Safety, Tolerability, Pharmacokinetics, and Concentration-QTc Analysis of Tetrodotoxin: A Randomized, Dose Escalation Study in Healthy Adults
Abstract
1. Introduction
2. Results
2.1. Subject Demographics
2.2. Dose Selection
2.3. Safety and Tolerability
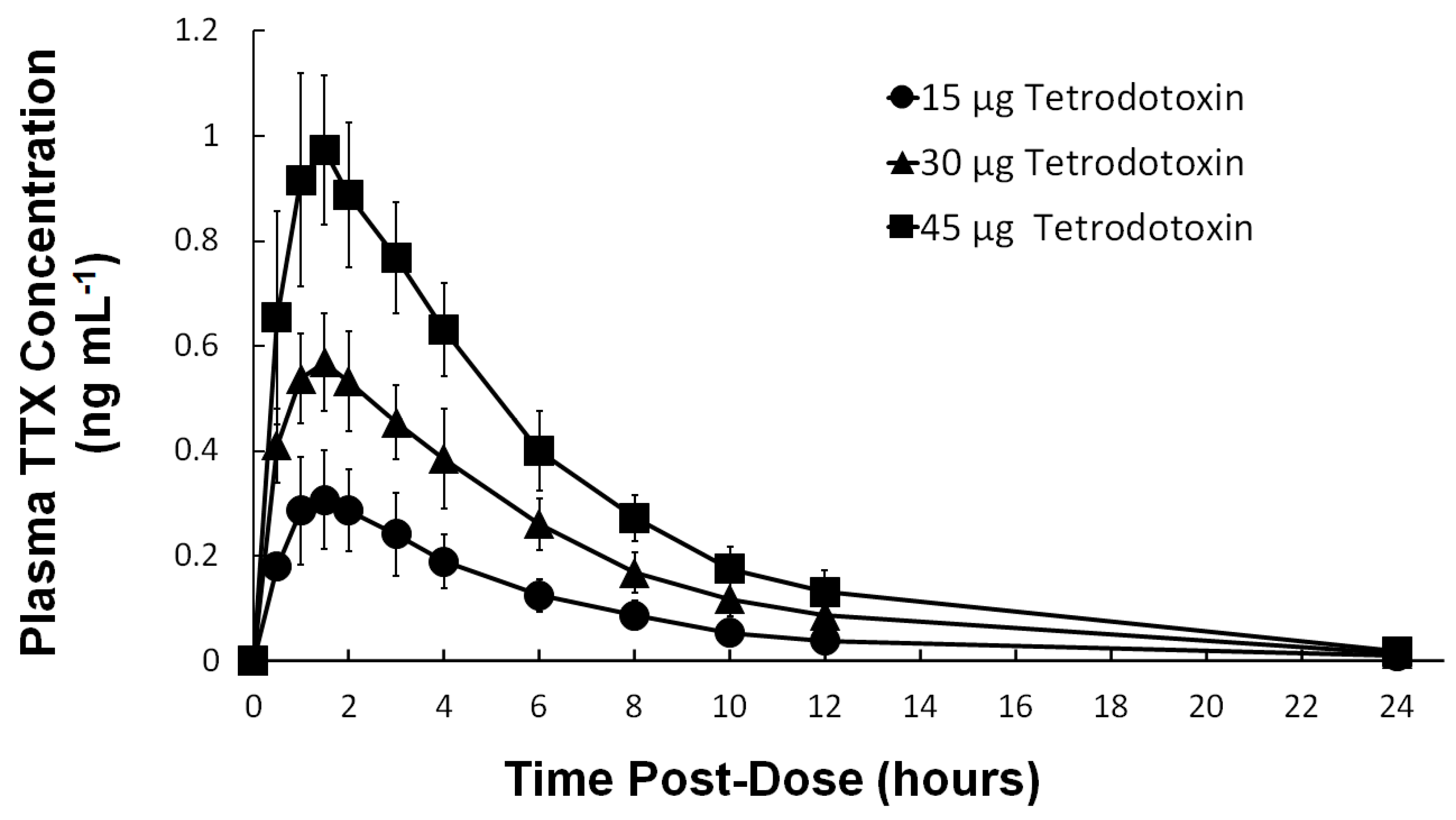
2.4. Pharmacokinetics
2.5. QT Interval Correction
2.6. QT/QTc Analysis
2.7. Concentration-QT Modeling
2.8. Assay Sensitivity
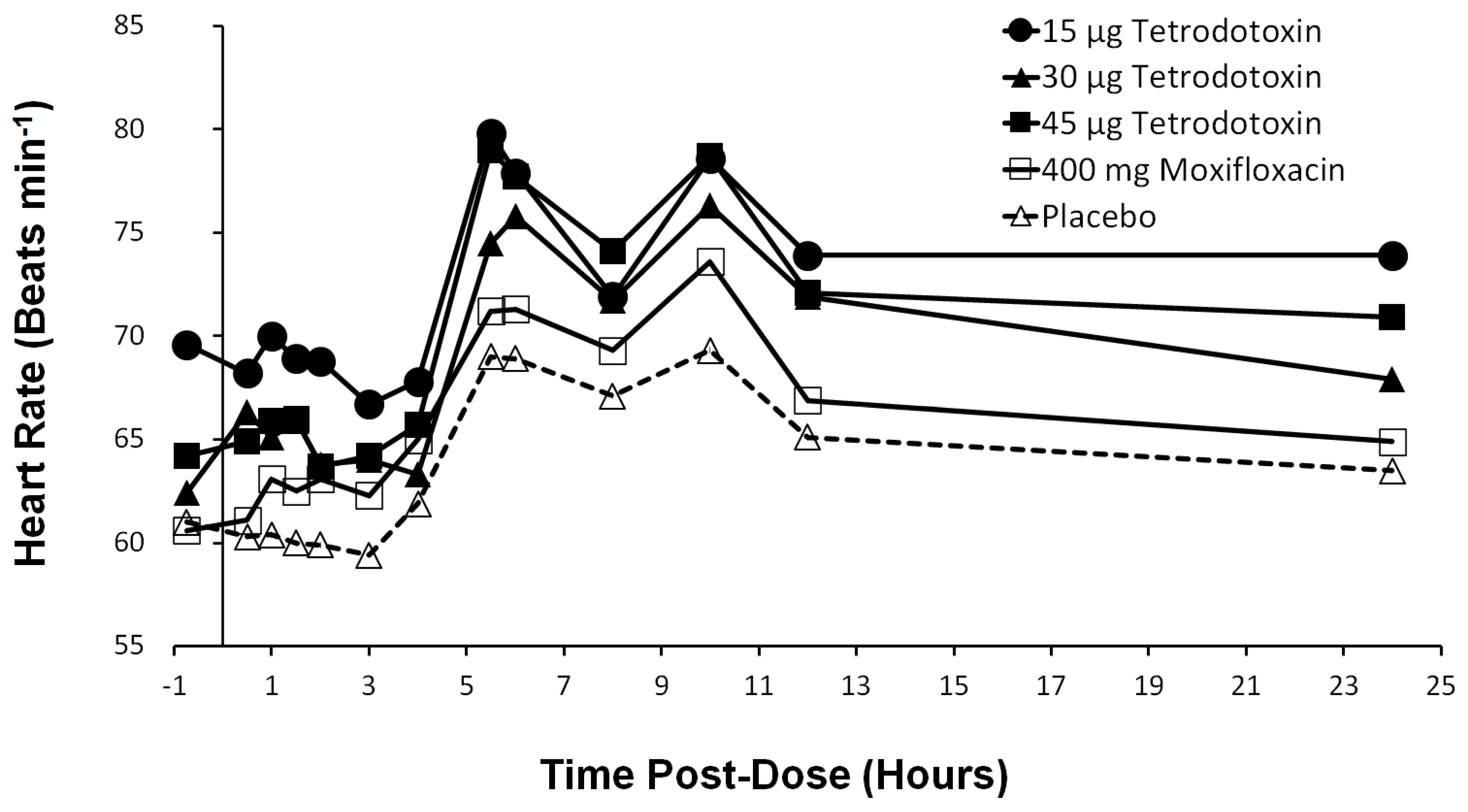
2.9. Heart Rate Effects
2.10. PR and QRS Intervals
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Population
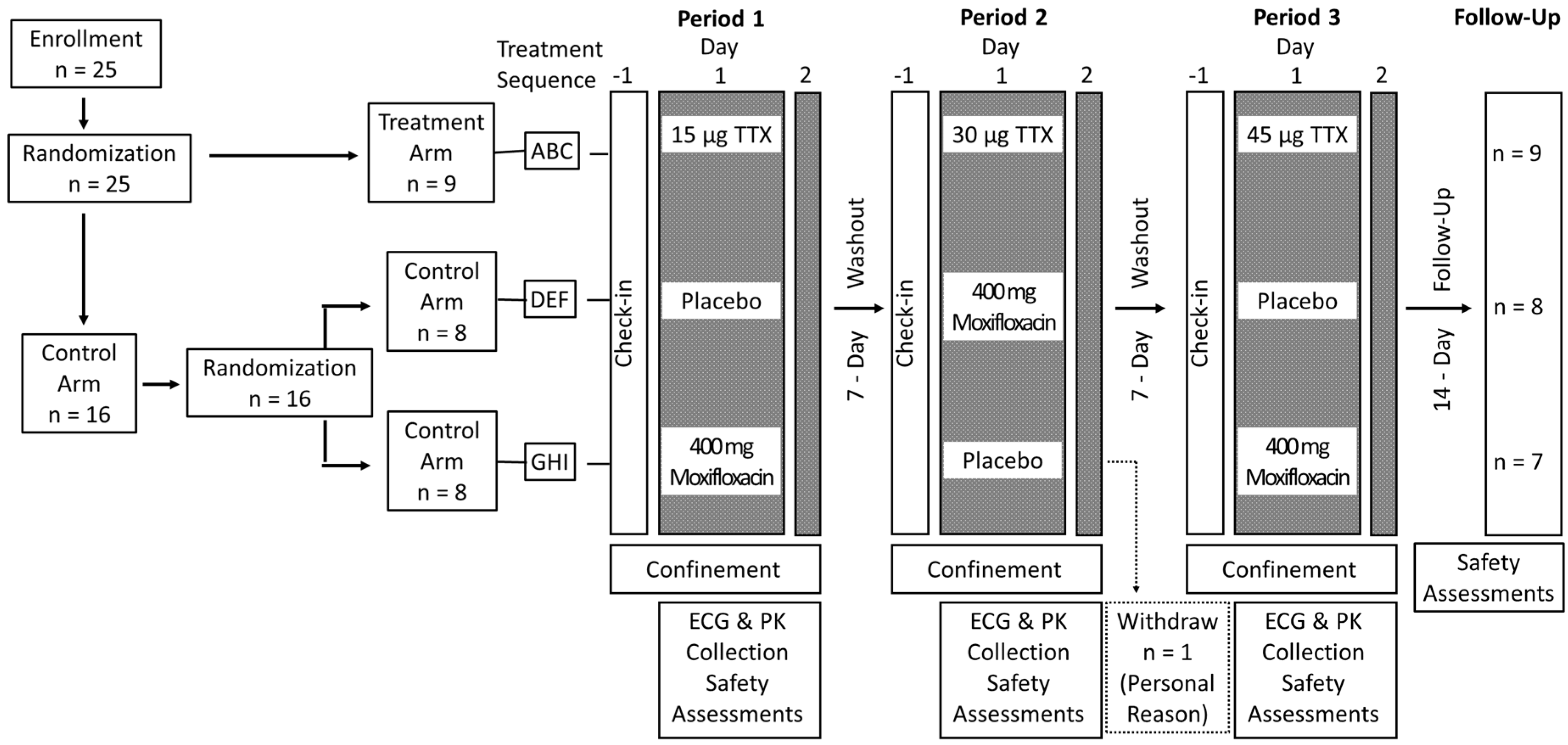
5.2. Study Design
5.3. Randomization and Blinding
5.4. Safety and Tolerability
5.5. Pharmacokinetics
5.6. Electrocardiography
5.7. Data and Statistical Analysis
5.8. QT Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AE | adverse event |
| AUC | area under the curve |
| bpm | beats per minute |
| Cmax | maximum plasma concentration |
| CI | confidence interval |
| C-QTc | corrected concentration-QT interval |
| CV% | coefficient of variance |
| hERG | Human Ether-à-go-go-Related Gene |
| ICH E14 | International Conference on Harmonization E14 guidance |
| PK | pharmacokinetics |
| QTcF | Fridericia corrected QT interval |
| ΔQTcF | Baseline adjusted Fridericia-corrected QTc |
| ΔΔQTcF | Baseline and placebo adjusted Fridericia-corrected QTc |
| TEAE | treatment emergent adverse event |
| tmax | time at Cmax |
| TTX | tetrodotoxin |
| VGSC | voltage-gated sodium channel |
References
- Goldberg, D.S.; McGee, S.J. Pain as a global public health priority. BMC Public Health 2011, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.; Argoff, C.E.; Bennett, G.J.; Cummins, T.R.; Durieux, M.E.; Gerner, P.; Gold, M.S.; Porreca, F.; Strichartz, G.R. The role of sodium channels in chronic inflammatory and neuropathic pain. J. Pain 2006, 7, S1–S29. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.C.; Lewis, R.J. Sodium channels and pain: From toxins to therapies. Br. J. Pharmacol. 2018, 175, 2138–2157. [Google Scholar] [CrossRef] [PubMed]
- Cummins, T.R.; Sheets, P.L.; Waxman, S.G. The roles of sodium channels in nociception: Implications for mechanisms of pain. Pain 2007, 131, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Reimann, F.; Cox, J.J.; Belfer, I.; Diatchenko, L.; Zaykin, D.V.; McHale, D.P.; Drenth, J.P.; Dai, F.; Wheeler, J.; Sanders, F.; et al. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc. Natl. Acad. Sci. USA 2010, 107, 5148–5153. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Wickenden, A.D.; Chaplan, S.R. Sodium channel blockers for the treatment of neuropathic pain. Neurotherapeutics 2009, 6, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Murtha, E.F. Pharmacological study of poisons from shellfish and puffer fish. Ann. N. Y. Acad. Sci. 1960, 90, 820–836. [Google Scholar] [CrossRef]
- Noguch, T.; Arakawa, O. Tetrodotoxin–distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef]
- Stokes, A.N.; Ducey, P.K.; Neuman-Lee, L.; Hanifin, C.T.; French, S.S.; Pfrender, M.E.; Brodie, E.D., III; Brodie, E.D., Jr. Confirmation and distribution of tetrodotoxin for the first time in terrestrial invertebrates: Two terrestrial flatworm species (Bipalium adventitium and Bipalium kewense). PLoS ONE 2014, 9, e100718. [Google Scholar] [CrossRef]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef]
- Lee, C.H.; Ruben, P.C. Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin. Channels 2008, 2, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Berde, C.B.; Athiraman, U.; Yahalom, B.; Zurakowski, D.; Corfas, G.; Bognet, C. Tetrodotoxin-bupivacaine-epinephrine combinations for prolonged local anesthesia. Mar. Drugs 2011, 9, 2717–2728. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.S.; Park, S.K.; Chung, K.; Chung, J.M. Low dose of tetrodotoxin reduces neuropathic pain behaviors in an animal model. Brain Res. 2000, 871, 98–103. [Google Scholar] [CrossRef]
- Omana-Zapata, I.; Khabbaz, M.A.; Hunter, J.C.; Clarke, D.E.; Bley, K.R. Tetrodotoxin inhibits neuropathic ectopic activity in neuromas, dorsal root ganglia and dorsal horn neurons. Pain 1997, 72, 41–49. [Google Scholar] [CrossRef]
- Marcil, J.; Walczak, J.S.; Guindon, J.; Ngoc, A.H.; Lu, S.; Beaulieu, P. Antinociceptive effects of tetrodotoxin (TTX) in rodents. Br. J. Anaesth. 2006, 96, 761–768. [Google Scholar] [CrossRef]
- Melnikova, D.I.; Khotimchenko, Y.S.; Magarlamov, T.Y. Addressing the issue of tetrodotoxin targeting. Mar. Drugs 2018, 16, 352. [Google Scholar] [CrossRef]
- Alvarez, P.; Levine, J.D. Antihyperalgesic effect of tetrodotoxin in rat models of persistent muscle pain. Neuroscience 2015, 311, 499–507. [Google Scholar] [CrossRef]
- Hagen, N.A.; Fisher, K.M.; Lapointe, B.; du Souich, P.; Chary, S.; Moulin, D.; Sellers, E.; Ngoc, A.H.; Canadian Tetrodotoxin Study Group. An open-label, multi-dose efficacy and safety study of intramuscular tetrodotoxin in patients with severe cancer-related pain. J. Pain Symptom Manag. 2007, 34, 171–182. [Google Scholar] [CrossRef]
- Hagen, N.A.; du Souich, P.; Lapointe, B.; Ong-Lam, M.; Dubuc, B.; Walde, D.; Love, R.; Ngoc, A.H.; Canadian Tetrodotoxin Study Group. Tetrodotoxin for moderate to severe cancer pain: A randomized, double blind, parallel design multicenter study. J. Pain Symptom Manag. 2008, 35, 420–429. [Google Scholar] [CrossRef]
- Hagen, N.A.; Lapointe, B.; Ong–Lam, M.; Dubuc, B.; Walde, D.; Gagnon, B.; Love, R.; Goel, R.; Hawley, P.; Ngoc, A.H.; et al. A multicentre open-label safety and efficacy study of tetrodotoxin for cancer pain. Curr. Oncol. 2011, 18, e109–e116. [Google Scholar] [CrossRef]
- Hagen, N.A.; Cantin, L.; Constant, J.; Haller, T.; Blaise, G.; Ong-Lam, M.; du Souich, P.; Korz, W.; Lapointe, B. Tetrodotoxin for moderate to severe cancer-related pain: A multicentre, randomized, double-blind, placebo-controlled, parallel-design trial. Pain Res. Manag. 2017, 2017, 7212713. [Google Scholar] [CrossRef] [PubMed]
- Eijkelkamp, N.; Linley, J.E.; Baker, M.D.; Minett, M.S.; Cregg, R.; Werdehausen, R.; Rugiero, F.; Wood, J.N. Neurological perspectives on voltage-gated sodium channels. Brain 2012, 135, 2585–2612. [Google Scholar] [CrossRef] [PubMed]
- Scholz, A. Nav1.4 Voltage—Gated Sodium Channel. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–7. [Google Scholar]
- Kollarik, M.; Sun, H.; Herbstsomer, R.A.; Ru, F.; Kocmalova, M.; Meeker, S.N.; Undem, B.J. Different role of TTX-sensitive voltage-gated sodium channel (NaV1) subtypes in action potential initiation and conduction in vagal airway nociceptors. J. Physiol. 2018, 596, 1419–1432. [Google Scholar] [CrossRef]
- Gaborit, N.; Le Bouter, S.; Szuts, V.; Varro, A.; Escande, D.; Nattel, S.; Demolombe, S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 2007, 582, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Blechschmidt, S.; Haufe, V.; Benndorf, K.; Zimmer, T. Voltage-gated Na+ channel transcript patterns in the mammalian heart are species-dependent. Prog. Biophys. Mol. Biol. 2008, 98, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Gellens, M.E.; George, A.L.; Chen, L.Q.; Chahine, M.; Horn, R.; Barchi, R.L.; Kallen, R.G. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc. Natl. Acad. Sci. USA 1992, 89, 554–558. [Google Scholar] [CrossRef] [PubMed]
- ICH E14 Implementation Working Group. ICH E14 Guideline: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs Questions & Answers (R3) 2015 [2/6/2016]. [61134]. Available online: https://database.ich.org/sites/default/files/E14_Q%26As_R3_Q%26As.pdf (accessed on 19 May 2017).
- Cirincione, B.; Sager, P.T.; Mager, D.E. Influence of meals and glycemic changes on QT interval dynamics. J. Clin. Pharmacol. 2017, 57, 966–976. [Google Scholar] [CrossRef]
- Taubel, J.; Wong, A.H.; Naseem, A.; Ferber, G.; Camm, A.J. Shortening of the QT interval after food can be used to demonstrate assay sensitivity in thorough QT studies. J. Clin. Pharmacol. 2012, 52, 1558–1565. [Google Scholar] [CrossRef]
- Makarova, M.; Rycek, L.; Hajicek, J.; Baidilov, D.; Hudlicky, T. Tetrodotoxin: History, Biology, and Synthesis. Angew. Chem. Int. Ed. 2019, 58, 18338–18387. [Google Scholar] [CrossRef]
- Suehiro, M. Historical review on chemical and medical studies of globefish toxin before World War II. Yakushigaku Zasshi 1994, 29, 428–434. [Google Scholar]
- Nieto, F.R.; Entrena, J.M.; Cendán, C.M.; Del Pozo, E.; Vela, J.M.; Baeyens, J.M. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain 2008, 137, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.R.; Cobos, E.J.; Tejada, M.Á.; Sánchez-Fernández, C.; González-Cano, R.; Cendán, C.M. Tetrodotoxin (TTX) as a therapeutic agent for pain. Mar. Drugs 2012, 10, 281–305. [Google Scholar] [CrossRef] [PubMed]
- González-Cano, R.; Tejada, M.Á.; Artacho-Cordón, A.; Nieto, F.R.; Entrena, J.M.; Wood, J.N.; Cendán, C.M. Effects of tetrodotoxin in mouse models of visceral pain. Mar. Drugs 2017, 15, 188. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.M.; McIntyre, M.K.; Petz, L.N.; Korz, W.; Wong, D.; Clifford, J.L. Tetrodotoxin suppresses thermal hyperalgesia and mechanical allodynia in a rat full thickness thermal injury pain model. Neurosci. Lett. 2015, 607, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, A.; de Henestrosa, A.R.F.; Marín, A.P.; Ho, A.; Borroto, J.I.G.; Carasa, I.; Pritchard, L. Evaluation of the genotoxic potential of the natural neurotoxin Tetrodotoxin (TTX) in a battery of in vitro and in vivo genotoxicity assays. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 634, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Padera, R.F.; Tse, J.Y.; Bellas, E.; Kohane, D.S. Tetrodotoxin for prolonged local anesthesia with minimal myotoxicity. Muscle Nerve 2006, 34, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Shomorony, A.; Santamaria, C.M.; Zhao, C.; Rwei, A.Y.; Mehta, M.; Zurakowski, D.; Kohane, D.S. Prolonged Duration Local Anesthesia by Combined Delivery of Capsaicin-and Tetrodotoxin-Loaded Liposomes. Anesth. Analg. 2019, 129, 709–717. [Google Scholar] [CrossRef]
- Stevens, M.F.; Hoppe, M.; Holthusen, H.; Lipfert, P. Tetrodotoxin-induced conduction blockade is prolonged by hyaluronic acid with and without bupivacaine. Acta Anaesthesiol. Scand. 2004, 48, 128–134. [Google Scholar] [CrossRef]
- Rook, M.B.; Evers, M.M.; Vos, M.A.; Bierhuizen, M.F. Biology of cardiac sodium channel Nav1. 5 expression. Cardiovasc. Res. 2012, 93, 12–23. [Google Scholar] [CrossRef]
- Bloomfield, D.M.; Kost, J.T.; Ghosh, K.; Hreniuk, D.; Hickey, L.A.; Guitierrez, M.J.; Gottesdiener, K.; Wagner, J.A. The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin. Pharmacol. Ther. 2008, 84, 475–480. [Google Scholar] [CrossRef]
- Bednar, M.M.; Harrigan, E.P.; Ruskin, J.N. Torsades de pointes associated with nonantiarrhythmic drugs and observations on gender and QTc. Am. J. Cardiol. 2002, 89, 1316–1319. [Google Scholar] [CrossRef]
- Burke, J.H.; Ehlert, F.A.; Kruse, J.T.; Parker, M.A.; Goldberger, J.J.; Kadish, A.H. Gender-specific differences in the QT interval and the effect of autonomic tone and menstrual cycle in healthy adults. Am. J. Cardiol. 1997, 79, 178–181. [Google Scholar] [CrossRef]
- Ebert, S.N.; Liu, X.K.; Woosley, R.L. Female gender as a risk factor for drug-induced cardiac arrhythmias: Evaluation of clinical and experimental evidence. J. Womem’s Health 1998, 7, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, L.; Garnett, C.; Luo, M.; Targum, S.; Sørensen, J.S.; Mehrotra, N. Quantitative understanding of QTc prolongation and gender as risk factors for torsade de pointes. Clin. Pharmacol. Ther. 2018, 103, 304–309. [Google Scholar] [CrossRef]
- Darpo, B.; Karnad, D.R.; Badilini, F.; Florian, J.; Garnett, C.E.; Kothari, S.; Panicker, G.K.; Sarapa, N. Are women more susceptible than men to drug-induced QT prolongation? Concentration–QTc modelling in a phase 1 study with oral rac-sotalol. Br. J. Clin. Pharmacol. 2014, 77, 522–531. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fromm, B.S.; Steinman, R.T.; Meissner, M.D.; Lehmann, M.H. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. Jama 1993, 270, 2590–2597. [Google Scholar] [CrossRef]
- Kang, J.; Luo, Y.; Searles, M.; Rampe, D. Observations on conducting whole-cell patch clamping of the hERG cardiac K+ channel in pure human serum. J. Appl. Toxicol. 2017, 37, 445–453. [Google Scholar] [CrossRef]
- Zimmer, T. Effects of tetrodotoxin on the mammalian cardiovascular system. Mar. Drugs 2010, 8, 741–762. [Google Scholar] [CrossRef]
- Chang, F.C.T.; Benton, B.J.; Salyer, J.L.; Foster, R.E.; Franz, D.R. Respiratory and cardiovascular effects of tetrodotoxin in urethane-anesthetized guinea pigs. Brain Res. 1990, 528, 259–268. [Google Scholar] [CrossRef]
- Mackenzie, C.E.; Smalley, A.J.; Barnas, G.M.; Park, S.G. Tetrodotoxin infusion: Nonventilatory effects and role in toxicity models. Acad. Emerg. Med. 1996, 3, 1106–1112. [Google Scholar] [CrossRef]
- Darpo, B.; Garnett, C.; Benson, C.T.; Keirns, J.; Leishman, D.; Malik, M.; Mehrotra, N.; Prasad, K.; Riley, S.; Rodriguez, I.; et al. Cardiac Safety Research Consortium: Can the thorough QT/QTc study be replaced by early QT assessment in routine clinical pharmacology studies? Scientific update and a research proposal for a path forward. Am. Heart J. 2014, 168, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Darpo, B.; Benson, C.; Dota, C.; Ferber, G.; Garnett, C.; Green, C.L.; Jarugula, V.; Johannesen, L.; Keirns, J.; Krudys, K.; et al. Results from the IQ-CSRC prospective study support replacement of the thorough QT study by QT assessment in the early clinical phase. Clin. Pharmacol. Ther. 2015, 97, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Darpo, B.; Garnett, C.; Keirns, J.; Stockbridge, N. Implications of the IQ-CSRC prospective study: Time to revise ICH E14. Drug Saf. 2015, 38, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration, HHS. International conference on harmonisation; guidance on E14 Clinical Evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs; availability notice. Fed. Regist. 2005, 70, 61134. [Google Scholar]
- Garnett, C.; Bonate, P.L.; Dang, Q.; Ferber, G.; Huang, D.; Liu, J.; Mehrotra, D.; Riley, S.; Sager, P.; Tornoe, C.; et al. Scientific white paper on concentration-QTc modeling. J. Pharmacokinet. Pharmacodyn. 2018, 45, 383–397. [Google Scholar] [CrossRef]
- Darpo, B. The thorough QT/QTc study 4 years after the implementation of the ICH E14 guidance. Br. J. Pharmacol. 2010, 159, 49–57. [Google Scholar] [CrossRef]
- Panicker, G.K.; Karnad, D.R.; Kadam, P.; Badilini, F.; Damle, A.; Kothari, S. Detecting moxifloxacin-induced QTc prolongation in thorough QT and early clinical phase studies using a highly automated ECG analysis approach. Br. J. Pharmacol. 2016, 173, 1373–1380. [Google Scholar] [CrossRef]
- Gough, K.; Hutchison, M.; Keene, O.; Byrom, B.; Ellis, S.; Lacey, L.; McKellar, J. Assessment of dose proportionality: Report from the statisticians in the pharmaceutical industry/pharmacokinetics UK joint working party. Drug Inf. J. 1995, 29, 1039–1048. [Google Scholar] [CrossRef]
- Kligfield, P.; Tyl, B.; Maarek, M.; Maison-Blanche, P. Magnitude, mechanism, and reproducibility of QT interval differences between superimposed global and individual lead ECG complexes. Ann. Noninvasive Electrocardiol. 2007, 12, 145–152. [Google Scholar] [CrossRef]
- Shah, R.R.; Maison-Blanche, P.; Duvauchelle, T.; Robert, P.; Denis, E. Establishing assay sensitivity in QT studies: Experience with the use of moxifloxacin in an early phase clinical pharmacology study and comparison with its effect in a thorough QT study. Eur. J. Clin. Pharmacol. 2015, 71, 1451–1459. [Google Scholar] [CrossRef]
- Ferber, G.; Lorch, U.; Täubel, J. The power of phase I studies to detect clinical relevant QTc prolongation: A resampling simulation study. BioMed Res. Int. 2015, 2015, 293564. [Google Scholar] [CrossRef] [PubMed]
- Ferber, G.; Zhou, M.; Darpo, B. Detection of QTc effects in small studies—Implications for replacing the thorough QT study. Ann. Noninvasive Electrocardiol. 2015, 20, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Tornøe, C.W.; Garnett, C.E.; Wang, Y.; Florian, J.; Li, M.; Gobburu, J.V. Creation of a knowledge management system for QT analyses. J. Clin. Pharmacol. 2011, 51, 1035–1042. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Treatment Arm | Control Arm | All Subjects | |
|---|---|---|---|---|
| ABC | DEF | GHI | Total | |
| n = 9 | n = 8 | n = 8 | n = 25 | |
| Female, n (%) | 8 (89%) | 5 (63%) | 6 (75%) | 19 (76%) |
| Male, n (%) | 1 (11%) | 3 (38%) | 2 (25%) | 6 (24%) |
| Race, n (%) Black/African American | 0 (0%) | 1 (13%) | 1 (13%) | 2 (8%) |
| Caucasian | 9 (100%) | 7 (88%) | 7 (88%) | 23 (92%) |
| Ethnicity, n (%) Hispanic/Latino | 8 (89%) | 5 (63%) | 6 (75%) | 19 (76%) |
| Not Hispanic/Latino | 1 (11%) | 3 (38%) | 2 (25%) | 6 (24%) |
| Age (years) a Mean ± SD (Range) | 38.7 ± 9.90 (24–53) | 36.6 ± 9.61 (24–49) | 34.5 ± 11.39 (18–46) | 36.7 ± 10.02 (18–53) |
| Weight (kg) Mean ± SD (Range) | 76.1 ± 13.86 (54.0–104.6) | 77.3 ± 14.65 (53.9–98.7) | 76.2 ± 13.21 (51.2–96.9) | 76.5 ± 13.34 (51.2–104.6) |
| Height (cm) Mean ± SD (Range) | 160.7 ± 9.63 (145–181) | 165.1 ± 11.76 (156–184) | 161.0 ± 7.35 (153–174) | 162.2 ± 9.55 (145–184) |
| BMI (kg m−2) b Mean ± SD (Range) | 29.24 ± 2.277 (25.68–31.97) | 28.19 ± 3.326 (22.03–31.77) | 29.22 ± 3.532 (21.38–31.93) | 28.90 ± 2.973 (21.38–31.97) |
| Adverse Event | 15 µg TTX a (%) (Severity/Relationship) | 30 µg TTX (%) (Severity/Relationship) | 45 µg TTX (%) (Severity/Relationship) | Overall TTX (%) | 400 mg b Moxifloxacin (%) | Placebo c (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Total TEAEs d | 9 (100%) | 24 (100%) | 46 (100%) | 79 (100%) | 14 (100%) | 8 (100%) | |||
| Arthralgia | 0 | 0 | 1 (2%) | 1 (1%) | 0 | 0 | |||
| Mild | Possibly | ||||||||
| Back Pain | 0 | 1 (4%) | 1 (2%) | 2 (3%) | 0 | 0 | |||
| Mild | Unlikely | Moderate | Unlikely | ||||||
| Chest Discomfort | 0 | 0 | 1 (2%) | 1 (1%) | 0 | 0 | |||
| Mild | Possibly | ||||||||
| Chills | 0 | 0 | 1 (2%) | 1 (1%) | 1 (7%) | 0 | |||
| Mild | Possibly | ||||||||
| Cough | 0 | 1 (4%) | 0 | 1 (1%) | 0 | 0 | |||
| Mild | Unlikely | ||||||||
| Dizziness | 1 (11%) | 3 (13%) | 4 (9%) | 8 (10%) | 2 (14%) | 1 (13%) | |||
| Mild | Probably | 3x Mild | 3x Probably | 4x Mild | 4x Probably | ||||
| Dry Throat | 0 | 0 | 1 (2%) | 1 (1%) | 0 | 0 | |||
| Mild | Probably | ||||||||
| Ear Pruritus | 0 | 1 (4%) | 0 | 1 (1%) | 0 | 0 | |||
| Mild | Possibly | ||||||||
| Fatigue | 0 | 1 (4%) | 0 | 1 (1%) | 0 | 0 | |||
| Mild | Unlikely | ||||||||
| Feeling Hot | 0 | 0 | 1 (2%) | 1 (1%) | 0 | 1 (13%) | |||
| Mild | Possibly | ||||||||
| Headache | 3 (33%) | 2 (8%) | 4 (9%) | 9 (11%) | 1 (7%) | 2 (25%) | |||
| 2x Mild 1x Moderate | 2x Probably1x Possibly | 1x Mild 1x Moderate | 1x Probably 1x Possibly | 2x Mild 2x Moderate | 4x Probably | ||||
| Hyperhidrosis | 1 (11%) | 0 | 0 | 1 (1%) | 0 | 0 | |||
| Mild | Possibly | ||||||||
| Myalgia | 1 (11%) | 0 | 3 (7%) | 4 (5%) | 2 (14%) | 1 (13%) | |||
| Mild | Possibly | 3x Mild | 1x Probably 2x Unlikely | ||||||
| Nausea | 0 | 2 (8%) | 2 (4%) | 4 (5%) | 1 (7%) | 0 | |||
| 2x Mild | 2x Probably | 2x Mild | 2x Probably | ||||||
| Papule | 0 | 2 (8%) | 0 | 2 (3%) | 0 | 0 | |||
| 2x Mild | 2x Possibly | ||||||||
| Paresthesia | 2 (22%) | 6 (25%) | 10 (22%) | 18 (23%) | 0 | 0 | |||
| 2x Mild | 2x Probably | 6x Mild | 6x Probably | 10x Mild | 9x Probably 1x Possibly | ||||
| Paresthesia Oral | 0 | 3 (13%) | 8 (17%) | 11 (14%) | 0 | 0 | |||
| 3x Mild | 3x Probably | 8x Mild | 8x Probably | ||||||
| Pharyngeal Paresthesia | 0 | 0 | 3 (7%) | 3 (4%) | 0 | 0 | |||
| 3x Mild | 3x Probably | ||||||||
| Productive Cough | 0 | 0 | 1 (2%) | 1 (1%) | 0 | 0 | |||
| Mild | Possibly | ||||||||
| Pruritus | 0 | 1 (4%) | 2 (4%) | 3 (4%) | 0 | 0 | |||
| Mild | Possibly | 2x Mild | 2x Possibly | ||||||
| Rash Erythematous | 0 | 0 | 1 (2%) | 1 (1%) | 0 | 0 | |||
| Mild | Possibly | ||||||||
| Tachycardia | 1 (11%) | 0 | 0 | 1 (1%) | 0 | 0 | |||
| Mild | Possibly | ||||||||
| Throat Tightness | 0 | 0 | 1 (2%) | 1 (1%) | 0 | 0 | |||
| Mild | Probably | ||||||||
| Vessel Puncture Site Pain | 0 | 1 (4%) | 0 | 1 (1%) | 1 (7%) | 0 | |||
| Mild | Unrelated | ||||||||
| Wheezing | 0 | 0 | 1 (2%) | 1 (1%) | 0 | 0 | |||
| Mild | Possibly | ||||||||
| Parameters | 15 µg TTX a (n = 9) | 30 µg TTX (n = 9) | 45 µg TTX (n = 9) |
|---|---|---|---|
| AUC0–∞ (h × ng mL−1) | |||
| Geometric Mean | 1.9612 | 4.0094 | 6.4983 |
| Geometric (CV%) | (31.8) | (17.6) | (10.0) |
| Cmax (ng mL−1) | |||
| Geometric Mean | 0.3046 | 0.5807 | 0.9914 |
| Geometric (CV%) | (30.3) | (17.4) | (16.2) |
| Tmax (h) | |||
| Median | 1.50 | 1.50 | 1.50 |
| (Min, Max) | (1.00, 2.00) | (1.00, 1.51) | (1.00, 2.00) |
| t½ (h) | |||
| Arithmetic Mean | 4.62 | 4.54 | 4.28 |
| Standard Deviation | (±1.84) | (±0.39) | (±1.35) |
| Treatment | Geometric Mean Cmax (ng mL−1) | Predicted ΔΔQTcF (ms) (90% CI) | Model Slope (ms (ng mL−1)−1) (90% CI) | Model Intercept (ms) (90% CI) |
|---|---|---|---|---|
| 15 µg TTX a | 0.3046 | 0.285 (−0.255, 0.825) | −1.378 (−3.266, 0.510) | −0.705 (−0.131, 1.540) |
| 30 µg TTX | 0.5807 | −0.096 (−0.798, 0.607) | ||
| 45 µg TTX | 0.9914 | −0.661 (−2.003, 0.680) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavoosi, M.; O’Reilly, T.E.; Kavoosi, M.; Chai, P.; Engel, C.; Korz, W.; Gallen, C.C.; Lester, R.M. Safety, Tolerability, Pharmacokinetics, and Concentration-QTc Analysis of Tetrodotoxin: A Randomized, Dose Escalation Study in Healthy Adults. Toxins 2020, 12, 511. https://doi.org/10.3390/toxins12080511
Kavoosi M, O’Reilly TE, Kavoosi M, Chai P, Engel C, Korz W, Gallen CC, Lester RM. Safety, Tolerability, Pharmacokinetics, and Concentration-QTc Analysis of Tetrodotoxin: A Randomized, Dose Escalation Study in Healthy Adults. Toxins. 2020; 12(8):511. https://doi.org/10.3390/toxins12080511
Chicago/Turabian StyleKavoosi, Mojgan, Terry E. O’Reilly, Mehran Kavoosi, Peng Chai, Caroline Engel, Walter Korz, Christopher C. Gallen, and Robert M. Lester. 2020. "Safety, Tolerability, Pharmacokinetics, and Concentration-QTc Analysis of Tetrodotoxin: A Randomized, Dose Escalation Study in Healthy Adults" Toxins 12, no. 8: 511. https://doi.org/10.3390/toxins12080511
APA StyleKavoosi, M., O’Reilly, T. E., Kavoosi, M., Chai, P., Engel, C., Korz, W., Gallen, C. C., & Lester, R. M. (2020). Safety, Tolerability, Pharmacokinetics, and Concentration-QTc Analysis of Tetrodotoxin: A Randomized, Dose Escalation Study in Healthy Adults. Toxins, 12(8), 511. https://doi.org/10.3390/toxins12080511




