Extensive Variation in the Activities of Pseudocerastes and Eristicophis Viper Venoms Suggests Divergent Envenoming Strategies Are Used for Prey Capture
Abstract
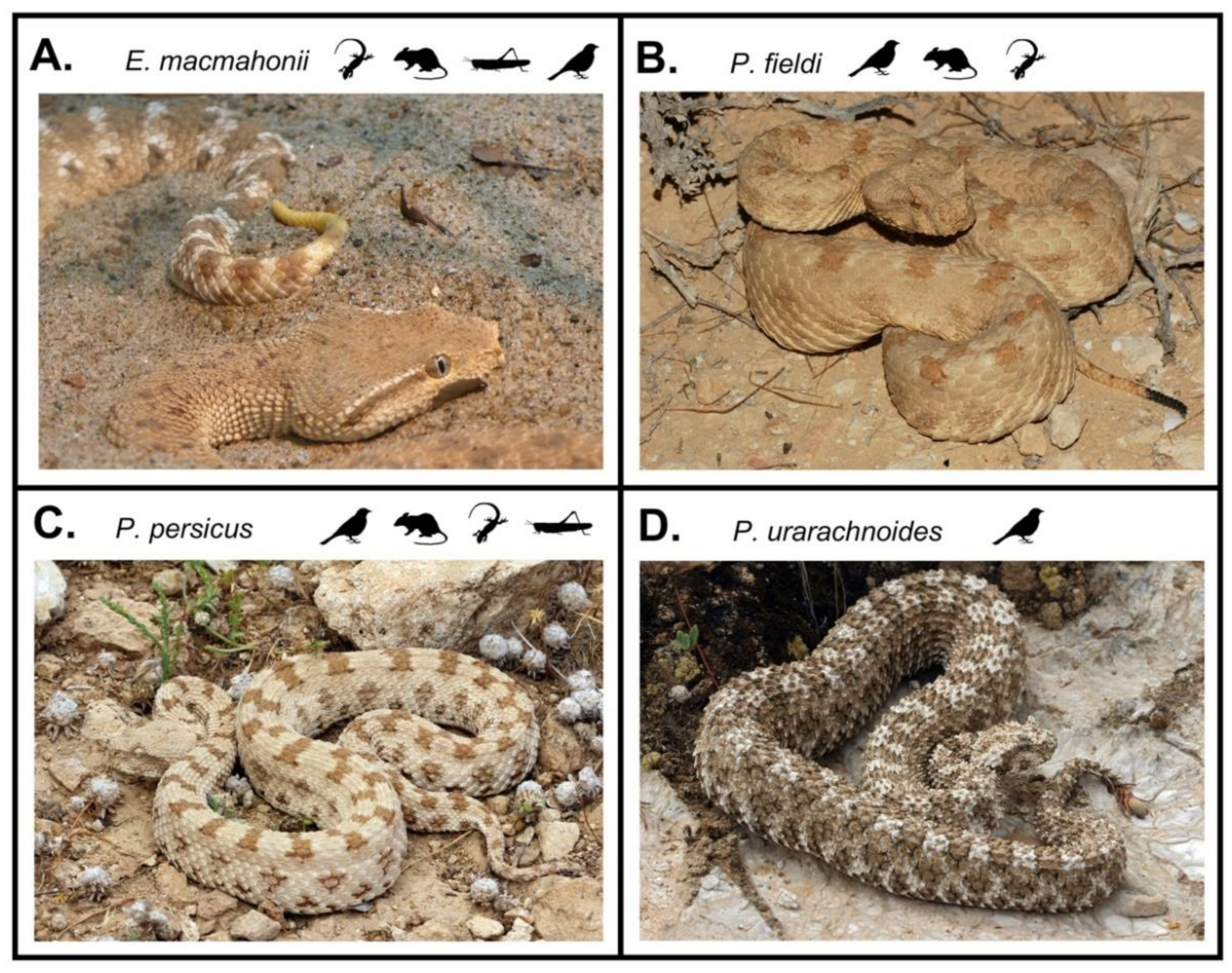
1. Introduction
2. Results and Discussion
2.1. Venom Proteomics
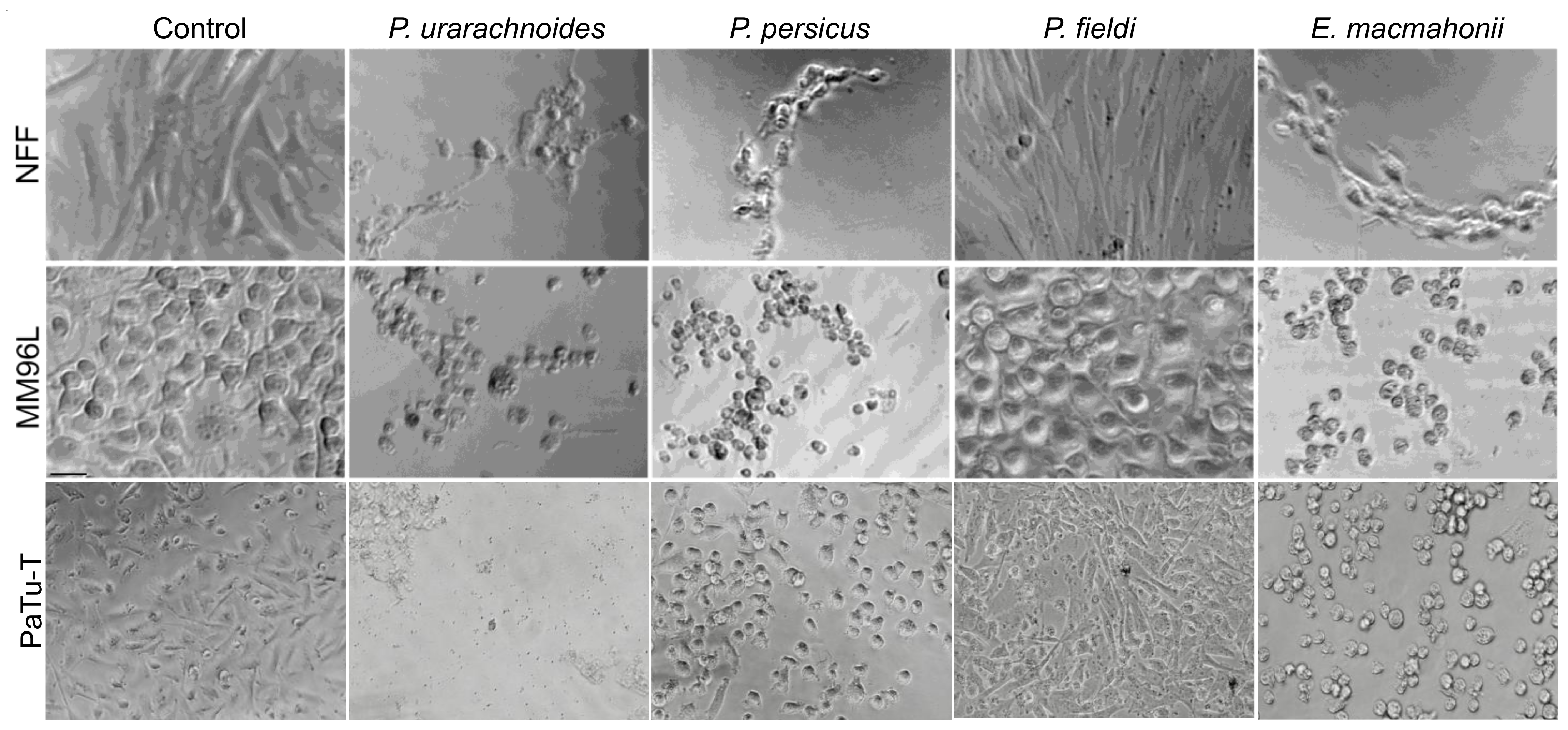
2.2. Venom-Induced Cytotoxicity
2.3. Venom-Induced Neurotoxicity
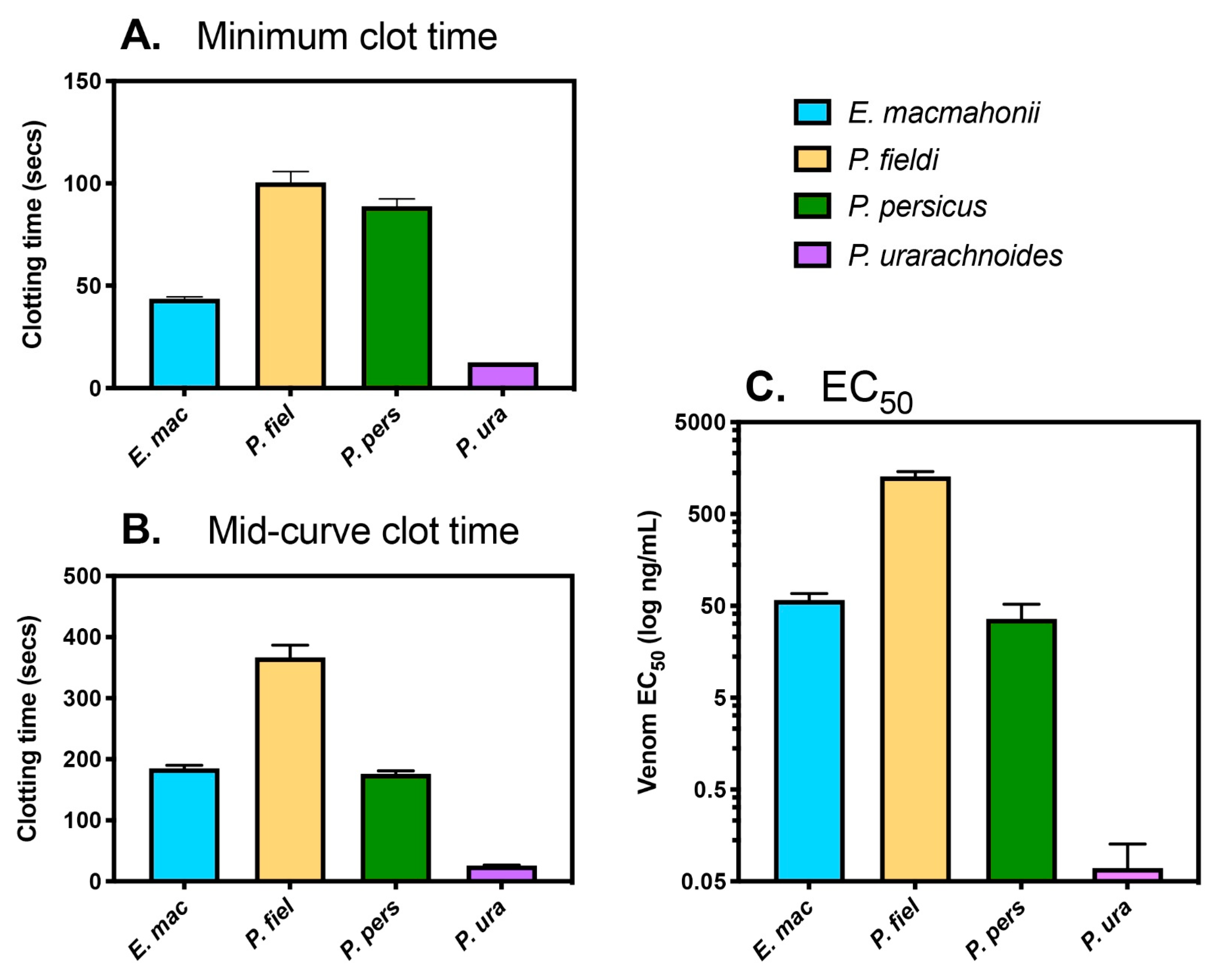
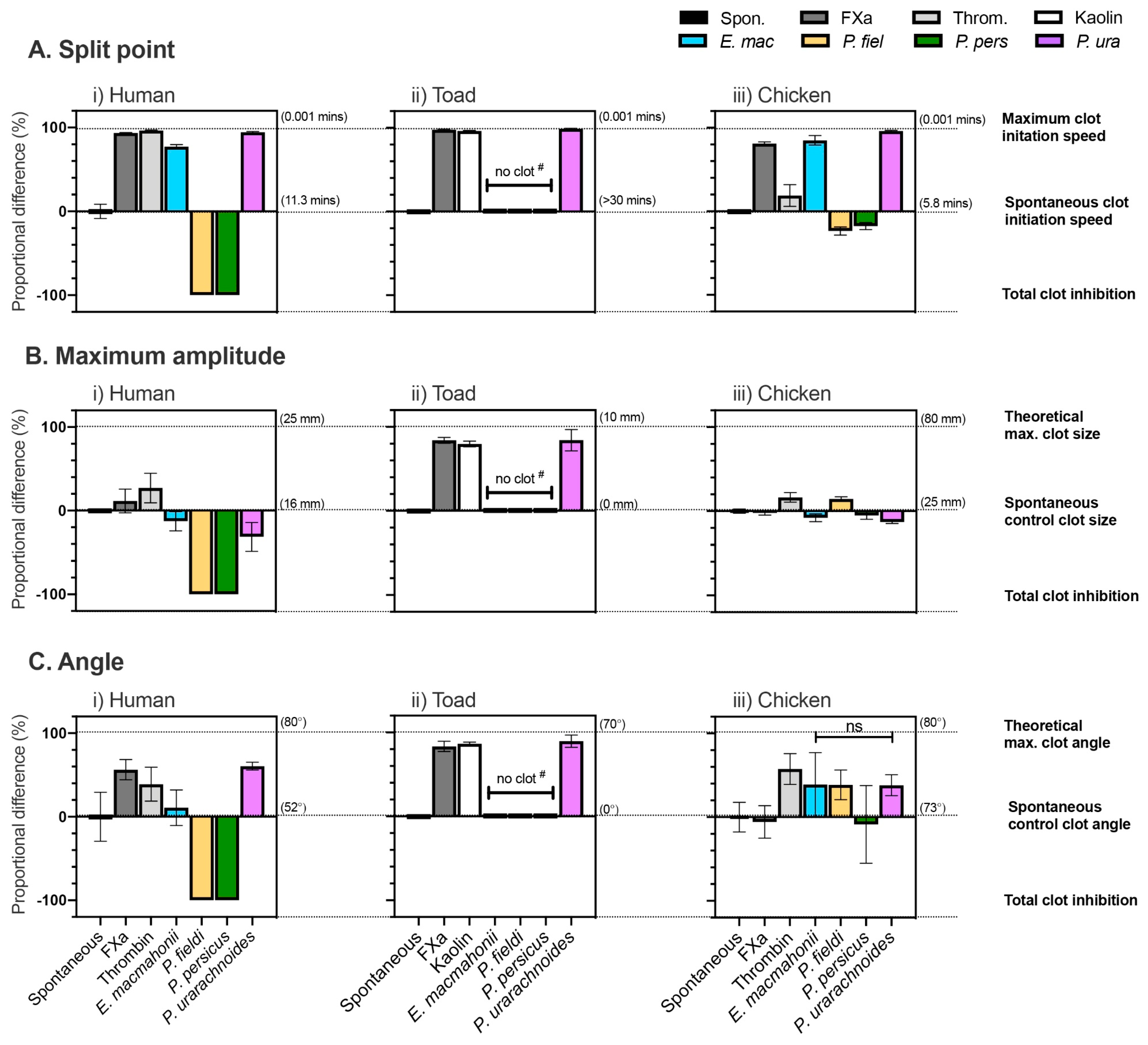
2.4. Venom-Induced Coagulant Activity
2.4.1. Coagulation Time, Strength, and Potency
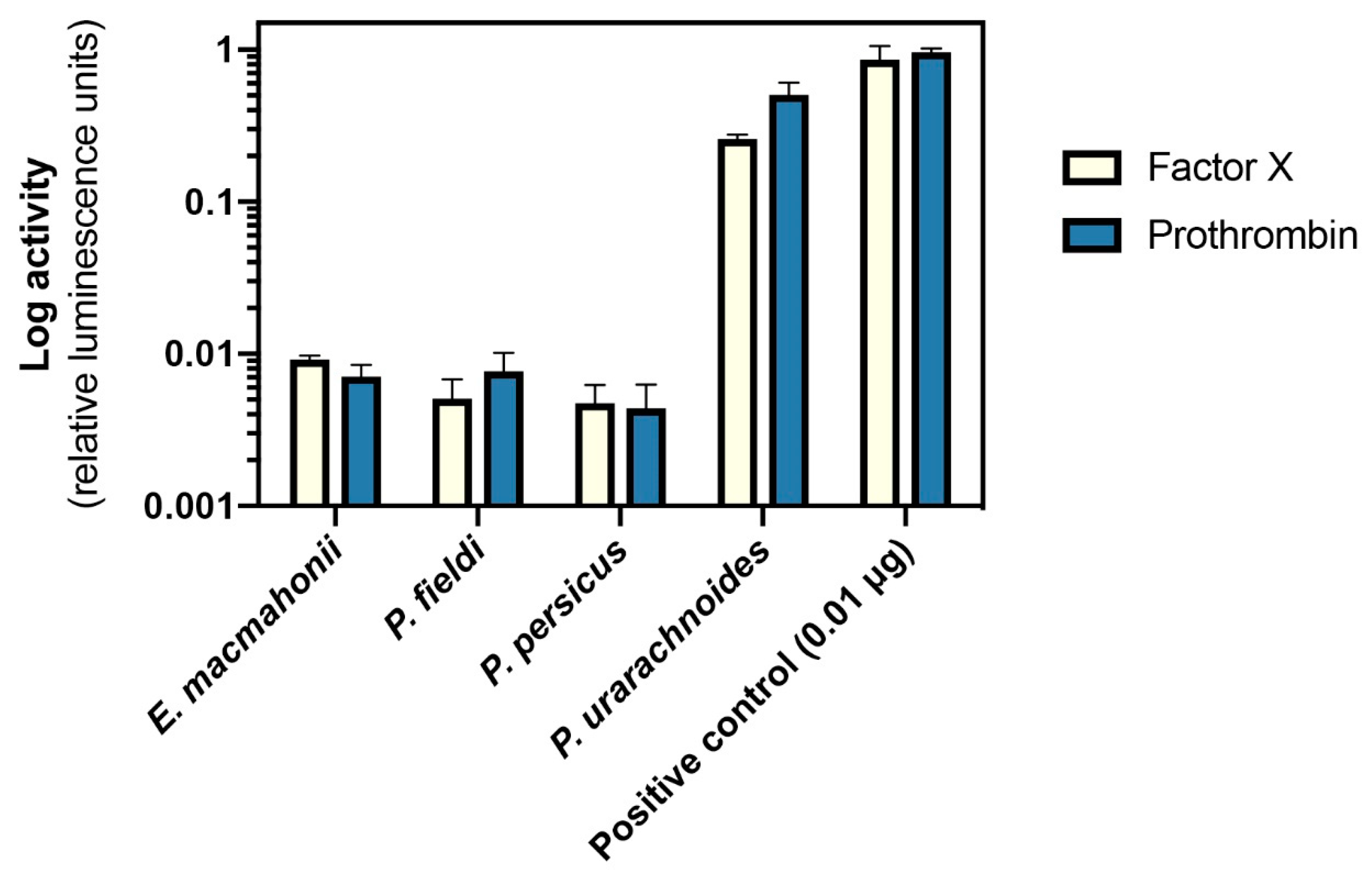
2.4.2. Activation of Factor X and Prothrombin
2.4.3. Thromboelastography
3. Conclusions
4. Materials and Methods
4.1. Venom Samples
4.2. Plasma
4.3. Proteomics
4.3.1. Venom SDS-PAGE Gel Electrophoresis
4.3.2. Liquid Chromatography-Mass Spectrometry
4.4. Cytotoxicity
4.5. Neurotoxicity
4.6. Coagulation Analyses
4.6.1. Clot Formation Time
4.6.2. Factor X and Prothrombin Activation
4.6.3. Thromboelastography
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef]
- Phelps, T. Old World Vipers, A Natural History of the Azemiopinae and Viperinae; Edition Chimaira: Frankfurt am Main, Germany, 2010. [Google Scholar]
- Bostanchi, H.; Anderson, S.C.; Kami, H.G.; Papenfuss, T.J. A new species of Pseudocerastes with elaborate tail ornamentation from western Iran (Squamata: Viperidae). Proc. Cal. Acad. Sci. 2006, 57, 443. [Google Scholar]
- Fathinia, B.; Rastegar-Pouyani, N.; Rastegar-Pouyani, E. Molecular phylogeny and historical biogeography of genera Eristicophis and Pseudocerastes (Ophidia, Viperidae). Zool. Scr. 2018, 47, 673–685. [Google Scholar] [CrossRef]
- Wüster, W.; Peppin, L.; Pook, C.E.; Walker, D.E. A nesting of vipers: Phylogeny and historical biogeography of the Viperidae (Squamata: Serpentes). Mol. Phylogenet. Evol. 2008, 49, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Alencar, L.R.; Quental, T.B.; Grazziotin, F.G.; Alfaro, M.L.; Martins, M.; Venzon, M.; Zaher, H. Diversification in vipers: Phylogenetic relationships, time of divergence and shifts in speciation rates. Mol. Phylogenet. Evol. 2016, 105, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Smid, J.; Tolley, K.A. Calibrating the tree of vipers under the fossilized birth-death model. Sci. Rep. 2019, 9, 5510. [Google Scholar] [CrossRef] [PubMed]
- Mallow, D.; Ludwig, D.; Nilson, G. True Vipers: Natural History and Toxinology of Old World Vipers; Kreiger Publishing Company: Malabar, FL, USA, 2003. [Google Scholar]
- Del Marmol, G.M.; Mozaffari, O.; Gállego, J. Pseudocerastes urarachnoides: The ambush specialist. Waterbirds 2016, 10, 117–126. [Google Scholar]
- Fathinia, B.; Anderson, S.C.; Rastegar-Pouyani, N.; Jahani, H.; Mohamadi, H. Notes on the natural history of Pseudocerastes urarachnoides (Squamata: Viperidae). Rus. J. Herpetol. 2009, 16, 134–138. [Google Scholar]
- Al-Sheikhly, O.F.; Al-Barazengy, A.N.; LAl-Haideri, M. First record of the Iranian Spider Viper Pseudocerastes urarachnoides Bostanchi, Anderson, Kami & Papenfuss, 2006 (Serpentes: Viperidae) in Iraq. SAURIA Berl. 2019, 41, 43–46. [Google Scholar]
- De Pous, P.; Simó-Riudalbas, M.; Els, J.; Jayasinghe, S.; Amat, F.; Carranza, S. Phylogeny and biogeography of Arabian populations of the Persian horned viper Pseudocerastes persicus (Duméril, Bibron & Duméril, 1854). Zool. Mid. East 2016, 62, 231–238. [Google Scholar]
- Bok, B.; Berroneau, M.; Yousefi, M.; Nerz, J.; Deschandol, F.; Berroneau, M.; Tiemann, L. Sympatry of Pseudocerastes persicus and P. urarachnoides in the western Zagros Mountains, Iran. Herpetol. Notes 2017, 10, 323–325. [Google Scholar]
- Gholamifard, A.; Esmaeili, H.R. First record and range extension of Field’s horned viper, Pseudocerastes fieldi Schmidt, 1930 (Squamata: Viperidae), from Fars province, southern Iran. Turk. J. Zool. 2010, 34, 551–552. [Google Scholar]
- Fathinia, B.; Rastegar-Pouyani, N. On the species of Pseudocerastes (Ophidia: Viperidae) in Iran. Russ. J. Herpetol. 2010, 17, 275–279. [Google Scholar]
- Baig, K.J.; Awan, M.R.; Ashraf, N. Ecological studies and zoogeographic affinities of the amphibians and reptiles found in Chagai desert, Balochistan, Pakistan. Pak. J. Zool. 2006, 38, 145. [Google Scholar]
- Khan, M.S. A Guide to the Snakes of Pakistan; Edition Chimaira: Frankfurt am Main, Germany, 2002. [Google Scholar]
- Minton, S.A. A Contribution to the Herpetology of West Pakistan; American Museum of Natural History: New York, NY, USA, 1966. [Google Scholar]
- Mendelssohn, H. On the biology of the venomous snakes of Israel. II. Isr. J. Ecol. Evol. 1965, 14, 185–212. [Google Scholar]
- Amr, Z.S.; Disi, A.M. Systematics, distribution and ecology of the snakes of Jordan. Vertebr. Zool. 2011, 61, 179–266. [Google Scholar]
- Ali, S.A.; Jackson, T.N.; Casewell, N.R.; Low, D.H.; Rossi, S.; Baumann, K.; Fathinia, B.; Visser, J.; Nouwens, A.; Hendrikx, I.; et al. Extreme venom variation in Middle Eastern vipers: A proteomics comparison of Eristicophis macmahonii, Pseudocerastes fieldi and Pseudocerastes persicus. J. Proteom. 2015, 116, 106–113. [Google Scholar] [CrossRef]
- Carranza, S.; Xipell, M.; Tarroso, P.; Gardner, A.; Arnold, E.N.; Robinson, M.D.; Simó-Riudalbas, M.; Vasconcelos, R.; de Pous, P.; Amat, F.; et al. Diversity, distribution and conservation of the terrestrial reptiles of Oman (Sauropsida, Squamata). PLoS ONE 2018, 13, e0190389. [Google Scholar] [CrossRef]
- Thomas, O. Persian horned viper (Pseudocerastes persicus): A record at unusually low elevation from the United Arab Emirates. Herpetol. Bull. 2019, 147, 28–29. [Google Scholar] [CrossRef]
- Fathinia, B.; Rastegar-Pouyani, N.; Rastegar-Pouyani, E.; Todehdehghan, F.; Mansouri, M. Annual activity pattern of Pseudocerastes urarachnoides Bostanchi, Anderson, Kami & Papenfuss, 2006, with notes on its natural history (squamata: Serpentes: Viperidae). Herpetozoa 2017, 29, 135–142. [Google Scholar]
- Fathinia, B.; Rastegar-pouyani, N.; Rastegar-pouyani, E. Avian deception using an elaborate caudal lure in Pseudocerastes urarachnoides (Serpentes:Viperidae). Amphib. Reptil. 2015, 36, 223–231. [Google Scholar] [CrossRef]
- Alam, J.M.; Ali, S.A. Age-dependent variability of biochemical and biological properties in venoms of Leaf-Nose Viper snakes, Eristicophis macmahoni. Pak. J. Zool. 2001, 33, 173–177. [Google Scholar]
- Bdolah, A. Comparison of venoms from two subspecies of the false horned viper (Pseudocerastes persicus). Toxicon 1986, 24, 726–729. [Google Scholar] [CrossRef]
- Batzri-Izraeli, R.; Bdolah, A. Isolation and characterization of the main toxic fraction from the venom of the false horned viper (Pseudocerastes fieldi). Toxicon 1982, 20, 867–875. [Google Scholar] [CrossRef]
- Francis, B.; Bdolah, A.; Kaiser, I.I. Amino acid sequences of a heterodimeric neurotoxin from the venom of the false horned viper (Pseudocerastes fieldi). Toxicon 1995, 33, 863–874. [Google Scholar] [CrossRef]
- Tsai, M.; Lee, C.; Bdolah, A. Mode of neuromuscular blocking action of a toxic phospholipase A2 from Pseudocerastes fieldi (Field’s horned viper) snake venom. Toxicon 1983, 21, 527–534. [Google Scholar] [CrossRef]
- Bdolah, A.; Kinamon, S.; Batzri-Izraeli, R. The neurotoxic complex from the venom of Pseudocerastes fieldi. Contribution of the nontoxic subunit. Biochem. Int. 1985, 11, 627–636. [Google Scholar]
- Latifi, M. Variation in yield and lethality of venoms from Iranian snakes. Toxicon 1984, 22, 373–380. [Google Scholar] [CrossRef]
- Strickland, J.L.; Smith, C.F.; Mason, A.J.; Schield, D.R.; Borja, M.; Castañeda-Gaytán, G.; Spencer, C.L.; Smith, L.L.; Trápaga, A.; Bouzid, N.M.; et al. Evidence for divergent patterns of local selection driving venom variation in Mojave Rattlesnakes (Crotalus scutulatus). Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- David, P.; Vogel, G. Venomous Snakes of Europe Northern, Central and Western Asia; Edition Chimaira: Frankfurt am Main, Germany, 2010. [Google Scholar]
- Maduwage, K.P.; Scorgie, F.E.; Lincz, L.F.; O’Leary, M.A.; Isbister, G.K. Procoagulant snake venoms have differential effects in animal plasmas: Implications for antivenom testing in animal models. Throm. Res. 2016, 137, 174–177. [Google Scholar] [CrossRef]
- Doolittle, R.F. Coagulation in vertebrates with a focus on evolution and inflammation. J. Innate Immun. 2010, 3, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, R.F. The evolution of the vertebrate plasma proteins. Biol. Bull. 1987, 172, 269–283. [Google Scholar] [CrossRef]
- Youngman, N.J.; Zdenek, C.N.; Dobson, J.S.; Bittenbinder, M.A.; Gillett, A.; Hamilton, B.; Dunstan, N.; Allen, L.; Veary, A.; Veary, E.; et al. Mud in the blood: Novel potent anticoagulant coagulotoxicity in the venoms of the Australian elapid snake genus Denisonia (mud adders) and relative antivenom efficacy. Toxicol. Lett. 2019, 302, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.V.; Abe, A.S. Relationship of venom ontogeny and diet in Bothrops. Herpetologica 1999, 55, 200–204. [Google Scholar]
- National Center for Biotechnology Information (NCBI). Bethesda (MD): National Library of Medicine (US). Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 12 August 2020).
- Coimbra, F.C.; Dobson, J.; Zdenek, C.N.; Op den Brouw, B.; Hamilton, B.; Debono, J.; Masci, P.; Frank, N.; Ge, L.; Kwok, H.F.; et al. Does size matter? Venom proteomic and functional comparison between night adder species (Viperidae: Causus) with short and long venom glands. Comp. Biochem. Phys. Part C Toxicol. Pharm. 2018, 211, 7–14. [Google Scholar] [CrossRef]
- Minton, S.A.; Minton, M.R. Toxicity of some Australian snake venoms for potential prey species of reptiles and amphibians. Toxicon 1981, 19, 749–755. [Google Scholar] [CrossRef]
- Davies, E.-L.; Arbuckle, K. Coevolution of snake venom toxic activities and diet: Evidence that ecological generalism favours toxicological diversity. Toxins 2019, 11, 711. [Google Scholar] [CrossRef]
- Barlow, A.; Pook, C.E.; Harrison, R.A.; Wüster, W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. R. Soc. B 2009, 276, 2443–2449. [Google Scholar] [CrossRef]
- Zancolli, G.; Calvete, J.J.; Cardwell, M.D.; Greene, H.W.; Hayes, W.K.; Hegarty, M.J.; Herrmann, H.W.; Holycross, A.T.; Lannutti, D.I.; Mulley, J.F.; et al. When one phenotype is not enough: Divergent evolutionary trajectories govern venom variation in a widespread rattlesnake species. Proc. R. Soc. B 2019, 286, 20182735. [Google Scholar] [CrossRef]
- Dessauer, H.C. Blood chemistry of reptiles: Physiological and evolutionary aspects. Biol. Reptil. 1970, 3, 1–72. [Google Scholar]
- Johnson, G.S.; Turrentine, M.A.; Swayne, D.E. Coagulation of plasma from the chicken (Gallus domesticus): Phospholipids influence clotting rates induced by components from Russell’s viper venom. Comp. Biochem. Phys. Part B Biochem. Mol. Biol. 1985, 82, 647–653. [Google Scholar] [CrossRef]
- Stopforth, A. A study of coagulation mechanisms in domestic chickens. J. Comp. Path. 1970, 80, 525–533. [Google Scholar] [CrossRef]
- Hackett, E.; Lepage, R. The clotting of the blood of an amphibian, Bufo marinus. Aust. J. Exp. Biol. Med. Sci. 1961, 39, 67–78. [Google Scholar] [CrossRef] [PubMed]



| P. urarachnoides Venom vs. Spontaneous Control Difference Between Means ± SD (%); Unpaired t-Test | ||
|---|---|---|
| Plasma (n = 4) | Clot Initiation Speed | Clot Formation Rate |
| Human | 95 ± 4.3%; t (6) = 22.08, p < 0.000001 | 61 ± 14.8%; t (6) = 4.08, p = 0.00651 |
| Toad | 99 ± 4.3%; t (6) = 23.15, p < 0.000001 | 63 ± 2.6%; t (6) = 24.37, p < 0.000001 |
| Chicken | 96 ± 4.3%; t (6) = 22.46, p < 0.000001 | 38 ± 10.9%; t (6) = 3.519, p = 0.01254 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
op den Brouw, B.; Coimbra, F.C.P.; Bourke, L.A.; Huynh, T.M.; Vlecken, D.H.W.; Ghezellou, P.; Visser, J.C.; Dobson, J.S.; Fernandez-Rojo, M.A.; Ikonomopoulou, M.P.; et al. Extensive Variation in the Activities of Pseudocerastes and Eristicophis Viper Venoms Suggests Divergent Envenoming Strategies Are Used for Prey Capture. Toxins 2021, 13, 112. https://doi.org/10.3390/toxins13020112
op den Brouw B, Coimbra FCP, Bourke LA, Huynh TM, Vlecken DHW, Ghezellou P, Visser JC, Dobson JS, Fernandez-Rojo MA, Ikonomopoulou MP, et al. Extensive Variation in the Activities of Pseudocerastes and Eristicophis Viper Venoms Suggests Divergent Envenoming Strategies Are Used for Prey Capture. Toxins. 2021; 13(2):112. https://doi.org/10.3390/toxins13020112
Chicago/Turabian Styleop den Brouw, Bianca, Francisco C. P. Coimbra, Lachlan A. Bourke, Tam Minh Huynh, Danielle H. W. Vlecken, Parviz Ghezellou, Jeroen C. Visser, James S. Dobson, Manuel A. Fernandez-Rojo, Maria P. Ikonomopoulou, and et al. 2021. "Extensive Variation in the Activities of Pseudocerastes and Eristicophis Viper Venoms Suggests Divergent Envenoming Strategies Are Used for Prey Capture" Toxins 13, no. 2: 112. https://doi.org/10.3390/toxins13020112
APA Styleop den Brouw, B., Coimbra, F. C. P., Bourke, L. A., Huynh, T. M., Vlecken, D. H. W., Ghezellou, P., Visser, J. C., Dobson, J. S., Fernandez-Rojo, M. A., Ikonomopoulou, M. P., Casewell, N. R., Ali, S. A., Fathinia, B., Hodgson, W. C., & Fry, B. G. (2021). Extensive Variation in the Activities of Pseudocerastes and Eristicophis Viper Venoms Suggests Divergent Envenoming Strategies Are Used for Prey Capture. Toxins, 13(2), 112. https://doi.org/10.3390/toxins13020112







