Abstract
The current study investigated the fungal diversity in freshly harvested oat samples from the two largest production regions in Brazil, Paraná (PR) and Rio Grande do Sul (RS), focusing primarily on the Fusarium genus and the presence of type B trichothecenes. The majority of the isolates belonged to the Fusarium sambucinum species complex, and were identified as F. graminearum sensu stricto (s.s.), F. meridionale, and F. poae. In the RS region, F. poae was the most frequent fungus, while F. graminearum s.s. was the most frequent in the PR region. The F. graminearum s.s. isolates were 15-ADON genotype, while F. meridionale and F. poae were NIV genotype. Mycotoxin analysis revealed that 92% and 100% of the samples from PR and RS were contaminated with type B trichothecenes, respectively. Oat grains from PR were predominantly contaminated with DON, whereas NIV was predominant in oats from RS. Twenty-four percent of the samples were contaminated with DON at levels higher than Brazilian regulations. Co-contamination of DON, its derivatives, and NIV was observed in 84% and 57.7% of the samples from PR and RS, respectively. The results provide new information on Fusarium contamination in Brazilian oats, highlighting the importance of further studies on mycotoxins.
Key Contribution:
Brazilian oat grains were highly contaminated by type B trichothecenes; F. graminearum s.s. and F. poae were the main recovered species able to produce deoxynivalenol and nivalenol, respectively.
1. Introduction
Oats (Avena sativa L.) have been consumed by humans and livestock since ancient times; it is considered a nutrient-rich cereal due to the high concentration of lipids, proteins, vitamins, antioxidants, minerals, and β-glucan [1]. The global production of oats in 2021/2021 was 25,570 thousand metric tons, with the European Union being the largest producer followed closely by Canada, Russia, Australia, United Kingdom and Brazil [2].
Over the last five years, oat production has increased in Brazil, with a reported grain productivity of 2426 kg/hectare and production of 1087.1 thousand tons [3]. However, it is important to highlight that over the last seasons, heavy rain conditions were reported in the southern region, where most of the small-grain cereals, such as oats, are produced. The increased humidity potentially favored fungal infections and reduced grain quality [3].
Cereals can be affected by fungal diseases, which can lead to lower nutritional values and mycotoxin accumulation in the grains, resulting in reduced product quality and economic losses [4]. Numerous fungi may be attributed to various oat diseases; the Fusarium genus, however, is considered one of the major threats. One of the most serious and economically important diseases caused by the genus is Fusarium head blight (FHB), which affects cereal production worldwide [5,6,7].
Fusarium head blight is primarily caused by species in the Fusarium graminearum species complex (FGSC); however, other Fusarium species may also be involved. FHB causes flower abortion and the formation of pitted, wrinkled, and rough grains that are “pinkish” in color [8]. Infection by these pathogens can also result in mycotoxin accumulation, mainly trichothecenes and zearalenone (ZEN) [9,10].
Trichothecenes produced by Fusarium species are classified into either type A or B; these compounds are differentiated by the C-8 function of the 12,13-epoxytrichothec-9-ene (EPT) core structure [11]. Members of the FGSC are able to produce type B trichothecenes such as deoxynivalenol (DON) and its acetylated derivatives (3 acetyl-DON and 15 acetyl-DON; 3-ADON and 15-ADON) as well as nivalenol (NIV) and its acetylated forms [11].
In animals, DON has been linked to feed refusal, vomiting, and weight reduction [12]. NIV can cause immunotoxicity and hematotoxicity, based on in vitro and in vivo tests [13]. The toxic effects of the acetylated DON forms are poorly documented; however, 15-ADON has been reported to be more toxic than DON and 3-ADON in ex vivo and in vivo tests using human intestinal cells and piglets [14]. Due to the toxic effects of DON in humans, a provisional maximum tolerable daily intake (PMTDI) of 1.0 μg/kg body weight/day has been set by the UN Food and Agriculture Organization/World Health Organization Joint Expert Committee on Food Additives (JECFA) [15]. For NIV, a tolerable daily intake (TDI) of 1.2 μg/kg body weight/day has been set by the European Food Safety Authority (EFSA) [13].
Zearalenone is a cyclic compound containing a resorcyclic acid lactone structure, and it is also primarily produced by the same fungi that produce type B trichothecenes. It is commonly found together with DON and NIV in cereals. ZEN is considered an estrogenic mycotoxin that causes abnormalities in the reproductive system, particularly in swine, leading to infertility, genital prolapse, and enlarged mammary glands [16]. Due to the fact of these effects, the JECFA established a PMTDI of 0.5 μg/kg body weight/day [17].
In the Northern Hemisphere, the main Fusarium species associated with oats are F. graminearum, F. avenaceum, F. sporotrichioides, F. langsethiae, F. poae, F. culmorum, and F. tricinctum [5,6,7,18,19,20]. This implies that a diverse range of mycotoxins may be found in oats. For example, F. sporotrichioides and F. langsethiae are responsible for T-2 and HT-2 (type A trichothecenes) accumulation in small grain cereals [21]; whereas F. graminearum and F. culmorum are able to produce ZEN and type B trichothecenes; F. poae mainly produces NIV [22]; finally, F. avenaceum and F. tricinctum are able to produce other Fusarium mycotoxins such as moniliformin (MON) and enniatins (ENNs) [23]. Indeed, several studies have already shown the occurrence of type A and type B trichothecenes in oats grown in colder climates [7,24,25,26,27,28].
In South America, a few studies have shown that Alternaria, Aspergillus, Penicillium, and Fusarium are prevalent in oats [29,30,31]. Regarding the Fusarium genus, F. graminearum, F. poae, and F. verticillioides have previously been recovered from freshly harvested grains [31]. In regard to mycotoxin contamination, aflatoxin B1 (AFB1), DON, fumonisin B1 (FB1), and ochratoxin A (OTA) have been detected in oat grains and products [29,31,32,33,34,35].
In Brazil, reports of fungi and mycotoxin contamination in oats are scarce, with a few only focusing either on the mycobiota or mycotoxin contamination. The majority of the associated fungi recovered were Alternaria, Drechslera, Fusarium, and Puccinia [29,30,36]. However, the only reported mycotoxin was FB1, while aflatoxins, ochratoxin A, and ZEN were not detected [29,33].
Due to the lack of information on fungal diversity and mycotoxin contamination in Brazilian oats, together with the increasing production of this cereal within the country, the objectives of the current study were to (i) characterize Fusarium species associated with freshly harvested oats, recovered from the largest production regions of Brazil; (ii) determine the levels of deoxynivalenol, its derivatives (3-acetildeoxynivalenol and 15-acetildeoxynivalenol), and nivalenol in the grain samples.
2. Results
2.1. Water Activity and Mycobiota of Freshly Harvested Oat Grains
Ninety-two percent of the oat samples were contaminated with fungi, predominantly by the Fusarium genus. In the RS region, samples were contaminated with Fusarium, followed by Phoma, Epicoccum, Alternaria, Cladosporium, Penicillium, Aspergillus, Drechslera, Pestalotiopsis, Mucor, Rhizopus, Curvularia, and Trichoderma. Water activity (aw) ranged from 0.4 to 0.6 (mean = 0.54). No correlation between aw and the occurrence of Fusarium was observed (p < 0.05). In the PR region, 33.1% of the samples were contaminated with Fusarium, followed by Alternaria, Nigrospora, Epicoccum, Phoma, Cladosporium, Rhizopus, Penicillium, Drechslera, Mucor, and Pestalotiopsis (Table 1). Water activity ranged from 0.5 to 0.6 (mean = 0.51). No correlation between aw and the occurrence of Fusarium was observed (p < 0.05).

Table 1.
Frequency and mean count of fungal genera and Fusarium species complexes isolated from oat samples from two different regions of Brazil: Paraná (PR—50 samples) and Rio Grande do Sul (RS—50 samples).
The F. sambucinum species complex (FSAMSC) was foremost in oat samples from both regions. In the RS region, F. poae was the primary species isolated, followed by the FGSC, F. avenaceum, and F. proliferatum. In the PR region, the majority of the isolates belonged to the FGSC, followed by F. poae, F. incarnatum-equiseti species complex (FIESC), F. verticillioides, F. subglutinans, and F. solani species complex.
2.2. Molecular Characterization of Fusarium Isolates
A subsample of Fusarium isolates were selected for molecular characterization. Isolates belonging to the FSAMSC were randomly selected for phylogenetic analysis of the second major subunit of the RNA polymerase locus (RPB2) and genotype characterization.
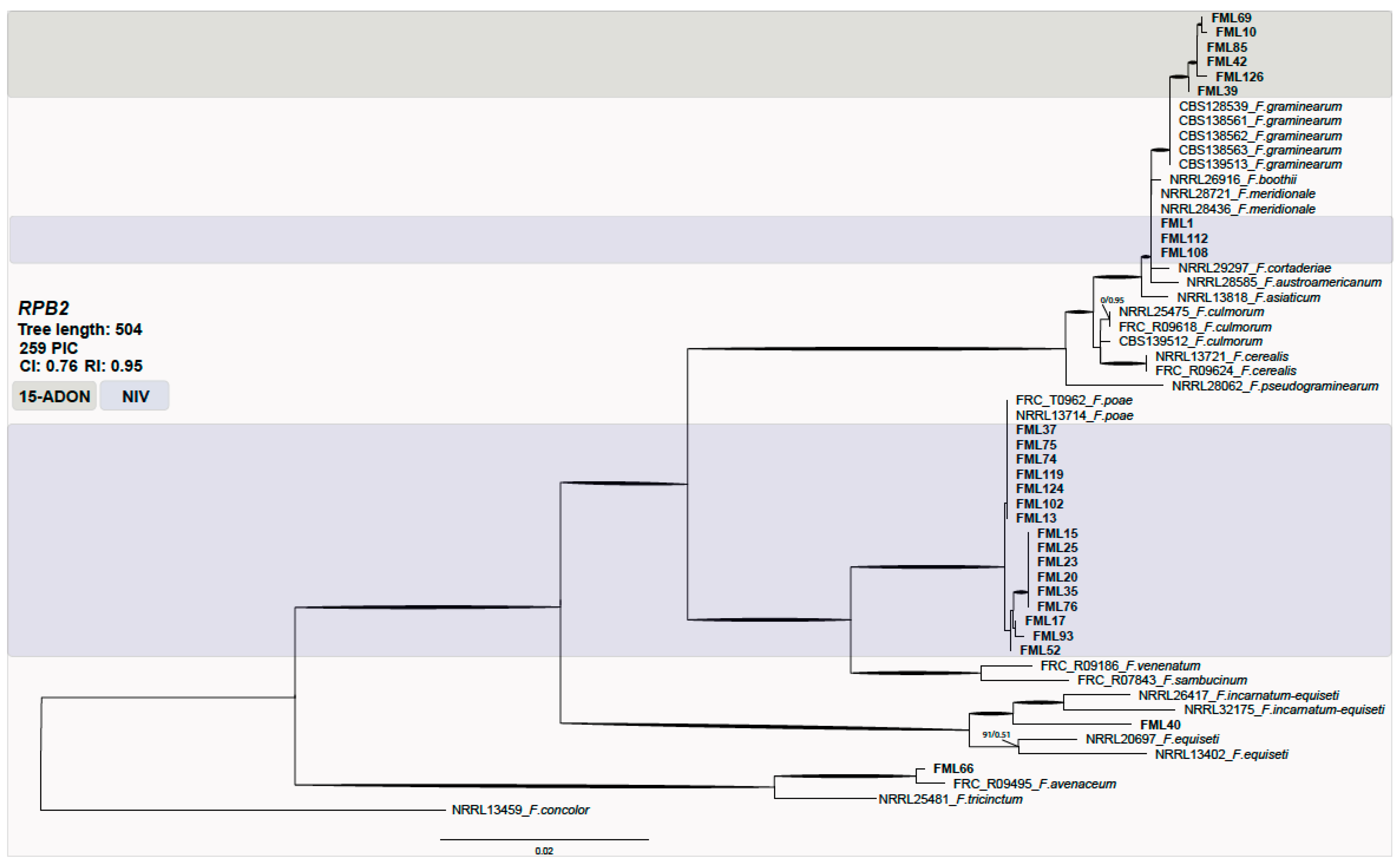
The phylogenetic analysis data set consisted of 55 taxa with 259 parsimony informative characters (PICs). The analysis resulted in one hundred of the most parsimonious trees (consistency index (CI) = 0.76; retention index (RI) = 0.95). No significant topological variations were detected between neighbor-joining, parsimony, and likelihood phylogenies (data not shown). Most of the isolates were clustered within F. graminearum and F. poae species and a few within F. meridionale (Figure 1).
Figure 1.
Maximum parsimony phylogeny inferred from the first fragment of the RPB2 locus. Bootstrap values above 70% and Bayesian posterior probabilities (BPPs) above 0.9 are assigned in bold branches. Support values above branches are bootstrap/BPP values. The outgroup is F. concolor. The NIV genotype is highlighted in blue and 15-ADON in green. FML: Food Microbiology Laboratory.
The FGSC isolates were predominantly of the 15-ADON (50%) genotype, followed by NIV (36.4%) and 3-ADON (13.6%). All strains identified as F. meridionale were of the NIV genotype. All F. graminearum s.s. were characterized as 15-ADON, while F. austroamericanum were of the 3-ADON genotype. As expected, all of the F. poae strains displayed the NIV genotype. The oat grain isolates from Rio Grande do Sul were identified mostly as of the NIV (75%) genotype, followed by 15-ADON (16.7%) and 3-ADON (8.3%). The isolates from Paraná mostly displayed the NIV genotype (70.4%), followed by 15-ADON (25.9%) and 3-ADON (3.7%).
2.3. Mycotoxin Analysis
Occurrence of Type B Trichothecenes
Type B trichothecenes were found in 92% and 100% of oat samples from PR and RS, respectively. In PR, DON was the predominant mycotoxin and was detected in 44.2% of the samples, followed by NIV (28.6%), 3-ADON (18.8%), and 15-ADON (7.7%). In RS, NIV was detected in 44.7% of the samples and was the predominant mycotoxin, followed by DON (35%), 15-ADON (3.6%), and 3-ADON (14.8%).
Table 2 shows the levels of DON, 3-ADON, 15-ADON, and NIV detected in the PR and RS regions. The mean contamination levels for mycotoxins in oat samples from PR were 45, 18.8, 7.7, and 28.6 µg/kg for DON, 3-ADON, 15-ADON, and NIV, respectively. Regarding the RS oat samples, the mean mycotoxin contamination levels were 35, 14.8, 3.6, and 46.7 µg/kg for DON, 3-ADON, 15-ADON, and NIV, respectively.

Table 2.
Occurrence of type B trichothecenes in oat grain samples from Rio Grande do Sul (RS) and Paraná (PR), Brazil.
In the current study, a higher frequency and average concentration levels of NIV were found in RS; however, no significant difference was observed between RS and PR for the other evaluated mycotoxins (p > 0.05).
The co-occurrence of trichothecenes was also observed. Eighty-four percent of the samples from the PR region simultaneously presented DON and NIV, whereas the co-occurrence of DON and NIV was observed in only 57.7% of the RS samples.
3. Discussion
The current study found a high diversity of fungi in Brazilian oat grains including potentially toxigenic fungi. The occurrence of the FSAMSC and its related mycotoxins, such as DON, 3-ADON, 15-ADON, and NIV, was the focus of the investigation. It is important to mention that there is a lack of research regarding mycotoxin contamination in Brazilian oat grains, despite high consumption by the population. Most of the previous studies were conducted in the Northern Hemisphere, and they have reported the presence of multiple Fusarium mycotoxins in oat grains [37,38].
A study conducted on Swiss oat samples from the 2013 to 2015 harvests reported the occurrence of nine different Fusarium species and a 97% frequency of Fusarium infection in the analyzed samples; similar to the frequency determined in this study (93.8% and 85.5% for samples from RS and PR, respectively). The same study pointed out that F. poae was the most predominant species in all three harvest years (2013, 2014 and 2015) with 55%, 57% and 87% isolation amongst Fusarium species [7].
In the current study, most of Fusarium isolates belonged to the FSAMSC and were characterized as F. graminearum s.s., F. meridionale and F. poae. The latter was frequently isolated from RS samples, in contrast to the PR samples, where F. meridionale was highly detected. Studies have highlighted F. poae as a frequent species found in oat samples [7,39]. These results suggest that different geographic origins, soil type, and environmental and harvest conditions could lead to a distinct predominant species and might influence the mycotoxin content of the grains [40].
In both studied regions, the NIV genotype was predominant. In RS, it was associated with the high frequency of F. poae and with the samples mostly contaminated with the NIV mycotoxin. The high occurrence of this genotype in PR was associated to F. meridionale and F. poae. This knowledge is relevant for determining a more efficient prediction of the contamination by NIV, and it may aid management strategies to control the occurrence of toxigenic fungi in barley from different geographic regions [41].
Mycotoxin analysis demonstrated that most of the samples were contaminated with type B trichothecenes. DON contamination was higher in samples from PR, while NIV was prevalent in RS. The presence of these mycotoxins conformed with the frequency of isolated fungi, as F. poae was the most isolated species in RS and F. graminearum s.s. in PR. It has been reported that the incidence of F. poae increases when the climatic conditions do not favor the proliferation of F. graminearum s.s., the dominant pathogen involved in FHB [42,43].
In Brazil, previous studies revealed a high frequency of the FSAMSC in wheat, barley, and rice, leading to the high occurrence of DON in grains. Despite this knowledge, information correlating mycotoxin contamination to the predominant species in Brazilian oats is still scarce [44,45,46,47,48,49,50,51]. In Europe, the high occurrence of F. poae in cereals is responsible for NIV contamination [37,52], while in Asia, NIV contamination is attributed to F. asiaticum [53,54]. In South America, NIV was found in wheat from Argentina and Brazil in lower frequency and levels than DON. Apparently, the higher frequency of DON is related to the higher risk of FHB epidemics caused by the predominance of no-till cropping and climate change in the subtropical environment of Southern Brazil [45,55]. Furthermore, the analysis detected the presence of 3-ADON and 15-ADON in oats, with high levels of 3-ADON in samples from both regions studied. This result corroborates with the occurrence of the FGSC 3-ADON genotype. In Europe, the acetylated DON forms are reported in cereals such as oats [52,56,57,58], maize, and wheat [59].
In our study, 24% of the samples presented DON levels higher than the maximum limit established by Brazilian legislation (750 µg/kg). Despite the absence of legislation for NIV globally, this mycotoxin was present in high levels, mainly in samples from RS. The toxic effects of NIV are still inconclusive, although it has displayed immunotoxic and hematotoxic effects, which can be critical to humans [13]. In the case of acetylated DON forms (i.e., 15-ADON and 3-ADON), high levels of 3-ADON were observed in the grains from both regions. Information about its toxic effects in animals and humans are still scarce, but a study demonstrated that 15-ADON was more toxic than DON and 3-ADON [14].
Co-contamination of DON, 15-ADON, 3-ADON, and NIV was observed in this study due to the presence of different fungi in the grains. To our knowledge, this is the first report demonstrating the co-occurrence of these mycotoxins in Brazilian oat grains and their correlation with associated Fusarium species. However, the co-occurrence of DON and NIV has already been reported in 86% of Brazilian wheat kernels analyzed [45] as well as in 29.6% of Brazilian barley samples [51]. The main concerns about co-contamination are the possible interactions and potential synergistic effects that these mycotoxins may have on animal and human health. Cheat et al. (2015) [60] reported that the toxic effects of DON were intensified when consumed with NIV in in vitro models.
Overall, studies about mycotoxin contamination in oat grains are relevant and necessary to determine an efficient risk control plan, as the consumption of oats in natura plant-based beverages or cereal-based foods has been increasing, boosted mainly by its good nutritional features such as high protein and dietary fiber content [61].
Since the levels of mycotoxin contamination and the dominant species in cereals can change according to various environmental parameters, studies that elucidate the prevalence of toxigenic fungi in different geographic regions are vital for designing efficient control management strategies, aiding producers in obtaining a safer product. The results of this study highlighted the importance of further research on the contamination of multiple Fusarium mycotoxins in oat grains and their by-products consumed in Brazil.
4. Conclusions
This study showed high recovery of F. graminearum s.s. and F. poae from Brazilian oat grains as well as contamination by the mycotoxins DON, 3-ADON, 15-ADON, and NIV. Samples were highly contaminated with type B trichothecenes, and 24% of the samples contaminated with DON were at concentrations higher than permitted by Brazilian legislation. Co-occurrence of these mycotoxins in oat grains samples was also observed; indicating the importance of further studies on trichothecene contamination in oat by-products as well as the toxic synergistic interactions of these mycotoxins to determine the potential risks to animal and human health.
5. Materials and Methods
5.1. Oat Samples
One hundred oat grain samples were collected from the states of Paraná and Rio Grande do Sul (50 samples from each region), the two largest oat-producing regions of Brazil. The grains were obtained after the cleaning and drying stages (up to maximum of 60 °C) of the 2018 harvest. Sampling was performed using a grain auger at different points of the harvest batches. At this stage, none of the grain samples appeared to have any visual fungal growth.
Each sample was homogenized and reduced to a subsample of 3 kg. Grains were transferred into polyethylene bags and kept at room temperature (for up to two days). The bags were then stored at −18 °C for mycotoxin analysis [46].
5.2. Water Activity and Identification of Mycobiota
Water activity analysis of the grain samples was conducted using the equipment Aqua-Lab CX-2, Decagon Devices. Samples were analyzed in triplicate. The serial dilution technique was used for fungal isolation [62]. Aliquots of each dilution were plated onto Dichloran Rose Bengal Chloramphenicol (DRBC, Oxoid) agar and incubated for 5 days at 25 °C, and the results are expressed in CFU/g.
Primary morphological characterization of the different genera was conducted according to [62], using Czapek yeast extract agar (CYA) and malt extract agar (MEA). Isolates belonging to the genus Fusarium were single-spored and plated onto potato dextrose agar (PDA) and carnation leaf agar (CLA) for further morphological characterization [63]. Isolates were stored in glycerol (60%) at −80 °C. For the FGSC strains, ma- croconidia formation was observed in CLA. PDA was used to verify the red pigment formed in the agar. F. poae was determined on CLA through visualization of the typical globose to napiform microconidia, formed in clusters on monophialides. In PDA, the mycelium color was pale to reddish-brown [63]
5.3. Characterization of Fusarium Isolates
The Fusarium isolates were initially identified as described above. Afterwards, 25 strains belonging to the F. sambucinum species complex (FGSC and F. poae isolates) were selected for sequencing and phylogenetic analyses. These isolates were selected in order to represent both regions studied (i.e., Paraná and Rio Grande do Sul). Sequencing reactions followed by phylogenetic analysis were performed on the RPB2 locus [64,65,66].
Isolates were also characterized based on trichothecene genotyping (3-ADON, 15-ADON, and NIV) by multiplex PCR, following the methodology proposed in [67].
5.4. DNA Extraction, PCR, and Sequencing Analyses of the RPB2 Gene
Fusarium cultures were grown on PDA for 5 days at 25 °C, and the DNA was extracted using a DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. PCR reactions and primer sets were performed according to [65,68]. PCR products were purified with QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) and sequenced using Applied Biosystems® 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) by the company Helixxa Bases for Life (Paulínia, SP, Brazil).
The sequences were analyzed using Geneious v.6.0.6 (Biomatters, Auckland, New Zealand), and polymorphisms were confirmed by examining the chromatograms. For multiple alignment, nucleotide sequences were downloaded from the National Centre for Biotechnology Information (NCBI) and aligned with the obtained Fusarium oat isolate sequences using the ClustalW plugin in Geneious v.6.0.6 (Supplementary Materials Table S1).
5.5. Phylogenetic Analysis
Maximum parsimony analysis was performed using PAUP 4.0b10 (Sinauer Associates, Sunderland, MA, USA) [69]. A heuristic search option with 1000 random additional sequences and tree-bisection–reconnection algorithm for branch-swapping were used to infer the most parsimonious tree. Gaps were treated as missing data. The consistency index (CI) and retention index (RI) were calculated to verify the homoplasy present. Clade stability was verified through bootstrap analysis with 1000 replicates (PAUP 4.0b10), Bayesian inference analysis was also performed using the MrBayes plugin in Geneious v.6.0.6, run with a 2,000,000 generation Monte Carlo Markov chain method with a burn-in of 10,000 trees. Fusarium concolor was used as the outgroup. The phylogenies were visualized using FigTree v.1.4 (University of Edinburgh, Edinburgh, UK) [70].
5.6. Mycotoxin Analysis
5.6.1. Mycotoxin Extraction
Mycotoxin extraction was conducted using QuEChERS, according to the manufacturer’s instructions. Initially, 300 g of oat grains were ground, and a subsequent subsample of 100 g was separated using a sieve (0.5 mm mesh 32, generating 0.5 mm particles) and homogenized. Then, 10 g of the ground sample was weighed and transferred into a 50 mL QuEChERS extraction tube, followed by 10 mL of ultrapure water and 10 mL of acetonitrile with 1.0% formic acid.
The sample was agitated vigorously for 1 min and then centrifuged for 5 min at 5000 rpm. After, 3 mL of supernatant was transferred to a 15 mL RoC QuEChERS centrifuge tube, containing 900 mg MgCl and 150 mg PSA (Primary and Secondary Amine Exchange Material—KS0-8924). This was shaken vigorously for 30 s and centrifuged for 5 min at 3700× g to separate the solid material. Finally, 1 mL of the supernatant was transferred into a flask for the solution to be evaporated in a heated sand bath at 60 °C.
Subsequently, the residue was diluted with 1 mL of acetonitrile:water (70:30 v/v), mixed and filtered through a 0.22 µm PTFE hydrophobic membrane filter and injected into a high-performance liquid chromatography with diode array detection.
5.6.2. Chromatography Conditions
Chromatographic separation was performed through a high-performance liquid chromatograph (Shimadzu, Kyoto, Japan), Gemini C18 5.0 µm (250 × 4.6 mm) chromatographic column, and an auto-injector for injection handling of 20 µL, equipped with a diode-array detector SPD-M20A [71].
The mobile phase was composed of acetonitrile:water (70:30 v/v), with elution in the isocratic mode and a flow rate of 0.5 mL min−1, with a total analysis time of 15 min. The maximum absorption wavelength was 220 nm for 3-ADON, 15-ADON, DON, and NIV.
Data were collected and processed using LC Solution-Shimadzu software. The limit of detection (LOD), limit of quantification (LOQ), and recovery were: 16.15, 2.5, 2.5, and 16.15 µg/kg; 53.3, 8.3, 8.3, and 53.3 µg/kg; 98%, 92%, 84%, and 70% for DON, 3-ADON, 15-ADON, and NIV, respectively.
5.7. Statistical Analysis
Statistical analysis was performed using Statistix v.10 software. ANOVA and the Kruskal–Wallis test were chosen to assess the differences in Fusarium occurrence between the two studied regions as well as the differences in mycotoxin levels between the two studied regions. Values of p < 0.05 were considered statistically significant.
Supplementary Materials
The Supplementary Table S1 is available online at https://www.mdpi.com/article/10.3390/toxins13120855/s1. Table S1: Fusarium strains isolated from oats and reference taxa used for phylogenetic analysis.
Author Contributions
M.P., Investigation, Methodology, Formal analysis, and Writing—original draft; C.H.T.I., Formal analysis and Writing—original draft; B.G.B., Formal analysis, Investigation, and Methodology; E.T.S.C., Formal analysis and Writing—review and editing; L.C.-Q., Investigation and Methodology; N.C.L., Funding acquisition and Resources; E.B.F., Funding acquisition and Resources. B.C., Conceptualization and Funding acquisition; L.O.R., Conceptualization, Funding acquisition, Resources, Project administration, Supervision and Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by São Paulo Research Foundation (FAPESP, Grant Number 2017/04811-4), the National Council for Scientific and Technological Development (CNPq, Scholarship Numbers 167055/2017-8 and 167039/2017-2) and Coordination for the Improvement of Higher Education Personnel (CAPES, finance code 001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequencing data are reported in Supplementary Table S1 and are available at the National Center for Biotechnology Information (NCBI) website (https://www.ncbi.nlm.nih.gov/, accessed on 23 November 2021), Accession Numbers: from MW929719 to MW929745.
Acknowledgments
The authors thank São Paulo Research Foundation (FAPESP), the National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES) for the financial support and Camila Siedlarczyk Martins and Maristela Cerqueira for the technical assistance.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could appear to have influenced the work reported in this paper.
References
- Kaur, S.; Bhardwaj, R.D.; Kapoor, R.; Grewal, S.K. Biochemical characterization of oat (Avena sativa L.) genotypes with high nutritional potential. LWT 2019, 110, 32–39. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Grain: World Markets and Trade. Foreign Agricultural Service/USDA, Global Market Analysis. Available online: https://apps.fas.usda.gov/psdonline/circulars/grain.pdf (accessed on 1 March 2021).
- Companhia Nacional de Abastecimento (CONAB). Acompanhamento da Safra Brasileira, Grãos, Safra 2021/22, 2° levantamento. Conab Reports 2021, 9. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 23 November 2021).
- Liu, L.; Ma, M.; Liu, Z.; Zhang, L.; Zhou, J. Community structure of fungal pathogens causing spikelet rot disease of naked oat from different ecological regions of China. Sci. Rep. 2021, 11, 1243. [Google Scholar] [CrossRef]
- Tamburic-Ilincic, L. Fusarium species and mycotoxins associated with oat in southwestern Ontario, Canada. Can. J. Plant Sci. 2010, 90, 211–216. [Google Scholar] [CrossRef]
- Hautsalo, J.; Jalli, M.; Manninen, O.; Veteläinen, M. Evaluation of resistance to Fusarium graminearum in oats. Euphytica 2018, 214, 139. [Google Scholar] [CrossRef]
- Schöneberg, T.; Jenny, E.; Wettstein, F.E.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Musa, T.; Seifert, K.; Gräfenhan, T.; Keller, B.; et al. Occurrence of Fusarium species and mycotoxins in Swiss oats—Impact of cropping factors. Eur. J. Agron. 2018, 92, 123–132. [Google Scholar] [CrossRef]
- Ghimire, B.; Sapkota, S.; Bahri, B.A.; Martinez-Espinoza, A.D.; Buck, J.W.; Mergoum, M. Fusarium Head Blight and rust diseases in soft red winter wheat in the Southeast United States: State of the art, challenges and future perspective for breeding. Front. Plant Sci. 2020, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Mylona, K.; Magan, N. Fusarium langsethiae: Storage environment influences dry matter losses and T2 and HT-2 toxin contamination of oats. J. Stored Prod. Res. 2011, 47, 321–327. [Google Scholar] [CrossRef]
- O’Donnel, K.; Rooney, A.P.; Proctor, R.H.; Brown, D.W.; McCormick, S.P.; Ward, T.J.; Frandsen, R.J.N.; Lysøe, E.; Rehner, S.A.; Aoki, T.; et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013, 52, 20–31. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef]
- Pereira, C.S.; Cunha, S.C.; Fernandes, J.O. Prevalent mycotoxins in animal feed: Occurrence and analytical methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Scientific opinion on risks for animal and public health related to the presence of nivalenol in food and feed. EFSA J. 2013, 11, 3262. [Google Scholar] [CrossRef]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Safety Evaluation of Certain Contaminants in Food; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Evaluation of Certain Food Additives and Contaminants; Fifty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Tekauz, A.; Mitchell Fetch, J.W.; Rossnagel, B.G.; Savard, M.E. Progress in assessing the impact of Fusarium head blight on oat in Western Canada and screening of avena germplasm for resistance. Cereal Res. Commun. 2008, 36, 49–56. [Google Scholar] [CrossRef]
- Yli-Mattila, T. Ecology and evolution of toxigenic Fusarium species in cereals in Northern Europe and Asia. J. Plant Pathol. 2010, 92, 7–18. [Google Scholar]
- Schöneberg, T.; Kibler, K.; Wettstein, F.E.; Bucheli, T.D.; Forrer, H.R.; Musa, T.; Mascher, F.; Bertossa, M.; Keller, B.; Vogelgsang, S. Influence of temperature, humidity duration and growth stage on the infection and mycotoxin production by Fusarium langsethiae and Fusarium poae in oats. Plant Pathol. 2019, 68, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Kokkonen, M.; Jestoi, M.; Laitila, A. Mycotoxin production of Fusarium langsethiae and Fusarium sporotrichioides on cereal-based substrates. Mycotoxin Res. 2012, 28, 25–35. [Google Scholar] [CrossRef]
- Stenglein, S.A.; Dinolfo, M.I.; Barros, G.; Bongiorno, F.; Chulze, S.N.; Moreno, M.V. Fusarium poae pathogenicity and mycotoxin accumulation on selected wheat and barley genotypes at a single location in Argentina. Plant Dis. 2014, 98, 1733–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munkvold, G.P. Fusarium species and their associated mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols; Moretti, A., Susca, A., Eds.; Human Press: Totowa, NJ, USA, 2017; pp. 51–106. [Google Scholar]
- Langseth, W.; Rundberget, T. The occurrence of HT-2 toxin and other trichothecenes in Norwegian cereals. Mycopathologia 1999, 147, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Hietaniemi, V.; Kontturi, M.; Rämö, S.; Eurola, M.; Kangas, A.; Niskanen, M.; Saastamoinen, M. Contents of trichothecenes in oats during official variety, organic cultivation and nitrogen fertilization trials in Finland. Agr. Food Sci. 2004, 13, 54–67. [Google Scholar] [CrossRef]
- Gottschalk, C.; Barthel, J.; Engelhardt, G.; Bauer, J.; Meyes, K. Occurrence of type A trichothecenes in conventionally and organically produced oats and oat products. Mol. Nutr. Food Res. 2007, 51, 1547–1553. [Google Scholar] [CrossRef]
- Nielsen, L.K.; Jensen, J.D.; Nielsen, G.C.; Jensen, J.E.; Spliid, N.H.; Thomsen, I.K.; Justesen, A.F.; Collinge, D.B.; Jørgensen, L.N. Fusarium head blight of cereals in Denmark: Species complex and related mycotoxins. Phytopathology 2011, 101, 960–969. [Google Scholar] [CrossRef] [Green Version]
- Polišenská, I.; Jirsa, O.; Vaculová, K.; Pospíchalová, M.; Wawroszova, S.; Frydrych, J. Fusarium mycotoxins in two hulles oat and barley cultivars used for food purposes. Foods 2020, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Rupollo, G.; Gutkoski, L.C.; Martins, I.R.; Elias, M.C. Efeito da umidade e do período de armazenamento hermético na contaminação natural por fungos e a produção de micotoxinas em grãos de aveia. Cienc. Agrotec. 2006, 30, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Marini, L.J.; Gutkoski, L.C.; Elias, M.C.; Santin, J.A. Qualidade de grãos de aveia sob secagem intermitente em altas temperaturas. Cienc. Rural 2007, 37, 1268–1273. [Google Scholar] [CrossRef]
- Sacchi, C.; González, H.H.L.; Broggi, L.E.; Pacin, A.; Resnik, S.L.; Cano, G.; Taglieri, D. Fungal contamination and mycotoxin natural occurrence in oats for race horses feeding in Argentina. Anim. Feed Sci. Tech. 2009, 152, 330–335. [Google Scholar] [CrossRef]
- Diaz, G.; Perilla, N.; Rojas, Y. Occurrence of aflatoxins in selected Colombian foods. Mycotoxin Res. 2001, 17, 15–20. [Google Scholar] [CrossRef]
- Mallmann, C.A.; Santurio, J.M.; Almeida, C.A.A.; Dilkin, P. Fumonisin B1 levels in cereals and feeds from Southern Brazil. Arq. Inst. Biol. 2001, 68, 41–45. [Google Scholar]
- Ortiz, J.; Camp, J.V.; Mestdagh, F.; Donoso, S.; Meulenaer, B. Mycotoxin co-occurrence in rice, oat flakes and wheat noodles used as staple foods in Ecuador. Food Addit. Contam. A 2013, 30, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.; Jacxsens, L.; Astudillo, G.; Ballesteros, A.; Donoso, S.; Huybregts, L.; Meulenaer, B. Multiple mycotoxin exposure of infants and young children via breastfeeding and complementary/weaning foods consumption in Ecuadorian highlands. Food Chem. Toxicol. 2018, 118, 541–548. [Google Scholar] [CrossRef]
- Graichen, F.A.S.; Martinelli, J.A.; Wesp, C.L.; Federizzi, L.C.; Chaves, M.S. Epidemiological and histological components of crown rust resistance in oat genotypes. Eur. J. Plant Pathol. 2011, 131, 497–510. [Google Scholar] [CrossRef]
- Fredlund, E.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Lindblad, M. Deoxynivalenol and other selected Fusarium toxins in Swedish oats—Occurrence and correlation to specific Fusarium species. Int. J. Food Microbiol. 2013, 167, 276–283. [Google Scholar] [CrossRef]
- Vidal, A.; Marín, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Determination of aflatoxins, deoxynivalenol, ochratoxin A and zearalenone in wheat and oat-based bran supplements sold in the Spanish market. Food Chem. Toxicol. 2013, 53, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Hofgaard, I.S.; Aamot, H.U.; Torp, T.; Jestoi, M.; Lattanzio, V.M.T.; Klemsdal, S.S.; Waalwijk, C.; Van Der Lee, T.; Brodal, G. Associations between Fusarium species and mycotoxins in oats and spring wheat from farmers fields in Norway over a six-year period. World Mycotoxin J. 2016, 9, 365–378. [Google Scholar] [CrossRef]
- Schöneberg, T.; Martin, C.; Wettstein, F.E.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Musa, T.; Keller, B.; Vogelgsang, S. Fusarium and mycotoxin spectra in Swiss barley are affected by various cropping techniques. Food Addit. Contam. A 2016, 33, 1608–1619. [Google Scholar] [CrossRef] [Green Version]
- Zingales, V.; Fernández-Franzón, M.; Ruiz, M.J. Occurrence, mitigation and in vitro cytotoxicity of nivalenol, a type B trichothecene mycotoxin—Updates from the last decade (2010–2020). Food Chem. Toxicol. 2021, 152, 112182. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Juan, C.; Ferrer, E.; Manes, J. Fusarium species, chemotype characterization and trichothecene contamination of durum and soft wheat in an area of Central Italy. J. Sci. Food Agr. 2015, 95, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Sumíková, T.; Chrpová, J.; Džuman, Z.; Salava, J.; Štĕrbová, L.; Palicová, J.; Slavíková, P.; Stránská-Zachariášová, M.; Hajšlová, J. Mycotoxins content and its association with changing patterns of Fusarium pathogens in wheat in the Czech Republic. World Mycotoxin J. 2017, 10, 143–151. [Google Scholar] [CrossRef]
- Almeida, M.I.; Almeida, N.G.; Carvalho, K.L.; Gonçalves, G.A.A.; Silva, C.N.; Santos, E.A.; Garcia, J.C.; Vargas, E.A. Co-occurrence of aflatoxins B1, B2, G1 and G2, ochratoxin A, zearalenone, deoxynivalenol, and citreoviridin in rice in Brazil. Food Addit. Contam. A 2012, 29, 694–703. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Garda-Buffon, J.; Badiale-Furlong, E. Deoxynivalenol and nivalenol in commercial wheat grain related to Fusarium head blight epidemics in southern Brazil. Food Chem. 2012, 132, 1087–1091. [Google Scholar] [CrossRef] [Green Version]
- Piacentini, K.C.; Savi, G.D.; Pereira, M.E.; Scussel, V.M. Fungi and the natural occurrence of deoxynivalenol and fumonisins in malting barley (Hordeum vulgare L.). Food Chem. 2015, 187, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Calori-Domingues, M.A.; Bernardi, C.M.; Nardin, M.S.; Souza, G.V.; Santos, F.G.R.; Stein, M.A.; Gloria, E.M.; Dias, C.T.S.; Camargo, A.C. Co-occurrence and distribution of deoxynivalenol, nivalenol and zearalenone in wheat from Brazil. Food Addit. Contam. B 2016, 9, 142–151. [Google Scholar] [CrossRef]
- Tralamazza, S.M.; Bemvenuti, R.H.; Zorzete, P.; Garcia, F.S.; Corrêa, B. Fungal diversity and natural occurrence of deoxynivalenol and zearalenone in freshly harvested wheat grains from Brazil. Food Chem. 2016, 196, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, K.C.; Rocha, L.O.; Savi, G.D.; Carnielli-Queiroz, L.; Almeida, F.G.; Minella, E.; Corrêa, B. Occurrence of deoxynivalenol and zearalenone in brewing barley grains from Brazil. Mycotoxin Res. 2018, 34, 173–178. [Google Scholar] [CrossRef]
- Savi, G.D.; Piacentini, K.C.; Rocha, L.O.; Carnielli-Queiroz, L.; Furtado, B.G.; Scussel, R.; Zanoni, E.T.; Machado-de-Ávila, R.A.; Corrêa, B.; Angioletto, E. Incidence of toxigenic fungi and zearalenone in rice grains from Brazil. Int. J. Food Microbiol. 2018, 270, 5–13. [Google Scholar] [CrossRef]
- Iwase, C.H.T.; Piacentini, K.C.; Giomo, P.P.; Čumová, M.; Wawroszová, S.; Běláková, S.; Minelli, E.; Rocha, L.O. Characterization of the Fusarium sambucinum species complex and detection of multiple mycotoxins in Brazilian barley samples. Food Res. Int. 2020, 136, 109336. [Google Scholar] [CrossRef] [PubMed]
- Scudamore, K.A.; Bailie, H.; Patel, S.; Edwards, S.G. Occurrence and fate of Fusarium mycotoxins during commercial processing of oats in the UK. Food Addit. Contam. 2007, 24, 1374–1385. [Google Scholar] [CrossRef]
- Ndoye, M.; Zhang, J.B.; Wang, J.H.; Gong, A.D.; Li, H.P.; Qu, B.; Li, S.J.; Liao, Y.C. Nivalenol and 15-acetyldeoxynivalenol chemotypes of Fusarium graminearum clade species are prevalent on maize throughout China. J. Phytopathol. 2012, 160, 519–524. [Google Scholar] [CrossRef]
- Jang, J.Y.; Baek, S.G.; Choi, J.H.; Kim, S.; Kim, J.; Kim, D.W.; Yun, S.H.; Lee, T. Characterization of nivalenol-producing Fusarium asiaticum that causes cereal head blight in Korea. Plant Pathol. J. 2019, 35, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pinto, V.E.; Terminiello, L.A.; Basilico, J.C.; Ritieni, A. Natural occurrence of nivalenol and mycotoxigenic potential of Fusarium graminearum strains in wheat affected by Head Blight in Argentina. Braz. J. Microbiol. 2008, 39, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Schollenberger, M.; Müller, H.M.; Rüfle, M.; Suchy, S.; Planck, S.; Drochner, W. Survey of Fusarium toxins in foodstuffs of plant origin marketed in Germany. Int. J. Food Microbiol. 2005, 97, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.G. Fusarium mycotoxin content of UK organic and conventional oats. Food Addit. Contam. A 2009, 26, 1063–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hietaniemi, V.; Rämö, S.; Yli-Matila, T.; Jestoi, M.; Peltonen, S.; Kartio, M.; Sieviläinen, E.; Koivisto, T.; Parikka, P. Update survey of Fusarium species and toxins in Finnish cereal grains. Food Addit. Contam. A 2016, 33, 831–848. [Google Scholar] [CrossRef]
- Yan, P.; Liu, Z.; Liu, S.; Yao, L.; Liu, Y.; Wu, Y.; Gong, Z. Natural occurrence of deoxynivalenol and its acetylated derivatives in Chinese maize and wheat collected in 2017. Toxins 2020, 12, 200. [Google Scholar] [CrossRef] [Green Version]
- Cheat, S.; Gerez, J.R.; Cognié, J.; Alassane-Kpembi, I.; Bracarense, A.P.F.L.; Raymond-Letron, I.; Oswald, I.P.; Kolf-Clauw, M. Nivalenol has a greater impact than deoxynivalenol on pig jejunum mucosa in vitro on explants and in vivo on intestinal loops. Toxins 2015, 7, 1945–1961. [Google Scholar] [CrossRef]
- Spaen, J.; Silva, J.V.C. Oat proteins: Review of extraction methods and techno-functionality for liquid and semi-solid applications. LWT 2021, 147, 111478. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: Boston, MA, USA, 2009. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Wiley-Blackwell: Iowa, NJ, USA, 2006. [Google Scholar]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.J.; Balajee, S.A.; Schroers, H.J.; Summerbell, R.C.; Robert, V.A.R.G.; Crous, P.W.; Zhang, N.; et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef] [Green Version]
- Laurence, M.H.; Summerell, B.A.; Liew, E.C.Y. Fusarium oxysporum f. sp. canariensis: Evidence for horizontal gene transfer of putative pathogenicity genes. Plant Pathol. 2015, 64, 1068–1075. [Google Scholar]
- Ward, T.J.; Bielawski, J.P.; Kistler, H.C.; Sullivan, E.; O’Donnell, K. Ancestral polymorphism and adaptative evolution in the trichothecene mycotoxin gene cluster of phytopathogenic Fusarium. Proc. Natl. Acad. Sci. USA 2002, 99, 9278–9283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, K.; Sarver, B.A.J.; Brandt, M.; Chang, D.C.; Noble-Wang, J.; Park, B.J.; Sutton, D.A.; Benjamin, L.; Lindsley, M.; Padhye, A.; et al. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. J. Clin. Microbiol. 2007, 45, 2235–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swofford, D.L. PAUP* Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Rambaut, A. FigTree v1.3.1; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, Scotland, 2010. [Google Scholar]
- Arraché, E.R.S.; Fontes, M.R.V.; Buffon, J.G.; Badiale-Furlong, E. Trichothecenes in wheat: Methodology, occurrence and human exposure risk. J. Cereal Sci. 2018, 82, 129–137. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).