Botulinum Toxin A and Osteosarcopenia in Experimental Animals: A Scoping Review
Abstract
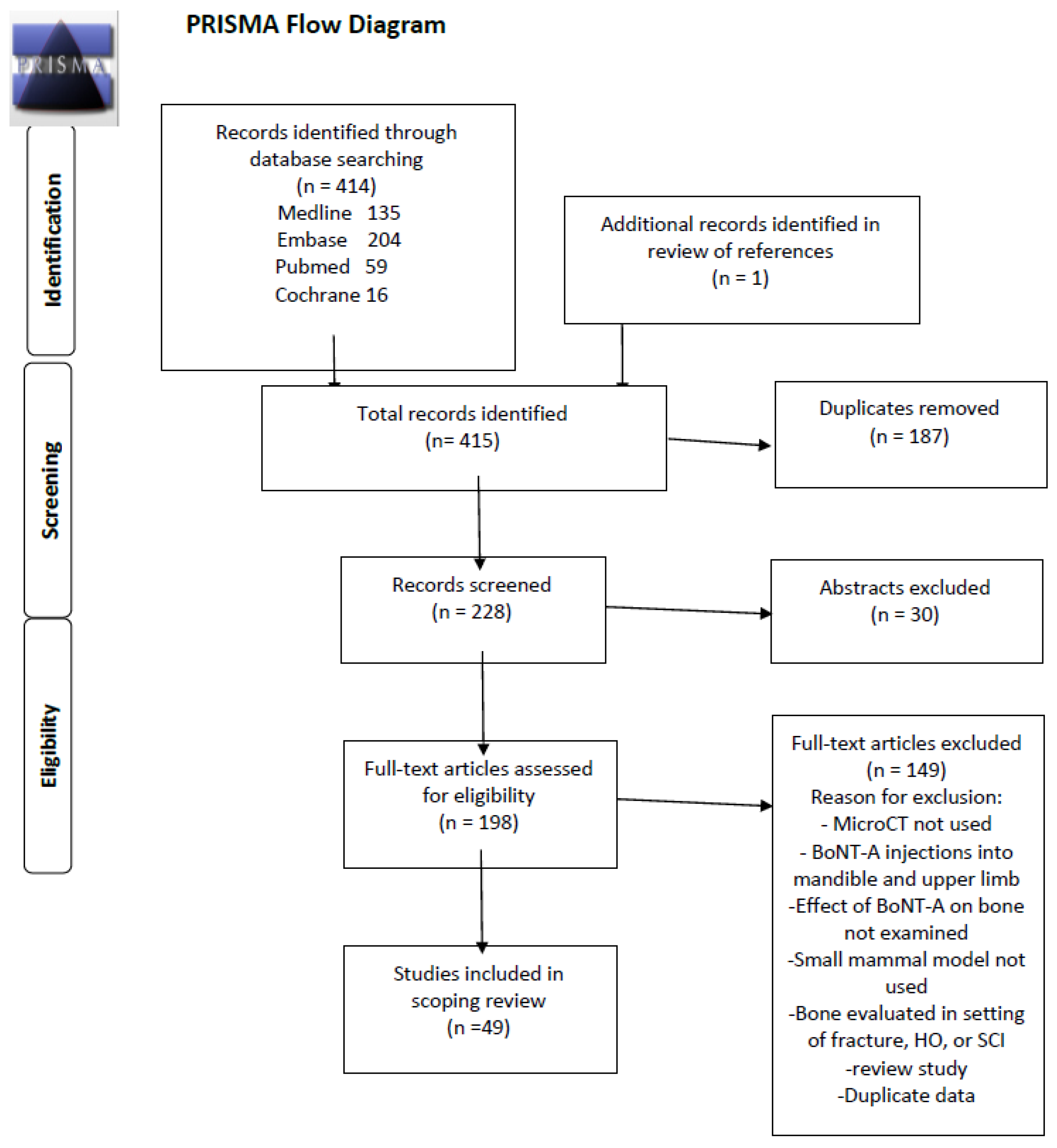
1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Inclusion Criteria
- Experimental studies with a full manuscript published in English that addressed effects of BoNT-A injections on bone in the hindlimb of mice or rats.
5.2. Exclusion Criteria
- Studies were excluded if BoNT-A injections were tested in non-small mammal animal models.
- Studies were excluded if the site of muscle injection was not in the lower limbs (e.g., masseter muscle to study the effects on the mandible).
- Studies were excluded if they did not include objective measurements of bone properties using micro-CT.
- Studies were excluded if they evaluated the effect of BoNT-A on fracture healing or in spinal cord injury.
5.3. Common Trabecular Measurements
- Bone volume/tissue volume (BV/TV): percentage of total tissue volume (cancellous space) occupied by trabecular bone.
- Trabecular thickness (Tb.Th): mean thickness of trabeculae.
- Trabecular number (Tb.N): average number of trabeculae per unit length.
- Trabecular separation (Tb.Sp): mean distance between trabeculae.
- Structural model index (SMI): indicates the structure of trabecular bone in the form of cylindrical rods or parallel plates—where rods confer a weaker formation (higher SMI) than plates (lower SMI).
- Connectivity density (CD): measure of the degree of connectivity of trabeculae (higher is stronger).
5.4. Common Cortical Measurements
- Cortical thickness (Ct.Th): mean cortical thickness.
- Cortical bone area (Ct.Ar): area occupied by cortical bone.
- Total cross-sectional tissue area (Tt.Ar): total area inside the periosteal envelope.
- Cortical area fraction (Ct.Ar/Tt.Ar): ratio of cortical bone to total tissue area inside periosteal envelope.
- Cortical marrow area (Ma.Ar): area occupied by medullary tissue.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BoNT-A | Botulinum toxin A |
| BMD | Bone mineral density |
| BMC | Bone mineral content |
| DEXA | Dual energy x-ray absorptiometry |
| CSA | Cross sectional area |
| BV/TV | Percentage of Bone volume to Total tissue volume |
| MAS | Modified Ashworth Scale |
| MTS | Modified Tardieu Scale |
| RCT | Randomized control trial |
Appendix A
| First Author/year | Species | Number | Follow Up (weeks) | Target Muscle(s) | BoNT-A Dose | Trabecular BV/TV Max. Loss | Cortical Area Max. Loss |
|---|---|---|---|---|---|---|---|
| Blouin 2006 [8] | Rat | 56 | 12 | Quad. | 2 U | 52% | N/A * |
| Warner 2006 [11] | Mouse | 20 | 3 | Quad, calf | 2 U/100 g | 54% | N/A |
| Grimston 2007 [12] | Mouse | 16 | 12 | Quad, ham, calf | 2 U/100 g | Decreased ** | 16% |
| Grimston 2011 [13] | Mouse | 40 | 19 | Quad, calf | 2 U/100 g | Decreased | Decreased |
| Liboubain 2008 [7] | Rat | 48 | 4 | Quad | 2 U | 30% | 9% |
| Liboubain 2018 [14] | Mouse | 21 | 12 | Quad | 2 U/100 g | 64% | N/A |
| Manske 2010 [15] | Mouse | 73 | 4 | Calf | 1 U/100 g | 22% | 8% |
| Manske 2010 [16] | Mouse | 36 | 16 | Calf | 1 U/100 g | 25% | 4% |
| Manske 2011 [17] | Mouse | 13 | 6 | Calf | 1 U/100 g | 28% | N/A |
| Manske 2012 [17] | Mouse | 27 | 3 | Calf | 1 U/100 g | Decreased | Decreased |
| Poliachik 2010 [18] | Mouse | 20 | 2 | Calf | 2 U/100 g | 81% | N/A |
| Poliachik 2014 [19] | Mouse | 64 | 1 | Calf | 2 U/100 g | Decreased | N/A |
| Agholme 2011 [20] | Rat | 40 | 4 | Quad, calf | 5 U | 66% | N/A |
| Agholme 2011 [21] | Rat | 48 | 4 | Quad, calf | 5 U | Decreased | N/A |
| Aliprantis 2012 [22] | Mouse | 36 | 2 | Quad, calf | 2 U/100 g | 51% | N/A |
| Bouvard 2012 [23] | Rat | 25 | 5 | Quad | 1.5 U | 55% | N/A |
| Sheng 2012 [24] | Rat | 21 | 9 | Quad | 2 U | 18% | 8% |
| Thomsen 2012 [25] | Rat | 108 | 4 | Quad, ham, calf | 1.7 U/100 g | 11% | N/A |
| Bruel 2013 [26] | Rat | 72 | 4 | Quad, ham, calf | 4 U | 34% | N/A |
| Marcias 2013 [27] | Rat | 80 | 1 | Quad, calf | 5 U | 58% | N/A |
| Marchand-Libouban 2013 [28] | Mouse | 40 | 4 | Quad | 2 U/100 g | 46% | 25% |
| Warden 2013 [29] | Mouse | 40 | 6 | Quad, ham, calf, Tib Ant | 0.5 U | Decreased | 9% |
| Ellman 2014 [30] | Mouse | 40 | 3 | Quad, calf | 2U/100 g | 66% | 24% |
| Grubbe 2014 [31] | Rat | 60 | 4 | Quad, ham, calf | 4 U | 26% | N/A |
| Morse 2014 [32] | Mouse | 20 | 2 | Quad, calf | 0.5 U | 25% | N/A |
| Sandberg 2014 [33] | Rat | 20 | 4 | Quad, calf | 5 U | 74% | N/A |
| Vegger 2014 [34] | Rat | 72 | 4 | Quad, ham, calf | 1.7 U/100 g | 25% | N/A |
| Vegger 2015 [35] | Rat | 57 | 8 | Quad, ham, calf | 1.7 U/100 g | 24% | N/A |
| Vegger 2016 [36] | Mouse | 35 | 3 | Quad, calf | 2 U/100 g | 62% | 15% |
| Vegger 2017 [37] | Mouse | 48 | 3 | Quad, calf | 2 U/100 g | 56% | 18% |
| Vegger 2018 [38] | Mouse | 42 | 3 | Quad, calf | 2 U/100 g | Decreased | 20% |
| Vegger 2018 [39] | Mouse | 48 | 3 | Quad, calf | 2 U/100 g | Decreased | 16% |
| Lodberg 2015 [40] | Mouse | 80 | 3 | Quad, calf | 2 U/100 g | 60% | N/A |
| Lodberg 2018 [41] | Mouse | 58 | 3 | Quad, calf | 2 U/100 g | Decreased | Decreased |
| Mabilleau 2015 [5] | Rat | 14 | 4 | Quad | 2 U | 17% | 5% |
| Niziolek 2015 [42] | Mouse | 24 | 3 | Quad, ham, calf, Tib Ant | 20 µL | 35% | 6% |
| Rucci 2015 [43] | Mouse | - | 3 | Quad, calf | 2 U/100 g | Decreased | N/A |
| Bach-Gansmo 2016 [44] | Rat | 77 | 28 | Quad, ham, calf | 1.7 U/100 g | N/A | N/A |
| Laurent 2016 [45] | Mouse | - | 19 | Quad, ham, calf | 2 U/100 g | Decreased | 8% |
| Canalis 2017 [46] | Rat | 12 | 3 | Quad, calf | 2 U/100 g | 59% | 16% |
| Brent 2018 [47] | Rat | 72 | 6 | Quad, ham, calf | 4 U | 28% | 8% |
| Omstrup 2018 [48] | Rat | 72 | 6 | Quad, ham, calf | 4 U | Decreased | 8% |
| Zhang 2018 [49] | Rat | 42 | 8 | Quad | 2 U | 46% | N/A |
| Bain 2019 [50] | Mouse | 16 | 4 | Calf | 2 U/100 g | 72% | N/A |
| Bullock 2019 [51] | Mouse | 80 | 4 | Quad, ham, calf, Tib Ant | 20 µL | 43% | N/A |
| Gatti 2019 [52] | Rat | 24 | 4 | Quad, ham, calf | 4 U | 18% | 14% |
| Xu 2019 [53] | Rat | 24 | 8 | Quad | 0.6 U/100 g | 22% | N/A |
| Liphardt 2020 [54] | Mouse | 33 | 2 | Calf | 2 U/100 g | Decreased | N/A |
| Sorensen 2020 [10] | Mouse | 81 | 3 | Rectus femoris, calf | 2 U/100 g | 57% | 11% |
References
- Graham, H.K.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.-P.; Damiano, D.L.; Becher, J.G.; Gaebler-Spira, D.; Colver, A.; Reddihough, D.S.; et al. Cerebral palsy. Nat. Rev. Dis. Primers 2016, 2, 15082. [Google Scholar] [CrossRef] [PubMed]
- Multani, I.; Manji, J.; Hastings-Ison, T.; Khot, A.; Graham, K. Botulinum Toxin in the Management of Children with Cerebral Palsy. Paediatr. Drugs 2019, 21, 261–281. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Truong, D.D. Expanding use of botulinum toxin. J. Neurol. Sci. 2005, 235, 1–9. [Google Scholar] [CrossRef]
- Tarabal, O.; Calderó, J.; Ribera, J.; Sorribas, A.; López, R.; Molgó, J.; Esquerda, J.E. Regulation of Motoneuronal Calcitonin Gene-related Peptide (CGRP) During Axonal Growth and Neuromuscular Synaptic Plasticity Induced by Botulinum Toxin in Rats. Eur. J. Neurosci. 1996, 8, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Multani, I.; Manji, J.; Tang, M.J.; Herzog, W.; Howard, J.J.; Graham, H.K. Sarcopenia, Cerebral Palsy, and Botulinum Toxin Type A. JBJS Rev. 2019, 7, e4. [Google Scholar] [CrossRef] [PubMed]
- Modlesky, C.M.; Zhang, C. Complicated Muscle-Bone Interactions in Children with Cerebral Palsy. Curr. Osteoporos. Rep. 2020, 18, 47–56. [Google Scholar] [CrossRef]
- Mabilleau, G.; Mieczkowska, A.; Libouban, H.; Simon, Y.; Audran, M.; Chappard, D. Comparison between quantitative X-ray imaging, dual energy X-ray absorptiometry and microCT in the assessment of bone mineral density in disuse-induced bone loss. J. Musculoskelet. Neuronal Interact. 2015, 15, 42–52. [Google Scholar]
- Blouin, S.; Gallois, Y.; Moreau, M.F.; Baslé, M.F.; Chappard, D. Disuse and orchidectomy have additional effects on bone loss in the aged male rat. Osteoporos. Int. 2006, 18, 85–92. [Google Scholar] [CrossRef]
- Libouban, H.; Blouin, S.; Moreau, M.F.; Basle, M.F.; Audran, M.; Chappard, D. Effects of risedronate in a rat model of osteo-penia due to orchidectomy and disuse: Densitometric, histomorphometric and microtomographic studies. Micron 2008, 39, 998–1007. [Google Scholar] [CrossRef]
- Sorensen, T.G.; Brent, M.B.; Thomsen, J.S.; Bruel, A. Disuse-induced loss of bone mineral density and bone strength is atten-uated by post-lactational bone gain in NMRI mice. Bone 2020, 131, 115183. [Google Scholar] [CrossRef]
- Warner, S.E.; Sanford, D.A.; Becker, B.A.; Bain, S.D.; Srinivasan, S.; Gross, T.S. Botox induced muscle paralysis rapidly de-grades bone. Bone 2006, 38, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Grimston, S.K.; Silva, M.J.; Civitelli, R. Bone Loss after Temporarily Induced Muscle Paralysis by Botox Is Not Fully Recovered After 12 Weeks. Ann. N. Y. Acad. Sci. 2007, 1116, 444–460. [Google Scholar] [CrossRef]
- Grimston, S.K.; Goldberg, D.B.; Watkins, M.; Brodt, M.D.; Silva, M.J.; Civitelli, R. Connexin43 deficiency reduces the sensi-tivity of cortical bone to the effects of muscle paralysis. J. Bone Miner. Res. 2011, 26, 2151–2160. [Google Scholar] [CrossRef]
- Libouban, H.; Guintard, C.; Minier, N.; Aguado, E.; Chappard, D. Long-Term Quantitative Evaluation of Muscle and Bone Wasting Induced by Botulinum Toxin in Mice Using Microcomputed Tomography. Calcif. Tissue Int. 2017, 102, 695–704. [Google Scholar] [CrossRef]
- Manske, S.L.; Boyd, S.K.; Zernicke, R.F. Muscle Changes Can Account for Bone Loss After Botulinum Toxin Injection. Calcif. Tissue Int. 2010, 87, 541–549. [Google Scholar] [CrossRef]
- Manske, S.L.; Boyd, S.K.; Zernicke, R.F. Muscle and bone follow similar temporal patterns of recovery from muscle-induced disuse due to botulinum toxin injection. Bone 2010, 46, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Manske, S.L.; Boyd, S.K.; Zernicke, R.F. Vertical ground reaction forces diminish in mice after botulinum toxin injection. J. Biomech. 2011, 44, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Poliachik, S.L.; Bain, S.D.; Threet, D.; Huber, P.; Gross, T.S. Transient muscle paralysis disrupts bone homeostasis by rapid degradation of bone morphology. Bone 2010, 46, 18–23. [Google Scholar] [CrossRef]
- Poliachik, S.L.; Khokhlova, T.D.; Wang, Y.N.; Simon, J.C.; Bailey, M.R. Pulsed focused ultrasound treatment of muscle miti-gates paralysis-induced bone loss in the adjacent bone: A study in a mouse model. Ultrasound Med. Biol. 2014, 40, 2113–2124. [Google Scholar] [CrossRef][Green Version]
- Agholme, F.; Isaksson, H.; Kuhstoss, S.; Aspenberg, P. The effects of Dickkopf-1 antibody on metaphyseal bone and implant fixation under different loading conditions. Bone 2011, 48, 988–996. [Google Scholar] [CrossRef]
- Agholme, F.; Isaksson, H.; Li, X.; Ke, H.Z.; Aspenberg, P. Anti-sclerostin antibody and mechanical loading appear to influence metaphyseal bone independently in rats. Acta Orthop. 2011, 82, 628–632. [Google Scholar] [CrossRef]
- Aliprantis, A.O.; Stolina, M.; Kostenuik, P.J.; Poliachik, S.L.; Warner, S.E.; Bain, S.D.; Gross, T.S. Transient muscle paralysis degrades bone via rapid osteoclastogenesis. FASEB J. 2011, 26, 1110–1118. [Google Scholar] [CrossRef]
- Bouvard, B.; Mabilleau, G.; Legrand, E.; Audran, M.; Chappard, D. Micro and macroarchitectural changes at the tibia after botulinum toxin injection in the growing rat. Bone 2012, 50, 858–864. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sheng, Z.-F.; Ma, Y.-L.; Tong, D.; Fang, D.-Y.; Liang, Q.-C.; Liu, L.-H.; Zhang, J.; Liao, E.-Y. Strontium Ranelate Prevents Bone Loss in a Rat Model of Localized Muscle Paralysis. Ann. Biomed. Eng. 2012, 40, 657–665. [Google Scholar] [CrossRef]
- Thomsen, J.S.; Christensen, L.L.; Vegger, J.B.; Nyengaard, J.R.; Brüel, A. Loss of Bone Strength is Dependent on Skeletal Site in Disuse Osteoporosis in Rats. Calcif. Tissue Int. 2012, 90, 294–306. [Google Scholar] [CrossRef]
- Brüel, A.; Vegger, J.B.; Raffalt, A.C.; Andersen, J.E.T.; Thomsen, J.S. PTH (1–34), but not strontium ranelate counteract loss of trabecular thickness and bone strength in disuse osteopenic rats. Bone 2013, 53, 51–58. [Google Scholar] [CrossRef]
- Macias, B.R.; Aspenberg, P.; Agholme, F. Paradoxical Sost gene expression response to mechanical unloading in metaphyse-al bone. Bone 2013, 53, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Marchand-Libouban, H.; Le Drevo, M.A.; Chappard, D. Disuse induced by botulinum toxin affects the bone marrow expres-sion profile of bone genes leading to a rapid bone loss. J. Musculoskelet. Neuronal Interact. 2013, 13, 27–36. [Google Scholar]
- Warden, S.J.; Galley, M.R.; Richard, J.S.; George, L.A.; Dirks, R.C.; Guildenbecher, E.A.; Judd, A.M.; Robling, A.G.; Fuchs, R.K. Reduced gravitational loading does not account for the skeletal effect of botu-linum toxin-induced muscle inhibition suggesting a direct effect of muscle on bone. Bone 2013, 54, 98–105. [Google Scholar] [CrossRef]
- Ellman, R.; Grasso, D.J.; Van Vliet, M.; Brooks, D.J.; Spatz, J.M.; Conlon, C.; Bouxsein, M.L. Combined Effects of Botulinum Toxin Injection and Hind Limb Unloading on Bone and Muscle. Calcif. Tissue Int. 2014, 94, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Grubbe, M.C.; Thomsen, J.S.; Nyengaard, J.R.; Duruox, M.; Bruel, A. Growth hormone mitigates loss of periosteal bone for-mation and muscle mass in disuse osteopenic rats. J. Musculoskelet. Neuronal Interact. 2014, 14, 473–483. [Google Scholar]
- Morse, A.; McDonald, M.M.; Kelly, N.H.; Melville, K.M.; Schindeler, A.; Kramer, I.; Kneissel, M.; Van Der Meulen, M.C.; Little, D.G. Mechanical Load Increases in Bone Formation via a Sclerostin-Independent Pathway. J. Bone Miner. Res. 2014, 29, 2456–2467. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, O.; Macias, B.R.; Aspenberg, P. Low dose PTH improves metaphyseal bone healing more when muscles are para-lyzed. Bone 2014, 63, 15–19. [Google Scholar] [CrossRef]
- Vegger, J.B.; Nielsen, E.S.; Brüel, A.; Thomsen, J.S. Additive effect of PTH (1–34) and zoledronate in the prevention of disuse osteopenia in rats. Bone 2014, 66, 287–295. [Google Scholar] [CrossRef]
- Vegger, J.B.; Bruel, A.; Thomsen, J.S. Vertical Trabeculae are Thinned More Than Horizontal Trabeculae in Skele-tal-Unloaded Rats. Calcif. Tissue Int. 2015, 97, 516–526. [Google Scholar] [CrossRef]
- Vegger, J.; Brüel, A.; Dahlgaard, A.; Thomsen, J. Alterations in gene expression precede sarcopenia and osteopenia in botulinum toxin immobilized mice. J. Musculoskelet. Neuronal Interact. 2016, 16, 355–368. [Google Scholar]
- Vegger, J.; Brüel, A.; Thomsen, J. Pantoprazole, a proton pump inhibitor, does not prevent botulinum toxin induced disuse osteopenia in mice. J. Musculoskelet. Neuronal Interact. 2017, 17, 162–175. [Google Scholar] [PubMed]
- Vegger, J.B.; Brüel, A.; Brent, M.B.; Thomsen, J.S. Disuse osteopenia induced by botulinum toxin is similar in skeletally mature young and aged female C57BL/6J mice. J. Bone Miner. Metab. 2017, 36, 170–179. [Google Scholar] [CrossRef]
- Vegger, J.B.; Brüel, A.; Thomsen, J.S. Zoledronic acid prevents disuse osteopenia and augments gene expression of osteoclastic differentiation markers in mice. J. Musculoskelet. Neuronal Interact. 2018, 18, 165–175. [Google Scholar]
- Lodberg, A.; Vegger, J.B.; Jensen, M.V.; Larsen, C.M.; Thomsen, J.S.; Brüel, A. Immobilization induced osteopenia is strain specific in mice. Bone Rep. 2015, 2, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lodberg, A.; Eijken, M.; van der Eerden, B.C.; Okkels, M.W.; Thomsen, J.S.; Brüel, A. A soluble activin type IIA receptor mitigates the loss of femoral neck bone strength and cancellous bone mass in a mouse model of disuse osteopenia. Bone 2018, 110, 326–334. [Google Scholar] [CrossRef]
- Niziolek, P.J.; Bullock, W.; Warman, M.L.; Robling, A.G. Missense Mutations in LRP5 Associated with High Bone Mass Pro-tect the Mouse Skeleton from Disuse- and Ovariectomy-Induced Osteopenia. PLoS ONE 2015, 10, e0140775. [Google Scholar] [CrossRef]
- Rucci, N.; Capulli, M.; Piperni, S.G.; Cappariello, A.; Lau, P.; Frings-Meuthen, P.; Heer, M.; Teti, A. Lipocalin 2: A New Mechanoresponding Gene Regulating Bone Homeostasis. J. Bone Miner. Res. 2015, 30, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Bach-Gansmo, F.L.; Wittig, N.K.; Brüel, A.; Thomsen, J.S.; Birkedal, H. Immobilization and long-term recovery results in large changes in bone structure and strength but no corresponding alterations of osteocyte lacunar properties. Bone 2016, 91, 139–147. [Google Scholar] [CrossRef]
- Laurent, M.R.; Jardí, F.; Dubois, V.; Schollaert, D.; Khalil, R.; Gielen, E.; Carmeliet, G.; Claessens, F.; Vanderschueren, D. Androgens have antiresorptive effects on trabecular disuse osteopenia independent from muscle atrophy. Bone 2016, 93, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Schilling, L.; Zanotti, S. Effects of Sex and Notch Signaling on the Osteocyte Cell Pool. J. Cell. Physiol. 2016, 232, 363–370. [Google Scholar] [CrossRef]
- Brent, M.B.; Brüel, A.; Thomsen, J.S. PTH (1–34) and growth hormone in prevention of disuse osteopenia and sarcopenia in rats. Bone 2018, 110, 244–253. [Google Scholar] [CrossRef]
- Ormstrup, M.J.; Bruel, A.; Thomsen, J.S.; Harslof, T.; Langdahl, B.L.; Pedersen, S.B. Long-Term High-Dose Resveratrol Sup-plementation Reduces Bone Mass and Fracture Strength in Rats. Calcif. Tissue Int. 2018, 102, 337–347. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, H.; Zhou, Y.; Liu, S.; Xie, D.; Fu, S.; Fu, S.; Xie, H.; Sheng, Z. Relationship of the expression of myogenic factors in disused rats with bone mass loss and microstructural degeneration. Osteoporos. Sarcopenia 2017, 3, S29–S30. [Google Scholar] [CrossRef]
- Bain, S.D.; Huber, P.; Ausk, B.J.; Kwon, R.Y.; Gardiner, E.M.; Srinivasan, S.; Gross, T.S. Neuromuscular dysfunction, independent of gait dysfunction, modulates trabecular bone homeostasis in mice. J. Musculoskelet. Neuronal Interact. 2019, 19, 79–93. [Google Scholar] [PubMed]
- Bullock, W.A.; Hoggatt, A.M.; Horan, D.J.; Lewis, K.J.; Yokota, H.; Hann, S.; Warman, M.L.; Sebastian, A.; Loots, G.G.; Pavalko, F.M.; et al. Expression of a Degradation-Resistant beta-Catenin Mutant in Osteocytes Protects the Skeleton From Mechanodeprivation-Induced Bone Wasting. J. Bone Miner. Res. 2019, 34, 1964–1975. [Google Scholar] [CrossRef]
- Gatti, V.; Ghobryal, B.; Gelbs, M.J.; Gerber, M.B.; Doty, S.B.; Cardoso, L.; Fritton, S.P. Botox-induced muscle paralysis alters intracortical porosity and osteocyte lacunar density in skeletally mature rats. J. Orthop. Res. 2019, 37, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; She, G.; Gui, T.; Hou, H.; Li, J.; Chen, Y.; Zha, Z. Knee muscle atrophy is a risk factor for development of knee osteoarthritis in a rat model. J. Orthop. Transl. 2020, 22, 67–72. [Google Scholar] [CrossRef]
- Liphardt, A.-M.; Windahl, S.H.; Sehic, E.; Hannemann, N.; Gustafsson, K.L.; Bozec, A.; Schett, G.; Engdahl, C. Changes in mechanical loading affect arthritis-induced bone loss in mice. Bone 2020, 131, 115149. [Google Scholar] [CrossRef] [PubMed]
- Kaňovský, P.; Bareš, M.; Severa, S.; Richardson, A.; On Behalf Of The Dysport Paediatric Limb Spasticity Study Group. Long-term efficacy and tolerability of 4-monthly versus yearly botulinum toxin type A treatment for lower-limb spasticity in children with cerebral palsy. Dev. Med. Child Neurol. 2009, 51, 436–445. [Google Scholar] [CrossRef]
- Hastings-Ison, T.; Blackburn, C.; Rawicki, B.; Fahey, M.; Simpson, P.; Baker, R.; Graham, K. Injection frequency of botulinum toxin A for spastic equinus: A randomized clinical trial. Dev. Med. Child Neurol. 2015, 58, 750–757. [Google Scholar] [CrossRef] [PubMed]
| BV/TV | Tb.Th | Tb.N | Tb.Sp | SMI | CD | |
|---|---|---|---|---|---|---|
| N (articles with objective measurements) | 31 | 28 | 30 | 26 | 17 | 14 |
| −43 (19) | −20 (11) | −15 (15) | 17 (28) | 126 (203) | 2 (50) | |
| Min % | −81 | −46 | −68 | 0 | −10 | −68 |
| Max % | −11 | 4 | 9 | 164 | 800 | 163 |
| N (articles with metaphyseal measurements) | 30 | 27 | 29 | 25 | 16 | 14 |
| −42 (19) | −17 (14) | −16 (16) | 20 (31) | 42 (78) | −21 (23) | |
| N (articles with epiphyseal measurements) | 4 | 4 | 6 | 6 | 5 | 4 |
| −46 (15) | −28 (13) | -10 (8) | 8 (5) | 440 (222) | 73 (48) |
| Ct.Th | Ct.Ar | Ma.Ar | Tt.Ar | |
|---|---|---|---|---|
| N (articles with objective measurements) | 17 | 18 | 11 | 14 |
| Min % | −27 | −25 | −3 | −6 |
| Max % | −2 | 2 | 14 | 5 |
| Ovid Search Number | Search Terms |
|---|---|
| 1 | exp Botulinum Toxins/ |
| 2 | (botox or botulinum or BTX-A or BTXA or BONT-A or BONTA or dysport or xeomin).tw,kf. |
| 3 | (rimobotulinum adj toxin adj b) or myobloc or (abobotulinum adj toxin adj a) or (onabotulinum adj toxin adj a) or (incobotulinum adj toxin adj a) or (letibotulinum adj toxin adj a) or (abobotulinumtoxin adj a) or (onabotulinumtoxin adj a) or (incobotulinumtoxin adj a) or (letibotulinumtoxin adj a)).tw,kf. |
| 4 | mouse or mice or rat or rats or murine).af. |
| 5 | osteoporosis/or bone resorption/or osteolysis/ |
| 6 | “bones of lower extremity”/or exp leg bones/ |
| 7 | Bone Density/ |
| 8 | Bone Diseases, Metabolic/ |
| 9 | (bone or bones or osteopenia or osteoporosis or osteolysis or femur or tibia).tw,kf,hw. |
| 10 | (1 or 2 or 3) and 4 and (5 or 6 or 7 or 8 or 9) |
| 11 | Limit 10 to (English language and yr = “1990–Current”) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.J.; Graham, H.K.; Davidson, K.E. Botulinum Toxin A and Osteosarcopenia in Experimental Animals: A Scoping Review. Toxins 2021, 13, 213. https://doi.org/10.3390/toxins13030213
Tang MJ, Graham HK, Davidson KE. Botulinum Toxin A and Osteosarcopenia in Experimental Animals: A Scoping Review. Toxins. 2021; 13(3):213. https://doi.org/10.3390/toxins13030213
Chicago/Turabian StyleTang, Min Jia, H. Kerr Graham, and Kelsey E. Davidson. 2021. "Botulinum Toxin A and Osteosarcopenia in Experimental Animals: A Scoping Review" Toxins 13, no. 3: 213. https://doi.org/10.3390/toxins13030213
APA StyleTang, M. J., Graham, H. K., & Davidson, K. E. (2021). Botulinum Toxin A and Osteosarcopenia in Experimental Animals: A Scoping Review. Toxins, 13(3), 213. https://doi.org/10.3390/toxins13030213




