R.; Lampert, A.; Huth, T.; Schuy, E.T.; Link, A.S.; Fleckenstein, J.; Alzheimer, C.; Grafe, P.; Carr, R.W. Anticancer Drug Oxaliplatin Induces Acute Cooling-Aggravated Neuropathy via Sodium Channel Subtype Na V1.6-Resurgent and Persistent Current. Proc. Natl. Acad. Sci. USA 2012, 109, 6704–6709. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.; Benson, C.; Lankford, K.L.; Zhao, P.; Carrara, J.; Tan, A.M.; Kocsis, J.D.; Waxman, S.G.; Dib-Hajj, S.D. Sodium Channel Nav1.6 in Sensory Neurons Contributes to Vincristine-Induced Allodynia. Brain 2020, 143, 2421–2436. [Google Scholar] [CrossRef] [PubMed]
- Lolignier, S.; Bonnet, C.; Gaudioso, C.; Noël, J.; Ruel, J.; Amsalem, M.; Ferrier, J.; Rodat-Despoix, L.; Bouvier, V.; Aissouni, Y.; et al. The Nav1.9 Channel Is a Key Determinant of Cold Pain Sensation and Cold Allodynia. Cell Rep. 2015, 11, 1067–1078. [Google Scholar] [CrossRef]
- Ghelardini, C.; Desaphy, J.F.; Muraglia, M.; Corbo, F.; Matucci, R.; Dipalma, A.; Bertucci, C.; Pistolozzi, M.; Nesi, M.; Norcini, M.; et al. Effects of a New Potent Analog of Tocainide on HNav1.7 Sodium Channels and in Vivo Neuropathic Pain Models. Neuroscience 2010, 169, 863–873. [Google Scholar] [CrossRef]
- Li, Y.; North, R.Y.; Laurence, X.; Rhines, D.; Claudio, X.; Tatsui, E.; Rao, G.; Edwards, D.D.; Cassidy, R.M.; Daniel, X.; et al. Neurobiology of Disease DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain. J. Neurosci 2018. [Google Scholar] [CrossRef]
- Chang, W.; Berta, T.; Kim, Y.H.; Lee, S.; Lee, S.Y.; Ji, R.R. Expression and Role of Voltage-Gated Sodium Channels in Human Dorsal Root Ganglion Neurons with Special Focus on Nav1.7, Species Differences, and Regulation by Paclitaxel. Neurosci. Bull. 2018, 34, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and Structure-Function Relationships of Voltage-Gated Sodium Channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef]
- Chong, H.L.; Ruben, P.C. Interaction between Voltage-Gated Sodium Channels and the Neurotoxin, Tetrodotoxin. Channels 2008, 2, 407–412. [Google Scholar] [CrossRef]
- Stevens, M.; Peigneur, S.; Tytgat, J. Neurotoxins and Their Binding Areas on Voltage-Gated Sodium Channels. Front. Pharmacol. 2011, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Alsaloum, M.; Higerd, G.P.; Effraim, P.R.; Waxman, S.G. Status of Peripheral Sodium Channel Blockers for Non-Addictive Pain Treatment. Nat. Rev. Neurol. 2020, 16, 689–705. [Google Scholar] [CrossRef]
- Craner, M.J.; Klein, J.P.; Renganathan, M.; Black, J.A.; Waxman, S.G. Changes of Sodium Channel Expression in Experimental Painful Diabetic Neuropathy. Ann. Neurol. 2002, 52, 786–792. [Google Scholar] [CrossRef]
- Lyu, Y.S.; Park, S.K.; Chung, K.; Chung, J.M. Low Dose of Tetrodotoxin Reduces Neuropathic Pain Behaviors in an Animal Model. Brain Res. 2000, 871, 98–103. [Google Scholar] [CrossRef]
- Omana-Zapata, I.; Khabbaz, M.A.; Hunter, J.C.; Clarke, D.E.; Bley, K.R. Tetrodotoxin Inhibits Neuropathic Ectopic Activity in Neuromas, Dorsal Root Ganglia and Dorsal Horn Neurons. Pain 1997, 72, 41–49. [Google Scholar] [CrossRef]
- Marcil, J.; Walczak, J.-S.S.; Guindon, J.; Ngoc, A.H.H.; Lu, S.; Beaulieu, P. Antinociceptive Effects of Tetrodotoxin (TTX) in Rodents. Br. J. Anaesth. 2006, 96, 761–768. [Google Scholar] [CrossRef]
- Min, S.S.; Wierzbicki, A.S. Radiotherapy, Chemotherapy and Atherosclerosis. Curr. Opin. Cardiol. 2017, 32, 441–447. [Google Scholar] [CrossRef]
- Hagen, N.A.; du Souich, P.; Lapointe, B.; Ong-Lam, M.; Dubuc, B.; Walde, D.; Love, R.; Ngoc, A.H. Tetrodotoxin for Moderate to Severe Cancer Pain: A Randomized, Double Blind, Parallel Design Multicenter Study. J. Pain Symptom Manag. 2008, 35, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.A.; Cantin, L.; Constant, J.; Haller, T.; Blaise, G.; Ong-Lam, M.; Du Souich, P.; Korz, W.; Lapointe, B. Tetrodotoxin for Moderate to Severe Cancer-Related Pain: A Multicentre, Randomized, Double-Blind, Placebo-Controlled, Parallel-Design Trial. Pain Res. Manag. 2017, 2017, 7212713–7212717. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.A.; Lapointe, B.; Ong-Lam Md, M.; Dubuc Md, B.; Walde, D.; Gagnon Md, B.; Love, R.; Goel, R.; Hawley Md, P.; Ho, A.; et al. A Multicentre Open-Label Safety and Efficacy Study of Tetrodotoxin for Cancer Pain. Curr. Oncol. 2011, 18, e109–e116. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.A.; Fisher, K.M.; Lapointe, B.; du Souich, P.; Chary, S.; Moulin, D.; Sellers, E.; Ngoc, A.H. An Open-Label, Multi-Dose Efficacy and Safety Study of Intramuscular Tetrodotoxin in Patients with Severe Cancer-Related Pain. J. Pain Symptom Manag. 2007, 34, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.R.; Entrena, J.M.; Cendán, C.M.; Del Pozo, E.; Vela, J.M.; Baeyens, J.M. Tetrodotoxin Inhibits the Development and Expression of Neuropathic Pain Induced by Paclitaxel in Mice. Pain 2008, 137, 520–531. [Google Scholar] [CrossRef]
- Deuis, J.R.; Zimmermann, K.; Romanovsky, A.A.; Possani, L.D.; Cabot, P.J.; Lewis, R.J.; Vetter, I. An Animal Model of Oxaliplatin-Induced Cold Allodynia Reveals a Crucial Role for Nav1.6 in Peripheral Pain Pathways. Pain 2013, 154, 1749–1757. [Google Scholar] [CrossRef]
- Kavoosi, M.; O’Reilly, T.E.; Kavoosi, M.; Chai, P.; Engel, C.; Korz, W.; Gallen, C.C.; Lester, R.M. Safety, Tolerability, Pharmacokinetics, and Concentration-QTc Analysis of Tetrodotoxin: A Randomized, Dose Escalation Study in Healthy Adults. Toxins 2020, 12, 511. [Google Scholar] [CrossRef]
- Dorsey, S.G.; Kleckner, I.R.; Barton, D.; Mustian, K.; O’Mara, A.; St. Germain, D.; Cavaletti, G.; Danhauer, S.C.; Hershman, D.L.; Hohmann, A.G.; et al. The National Cancer Institute Clinical Trials Planning Meeting for Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy. JNCI J. Natl. Cancer Inst. 2019, 111, 531–537. [Google Scholar] [CrossRef]
- Amir, R.; Argoff, C.E.; Bennett, G.J.; Cummins, T.R.; Durieux, M.E.; Gerner, P.; Gold, M.S.; Porreca, F.; Strichartz, G.R. The Role of Sodium Channels in Chronic Inflammatory and Neuropathic Pain. J. Pain 2006, 7, S1–S29. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Turk, D.C.; Mcdermott, M.P.; Peirce-Sandner, S.; Burke, L.B.; Cowan, P.; Farrar, J.T.; Hertz, S.; Raja, S.N.; Rappaport, B.A.; et al. Interpreting the Clinical Importance of Group Differences in Chronic Pain Clinical Trials: IMMPACT Recommendations. Pain 2009, 146, 238–244. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Peirce-Sandner, S.; Baron, R.; Bellamy, N.; Burke, L.B.; Chappell, A.; Chartier, K.; Cleeland, C.S.; Costello, A.; et al. Research Design Considerations for Confirmatory Chronic Pain Clinical Trials: IMMPACT Recommendations. Pain 2010, 149, 177–193. [Google Scholar] [CrossRef]
- Turk, D.C.; Dworkin, R.H.; Allen, R.R.; Bellamy, N.; Brandenburg, N.; Carr, D.B.; Cleeland, C.; Dionne, R.; Farrar, J.T.; Galer, B.S.; et al. Core Outcome Domains for Chronic Pain Clinical Trials: IMMPACT Recommendations. Pain 2003, 106, 337–345. [Google Scholar] [CrossRef]
- Postma, T.J.; Aaronson, N.K.; Heimans, J.J.; Muller, M.J.; Hildebrand, J.G.; Delattre, J.Y.; Hoang-Xuan, K.; Lantéri-Minet, M.; Grant, R.; Huddart, R.; et al. The Development of an EORTC Quality of Life Questionnaire to Assess Chemotherapy-Induced Peripheral Neuropathy: The QLQ-CIPN20. Eur. J. Cancer 2005, 41, 1135–1139. [Google Scholar] [CrossRef]
- Woolf, C.J. Central Sensitization: Implications for the Diagnosis and Treatment of Pain. Pain 2011, 152, S2. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Shafer, S.L.; Yaksh, T.L. Prolonged Alleviation of Tactile Allodynia by Intravenous Lidocaine in Neuropathic Rats. Anesthesiology 1995, 83, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Price, D.D.; Mayer, D.J.; Lu, J.; Hayes, R.L. Intrathecal MK-801 and Local Nerve Anesthesia Synergistically Reduce Nociceptive Behaviors in Rats with Experimental Peripheral Mononeuropathy. Brain Res. 1992, 576, 254–262. [Google Scholar] [CrossRef]
- Benedetti, F. Placebo and the New Physiology of the Doctor-Patient Relationship. Physiol. Rev. 2013, 93, 1207–1246. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A.; Deyo, R.A.; Loeser, J.D.; Von Korff, M.; Fordyce, W.E. The Importance of Placebo Effects in Pain Treatment and Research. JAMA J. Am. Med. Assoc. 1994, 271, 1609–1614. [Google Scholar] [CrossRef]
- Zou, K.; Wong, J.; Abdullah, N.; Chen, X.; Smith, T.; Doherty, M.; Zhang, W. Examination of Overall Treatment Effect and the Proportion Attributable to Contextual Effect in Osteoarthritis: Meta-Analysis of Randomised Controlled Trials. Ann. Rheum. Dis. 2016, 75, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Di Blasi, Z.; Harkness, E.; Ernst, E.; Georgiou, A.; Kleijnen, J. Influence of Context Effects on Health Outcomes: A Systematic Review. Lancet 2001, 357, 757–762. [Google Scholar] [CrossRef]
- Benedetti, F. How the Doctor’s Words Affect the Patient’s Brain. Eval. Health Prof. 2002, 25, 369–386. [Google Scholar] [CrossRef]
- Cochran, W.G. Some Methods for Strengthening the Common χ 2 Tests. Biometrics 1954, 10, 417. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical Aspects of the Analysis of Data from Retrospective Studies of Disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A. Statistical Methods for Research Workers; Oliver and Boyd: Edinburgh, UK, 1934. [Google Scholar]
| TTX Dosage | |||||
|---|---|---|---|---|---|
| Variable | 7.5 µg BID (N = 25) | 15 µg BID (N = 24) | 30 µg QD (N = 25) | 30 µg BID (N = 26) | Placebo (N = 25) |
| Age, mean (±SD), y | 59.1 (±10.3) | 61.4 (±10.0) | 60.4 (±10.3) | 60.6 (±11.1) | 59.0 (±10.3) |
| Female, n (%) | 16 (64.0) | 15 (62.5) | 15 (60.0) | 16 (61.5) | 15 (60.0) |
| BMI, mean (±SD), kg m−2 | 30.6 (±6.4) | 28.6 (±6.5) | 30.1 (±7.8) | 30.1 (±7.9) | 32.6 (±10.0) |
| Baseline NPRS, mean ±SD | 6.3 (±1.3) | 7.0 (±1.4) | 6.2 (±1.2) | 6.3 (±1.4) | 6.7 (±1.5) |
| CINP Duration, Mean (±SD), y | |||||
| Lower extremities | 3.4 (±5.4) | 2.1 (±2.3) | 1.7 (±2.1) | 2.4 (±1.9) | 3.1 (±3.1) |
| Upper extremities | 3.5 (±5.6) | 1.8 (±1.8) | 1.9 (±2.2) | 2.4 (±2.0) | 2.7 (±2.9) |
| Prior Medications | |||||
| Opiates, n (%) a | 7 (28.0) | 7 (29.2) | 8 (32.0) | 6 (23.1) | 9 (29.6) |
| SNRI, n (%) | 2 (8.0) | 2 (8.3) | 5 (20.0) | 4 (15.4) | 4 (16.0) |
| Anticonvulsants, n (%) b | 9 (36.0) | 8 (33.3) | 4 (16.0) | 10 (38.5) | 4 (16.0) |
| NSAIDs, n (%) c | 5 (20.0) | 7 (29.2) | 2 (8.0) | 3 (11.5) | 5 (20.0) |
| Prior radiation, n (%) | 13 (52.0) | 10 (41.7) | 13 (52.0) | 14 (53.8) | 11 (44.0) |
| Primary Cancer, n (%) | |||||
| Colon | 10 (40.0) | 7 (29.2) | 7 (28.0) | 8 (30.8) | 12 (48.0) |
| Breast | 9 (36.0) | 6 (25.0) | 9 (36.0) | 10 (38.5) | 5 (20.0) |
| Other | 6 (24.0) | 11 (45.8) | 9 (36.0) | 8 (30.8) | 8 (32.0) |
| Chemotherapy Considered to Cause Pain, n d | |||||
| Taxane | 7 | 10 | 12 | 11 | 6 |
| Platinum | 19 | 16 | 15 | 16 | 19 |
| TTX Dosage | |||||
|---|---|---|---|---|---|
| Time Period | 7.5 µg BID (N = 25) | 15 µg BID (N = 24) | 30 µg QD (N = 25) | 30 µg BID (N = 26) | Placebo (N = 25) |
| Days 1–7, n | 25 | 24 | 24 | 25 | 25 |
| Mean change in NPRS (±SD) | −0.8 (±1.0) | −0.9 (±0.8) | −1.0 (±1.6) | −1.2 (±1.6) | −0.9 (±1.1) |
| p-value for Difference in LSM vs. Placebo | 0.84 | 0.84 | 0.66 | 0.35 | |
| Days 8–14, n | 23 | 24 | 24 | 25 | 24 |
| Mean change in NPRS (±SD) | −1.2 (±1.4) | −1.2 (±1.1) | −1.5 (±1.8) | −1.4 (±1.8) | −1.4 (±1.7) |
| p-value for Difference in LSM vs. Placebo | 0.57 | 0.61 | 0.69 | 0.94 | |
| Days 15–21, n | 22 | 22 | 23 | 23 | 25 |
| Mean change in NPRS (±SD) | −1.2 (±1.5) | −1.3 (±1.6) | −1.7 (±2.0) | −1.6 (±1.6) | −1.4 (±1.9) |
| p-value for Difference in LSM vs. Placebo | 0.90 | 0.85 | 0.58 | 0.90 | |
| Days 22–28, n | 21 | 21 | 23 | 24 | 25 |
| Mean change in NPRS (±SD) | −1.3 (±1.4) | −1.1 (±1.6) | −1.7 (±2.3) | −1.5 (±1.8) | −1.3 (±2.1) |
| p-value for Difference in LSM vs. Placebo | 1.0 | 0.68 | 0.58 | 0.92 | |
| TTX Dosage | |||||
|---|---|---|---|---|---|
| 7.5 µg BID N = 25 | 15 µg BID N = 24 | 30 µg QD N = 25 | 30 µg BID N = 26 | Placebo N = 25 | |
| SF-36 Mental Component Score | |||||
| n | 23 | 24 | 24 | 24 | 24 |
| Mean change (±SD) | −0.23 (±8.8) | 4.1 (±8.0) | −0.08 (±9.1) | 2.6 (±9.3) | 0.03 (±7.6) |
| Difference in LSM vs. Placebo (95% CI) | 0.5 (−3.9, 4.9) | 3.6 (−0.8, 8.0) | −1.2 (−5.6, 3.2) | 2.7 (−1.7, 7.1) | |
| SF-36 Physical Component Score | |||||
| n | 23 | 24 | 24 | 24 | 24 |
| Mean change (±SD) | 2.1 (±8.7) | 4.2 (±7.6) | 2.3 (±5.3) | 6.8 (±7.3) | 3.1 (±7.2) |
| Difference in LSM vs. Placebo (95% CI) | −1.5 (−5.4, 2.4) | 0.37 (−3.5, 4.3) | −1.0 (−4.9, 2.8) | 3.5 (−0.4, 7.4) a | |
| SF-36 Mental Health Score | |||||
| n | 23 | 24 | 24 | 24 | 24 |
| Mean change (±SD) | 0.22 (±9.8) | 3.2 (±10.6) | −0.6 (±8.2) | 3.5 (±10.2) | 0.5 (±5.8) |
| Difference in LSM vs. Placebo (95% CI) | 0.4 (−4.3, 5.1) | 2.7 (−2.0, 7.3) | −1.6 (−6.3, 3.0) | 3.1 (−1.5, 7.7) | |
| SF-36 Role Emotional Score | |||||
| n | 23 | 24 | 24 | 25 | 24 |
| Mean change (±SD) | 0.66 (±13.4) | 6.8 (±10.1) | 2.2 (±9.5) | 2.4 (±13.1) | 0.16 (±11.9) |
| Difference in LSM vs. Placebo (95% CI) | 1.2 (−4.7, 7.0) | 4.2 (−1.6, 10.0) | −0.57 (−6.4, 5.2) | 2.5 (−3.3, 8.2) | |
| SF-36 Social Functioning Score | |||||
| n | 23 | 24 | 24 | 25 | 24 |
| Mean change (±SD) | 1.7 (±10.3) | 4.7 (±11.6) | 0.41 (±8.1) | 5.5 (±8.6) | 1.0 (±7.1) |
| Difference in LSM vs. Placebo (95% CI) | −0.35 (−5.1, 4.4) | 2.6 (−2.1, 7.3) | −1.2 (−5.8, 3.5) | 4.4 (−0.4, 9.0) | |
| SF-36 Vitality/Energy Score | |||||
| n | 23 | 24 | 24 | 24 | 24 |
| Mean change (±SD) | −0.95 (±10.7) | 2.6 (±9.2) | 1.3 (±10.1) | 5.0 (±8.7) | 3.1 (±6.2) |
| Difference in LSM vs. Placebo (95% CI) | −3.2 (−7.9, 1.6) | −0.55 (−5.2, 4.2) | −2.3 (−7.6, 1.8) | 2.1 (−2.6, 6.8) | |
| SF-36 General Health Score | |||||
| n | 23 | 24 | 24 | 25 | 25 |
| Mean change (±SD) | 0.04 (±6.3) | 3.1 (±8.9) | −0.90 (±5.6) | 1.5 (±6.1) | −0.25 (±6.6) |
| Difference in LSM vs. Placebo (95% CI) | 0.15 (−3.4, 3.7) | 3.4 (−0.2, 7.0) | −0.88 (−4.4, 2.7) | 2.3 (−1.3, 5.8) | |
| SF-36 Body Pain Score | |||||
| n | 23 | 24 | 24 | 25 | 24 |
| Mean (±SD) | 3.6 (±8.0) | 3.7 (±8.3) | 2.9 (±6.7) | 9.2 (±7.1) | 2.6 (±8.3) |
| Difference in LSM vs. Placebo (95% CI) | 0.11 (−4.0, 4.2) | −0.08 (−4.2, 4.0) | −0.07 (−4.1, 4.0) | 6.0 (2.0, 10.0) b | |
| SF-36 Role Physical Score | |||||
| n | 23 | 24 | 24 | 25 | 24 |
| Mean (±SD) | 2.1 (±9.4) | 6.6 (±13.2) | 2.1 (±7.4) | 6.9 (±9.7) | 3.3 (±8.0) |
| Difference in LSM vs. Placebo (95% CI) | −0.98 (−6.0, 4.0) | 1.2 (−3.8, 6.3) | −2.0 (−7.0, 3.0) | 3.6 (−1.3, 8.5) | |
| SF-36 Physical Functioning Score | |||||
| n | 23 | 24 | 24 | 25 | 25 |
| Mean (±SD) | 1.1 (±10.1) | 4.6 (±7.5) | 2.7 (±5.2) | 4.6 (±7.8) | 2.6 (±6.6) |
| Difference in LSM vs. Placebo (95% CI) | −2.1 (−6.1, 1.9) | 1.2 (−2.9, 5.2) | −0.80 (−4.8, 3.2) | 1.9 (−2.1, 5.9) | |
| CIPN20 Sensory Scale | |||||
| n | 23 | 24 | 23 | 24 | 25 |
| Mean (±SD) | −4.52 (±5.9) | −5.17 (±4.7) | −4.61 (±5.0) | −4.6 (±4.6) | −2.2 (±5.0) |
| Difference in LSM vs. Placebo (95% CI) | −1.7 (−4.3, 0.93) | −1.5 (−4.2, 1.2) | −1.6 (−4.3, 1.0) | −2.2 (−4.8, 0.37) c | |
| CIPN20 Motor Scale | |||||
| n | 23 | 24 | 23 | 24 | 25 |
| Mean (±SD) | −2.2 (±4.8) | −2.4 (±4.1) | −2.1 (±3.8) | −2.8 (±3.3) | −2.0 (±3.2) |
| Difference in LSM vs. Placebo (95% CI) | 0.53 (−1.4, 2.4) | 0.63 (−1.3, 2.5) | 0.58 (−1.3, 2.5) | −0.63 (−2.5, 1.3) | |
| CIPN20 Autonomic Scale | |||||
| n | 23 | 24 | 23 | 24 | 25 |
| Mean (±SD) | −0.83 (±1.6) | −0.33 (±1.4) | −0.74 (±1.5) | −0.33 (±0.6) | −0.08 (±1.2) |
| Difference in LSM vs. Placebo (95% CI) | −0.40 (−1.0, 0.2) | 0.08 (−0.5, 0.7) | −0.28 (−0.9, 0.3) | −0.27 (−0.9, 0.3) | |
| PGIC—Overall QoL | |||||
| Most Frequent Reported Impression of Change | About the same | About the same | About the same | Moderately better | About the same |
| n (%) | 11 (44.0%) | 8 (33.3%) | 9 (36.0%) | 10 (38.5%) | 9 (36.0%) |
| PGIC—Physical Condition | |||||
| Most Frequent Reported Impression of Change | About the same | About the same;A little better | About the same | Moderately better | About the same |
| n (%) | 13 (52.0%) | 8 (33.3%) 8 (33.3%) | 10 (40.0%) | 9 (34.6%) | 11 (44.0%) |
| PGIC—Emotional State | |||||
| Most Frequent Reported Impression of Change | About the same | About the same | About the same | About the same | About the same |
| n (%) | 17 (68.0%) | 15 (62.5%) | 14 (56.0%) | 12 (46.2%) | 11 (44.0%) |
| PGIC—Enjoy Social Life | |||||
| Most Frequent Reported Impression of Change | About the same | About the same | About the same | About the same | About the same |
| n (%) | 16 (64.0%) | 15 (62.5%) | 15 (60.0%) | 12 (46.2%) | 12 (48.0%) |
| PGIC—Numbness, Tingling or Pain in Hands or Feet | |||||
| Most Frequent Reported Impression of Change | About the same | About the same | About the same | About the same;Moderately better | About the same |
| n (%) | 10 (40.0%) | 8 (33.3%) | 8 (32.0%) | 6 (23.1%) 6 (23.1%) | 9 (36.0%) |
| PGIC—Believe Receiving Active Agent | |||||
| Yes, n (%) | 15 (60.0%) | 15 (62.5%) | 14 (56.0%) | 22 (84.6%) | 13 (52.0%) |
| No, n (%) | 8 (32.0%) | 7 (37.5%) | 8 (32.0%) | 3 (11.5%) | 12 (48.0%) |
| TTX Dosage | |||||
|---|---|---|---|---|---|
| 7.5 µg BID N = 25 | 15 µg BID N = 24 | 30 µg QD N = 25 | 30 µg BID N = 26 | Placebo N = 25 | |
| Time to Peak Pain Relief a | |||||
| n | 23 | 24 | 23 | 25 | 25 |
| Mean change (Weeks) (±SD) | 2.5 (±1.2) | 2.4 (±1.2) | 3.0 (±1.2) | 2.6 (±1.3) | 2.5 (±1.1) |
| Kaplan–Meier Estimate of weeks to peak pain relief | |||||
| Median (Weeks) (95% CI) | 3.00 (1.00, 3.00) | 2.50 (1.00, 3.00) | 3.00 (3.00, 4.00) | 3.00 (1.00, 4.00) | 3.00 (1.00, 3.00) |
| TTX Dosage | |||||
|---|---|---|---|---|---|
| Responders | 7.5 µg BID (n = 25) | 15 µg BID (n = 24) | 30 µg QD (n = 25) | 30 µg BID (n = 26) | Placebo (n = 25) |
| Any timepoint, n (%) a | 9 (36.0%) | 11 (45.8%) | 10 (40.0%) | 15 (57.7%) | 8 (32.0%) |
| Odds ratio vs. placebo | 1.12 | 1.99 | 1.65 | 3.39 a | |
| (95% CI) | (0.32, 3.96) | (0.56, 7.13) | (0.46, 5.90) | (0.96, 11.97) | |
| 5-day rolling averages, n (%) b | 10 (40.0%) | 12 (50.0%) | 10 (40.0%) | 16 (61.5%) | 8 (32.0%) |
| Odds ratio vs. placebo | 1.33 | 2.54 | 1.58 | 3.87 b | |
| (95% CI) | (0.38, 4.65) | (0.71, 9.06) | (0.45, 5.62) | (1.10, 13.61) | |
| 10-day rolling averages, n (%) c | 8 (32.0%) | 10 (41.7%) | 10 (40.0%) | 15 (57.7%) | 8 (32.0%) |
| Odds ratio vs. placebo | 0.90 | 1.68 | 1.63 | 3.90 c | |
| (95% CI) | (0.25, 3.24) | (0.47, 6.06) | (0.45, 5.83) | (1.08, 14.09) | |
| 15-day rolling averages, n (%) | 8 (32.0%) | 10 (41.7%) | 10 (40.0%) | 12 (46.2%) | 8 (32.0%) |
| Odds ratio vs. placebo | 0.92 | 1.59 | 1.66 | 2.50 | |
| (95% CI) | (0.26, 3.29) | (0.45, 5.69) | (0.47, 5.92) | (0.70, 8.91) | |
| 20-day rolling averages, n (%) d | 7 (28.0%) | 6 (25.0%) | 10 (40.0%) | 12 (46.2%) | 7 (28.0%) |
| Odds ratio vs. placebo | 1.03 | 0.93 | 1.68 | 2.90 d | |
| (95% CI) | (0.26, 4.04) | (0.23, 3.79) | (0.45, 6.24) | (0.77, 10.93) | |
| TTX Dosage | |||||
|---|---|---|---|---|---|
| 7.5 µg BID (N = 25) | 15 µg BID (N = 24) | 30 µg QD (N = 25) | 30 µg BID (N = 26) | Placebo (N = 25) | |
| Patients with ≥1 AE | 21 (84.0) | 22 (91.7) | 20 (80.0) | 24 (92.3) | 18 (72.0) |
| Common AEs, n (%) a | |||||
| Paresthesia oral | 4 (16.0) | 9 (37.5) | 10 (40.0) | 11 (42.3) | 3 (12.0) |
| Hypoesthesia oral | 5 (20.0) | 7 (29.2) | 6 (24.0) | 10 (38.5) | 3 (12.0) |
| Paresthesia | 5 (20.0) | 7 (29.2) | 5 (20.0) | 7 (26.9) | 6 (24.0) |
| Headache | 6 (24.0) | 3 (12.5) | 1 (4.0) | 9 (34.6) | 5 (20.0) |
| Dizziness | 3 (12.0) | 4 (16.7) | 3 (12.0) | 8 (30.8) | 5 (20.0) |
| Fatigue | 4 (16.0) | 5 (20.8) | 5 (20.0) | 3 (11.5) | 4 (16.0) |
| Nausea | 1 (4.0) | 5 (20.8) | 1 (4.0) | 6 (23.1) | 6 (24.0) |
| Pain in extremity | 1 (4.0) | 5 (20.8) | 4 (16.0) | 3 (11.5) | 2 (8.0) |
| SAEs, n (%) | |||||
| Metastatic colon cancer | 0 | 0 | 0 | 0 | 1 (4.0) |
| Metastatic bladder cancer | 0 | 1 (4.2) | 0 | 0 | 0 |
| Prostate cancer | 0 | 1 (4.2) | 0 | 0 | 0 |
| Viral upper respiratory tract infection | 0 | 0 | 0 | 1 (3.8) | 0 |
| Patients Reporting an AE by Severity, n (%) | |||||
| Mild | 14 (56.0) | 12 (50.0) | 13 (52.0) | 12 (46.2) | 9 (36.0) |
| Moderate | 7 (28.0) | 9 (37.5) | 7 (28.0) | 8 (30.8) | 9 (36.0) |
| Severe | 0 | 1 (4.2) | 0 | 1 (3.8) | 1 (4.0) |
| Life Threatening | 0 | 0 | 0 | 0 | 0 |
| Death | 0 | 1 (4.2) Unrelated | 0 | 0 | 0 |
| Patients Reporting an AE and Relationship to Treatment, n (%) | |||||
| Not related | 6 (24.0) | 5 (20.8) | 2 (8.0) | 4 (15.4) | 4 (16.0) |
| Unlikely related | 0 | 1 (4.2) | 0 | 2 (7.7) | 1 (4.0) |
| Possibly related | 8 (32.0) | 9 (37.5) | 5 (20.0) | 9 (34.6) | 10 (40.0) |
| Related | 7 (28.0) | 7 (29.2) | 13 (52.0) | 9 (34.6) | 3 (12.0) |
| TTX Dosage | |||||
|---|---|---|---|---|---|
| AE | 7.5 µg BID (N = 25) | 15 µg BID (N = 24) | 30 µg QD (N = 25) | 30 µg BID (N = 26) | Placebo (N = 25) |
| Hypoesthesia, n | 3 | 1 | 7 | 1 | 0 |
| Median Onset (h:mm) | 0:10 | 1:38 | 0:07 | 0:16 | |
| (Range) | (0:05–0:25) | (1:38–1:38) | (0:02–3:12) | (0:16–0:16) | |
| Median Duration (h:mm) | 0:40 | 0:12 | 3:00 | 1:10 | |
| (Range) | (0:35–0:40) | (0:12–0:12) | (2:30–22:10) | (1:10–1:10) | |
| Hypoesthesia Oral, n | 46 | 38 | 25 | 25 | 4 |
| Median Onset (h:mm) | 0:21 | 0:27 | 0:19 | 0:15 | 0:07 |
| (Range) | (0:00–3:35) | (0:07–7:03) | (0:00–1:30) | (0:05–6:25) | (0:02–1:27) |
| Median Duration (h:mm) | 0:39 | 1:15 | 2:51 | 1:30 | 4:22 |
| (Range) | (0:05–53:50) | (0:05–5:15) | (0:10–12:44) | (0:15–73:30) | (0:10–55:45) |
| Paresthesia, n | 21 | 14 | 12 | 12 | 9 |
| Median Onset (h:mm) | 0:30 | 0:34 | 0:21 | 0:16 | 0:26 |
| (Range) | (0:00–6:37) | (0:00–3:05) | (0:04–1:13) | (0:00–2:56) | (0:08–3:26) |
| Median Duration (h:mm) | 1:43 | 0:27 | 0:50 | 1:45 | 0:36 |
| (Range) | (0:03–48:00) | (0:01–59:35) | (0:10–5:28) | (0:05–12:00) | (0:15–4:00) |
| Paresthesia Oral, n | 21 | 28 | 23 | 55 | 8 |
| Median Onset (h:mm) | 0:19 | 0:22 | 0:24 | 0:44 | 0:10 |
| (Range) | (0:05–0:51) | (0:00–14:34) | (0:00–3:18) | (0:00–17:50) | (0:08–0:23) |
| Median Duration (h:mm) | 0:22 | 0:39 | 0:40 | 1:09 | 0:32 |
| (Range) | (0:04–3:30) | (0:02–3:20) | (0:01–11:00) | (0:01–4:52) | (0:10–2:40) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldlust, S.A.; Kavoosi, M.; Nezzer, J.; Kavoosi, M.; Korz, W.; Deck, K. Tetrodotoxin for Chemotherapy-Induced Neuropathic Pain: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Dose Finding Trial. Toxins 2021, 13, 235. https://doi.org/10.3390/toxins13040235
Goldlust SA, Kavoosi M, Nezzer J, Kavoosi M, Korz W, Deck K. Tetrodotoxin for Chemotherapy-Induced Neuropathic Pain: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Dose Finding Trial. Toxins. 2021; 13(4):235. https://doi.org/10.3390/toxins13040235
Chicago/Turabian StyleGoldlust, Samuel A., Mojgan Kavoosi, Jennifer Nezzer, Mehran Kavoosi, Walter Korz, and Kenneth Deck. 2021. "Tetrodotoxin for Chemotherapy-Induced Neuropathic Pain: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Dose Finding Trial" Toxins 13, no. 4: 235. https://doi.org/10.3390/toxins13040235
APA StyleGoldlust, S. A., Kavoosi, M., Nezzer, J., Kavoosi, M., Korz, W., & Deck, K. (2021). Tetrodotoxin for Chemotherapy-Induced Neuropathic Pain: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Dose Finding Trial. Toxins, 13(4), 235. https://doi.org/10.3390/toxins13040235




