Botulinum Toxin Therapy: A Series of Clinical Studies on Patients with Spasmodic Dysphonia in Japan
Abstract
:1. Introduction
2. Epidemiological Surveys of SD
2.1. Previous Studies Worldwide
2.2. Epidemiological Surveys in Japan
2.3. Epidemiological Characteristics of SD
3. Creation of the Diagnostic Criteria and a Severity Grading System
3.1. Diagnostic Criteria
3.2. Severity Grading
3.3. Significance of the Diagnostic Criteria and Severity Grading
4. Clinical Trial of BT Therapy
4.1. Study Design
4.2. Methods for Evaluation
4.2.1. The Mora Method
4.2.2. GRBAS Scale
4.2.3. Voice Handicap Index
4.2.4. Visual Analog Scale
4.3. Results
4.3.1. Treatment Effects and Clinical Courses
4.3.2. Adverse Events (AEs)
4.3.3. Factors Influencing the Treatment Effect
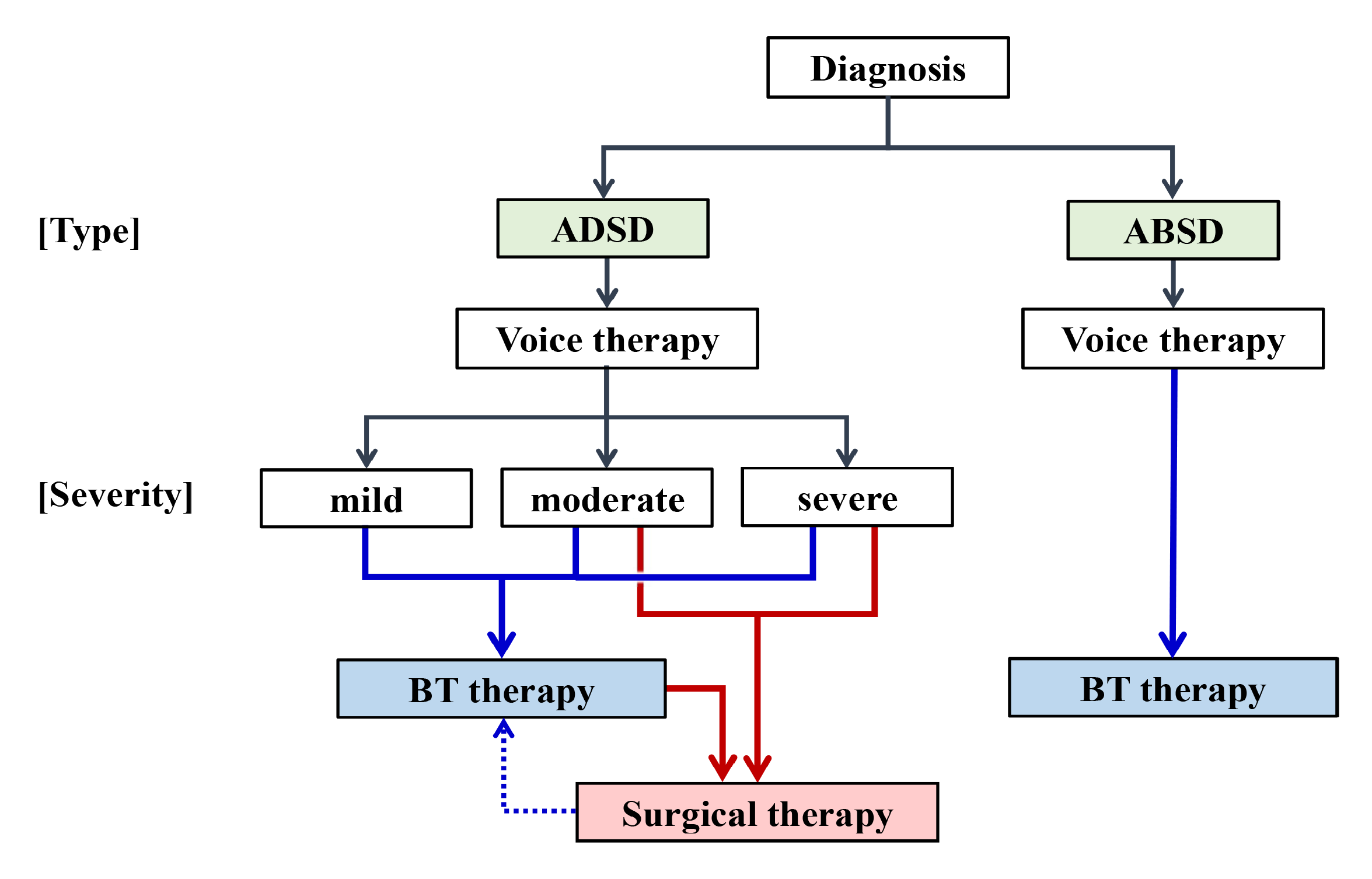
4.4. Role for BT Injection Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blitzer, A.; Brin, M.F.; Fahn, S.; Lovelace, R.E. Clinical and laboratory characteristics of laryngeal dystonia: A study of 110 cases. Laryngoscope 1988, 98, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Aronson, A.E.; Bless, D.M. Spasmodic Dysphonia, Clinical Voice Disorders, 4th ed.; Thieme Medical Publishers: New York, NY, USA, 2009; pp. 101–133. [Google Scholar]
- Ludlow, C.L. Spasmodic dysphonia: A laryngeal control disorder specific to speech. J. Neurosci. 2011, 31, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A. Spasmodic dystonia and botulinum toxin: Experience from the largest treatment series. Eur. J. Neurol. 2010, 17 (Suppl. 1), 28–30. [Google Scholar] [CrossRef]
- Patel, A.B.; Bansberg, S.F.; Adler, C.H.; Lott, D.G.; Crujido, L. The Mayo Clinic Arizona spasmodic dysphonia experience: A demographic analysis of 718 patients. Ann. Otol. Rhinol. Laryngol. 2015, 124, 859–863. [Google Scholar] [CrossRef]
- Hyodo, M.; Hisa, Y.; Nishizawa, N.; Omori, K.; Shiromoto, O.; Yumoto, E.; Sanuki, T.; Nagao, A.; Hirose, K.; Kobayashi, T.; et al. The prevalence and clinical features of spasmodic dysphonia: A review of epidemiological surveys conducted in Japan. Auris Nasus Larynx 2021, 48, 179–184. [Google Scholar] [CrossRef]
- Isshiki, N.; Haji, T.; Yamamoto, Y.; Mahieu, H.F. Thyroplasty for adductor spasmodic dysphonia: Further experiences. Laryngoscope 2001, 111 Pt 1, 615–621. [Google Scholar] [CrossRef]
- Sanuki, T.; Yumoto, E. Long-term evaluation of type 2 thyroplasty with titanium bridges for adductor spasmodic dysphonia. Otolaryngol. Head Neck Surg. 2017, 157, 80–84. [Google Scholar] [CrossRef]
- Koufman, J.A.; Rees, C.J.; Halum, S.L.; Blalock, D. Treatment of adductor-type spasmodic dysphonia by surgical myectomy: A preliminary report. Ann. Otol. Rhinol. Laryngol. 2006, 115, 97–102. [Google Scholar] [CrossRef]
- Nakamura, K.; Muta, H.; Watanabe, Y.; Mochizuki, R.; Yoshida, T.; Suzuki, M. Surgical treatment for adductor spasmodic dysphonia --efficacy of bilateral thyroarytenoid myectomy under microlaryngoscopy. Acta Otolaryngol. 2008, 128, 1348–1353. [Google Scholar] [CrossRef]
- Berke, G.S.; Blackwell, K.E.; Gerratt, B.R.; Verneil, A.; Jackson, K.S.; Sercarz, J.A. Selective laryngeal adductor denervation-reinnervation: A new surgical treatment for adductor spasmodic dysphonia. Ann. Otol. Rhinol. Laryngol. 1999, 108, 227–231. [Google Scholar] [CrossRef]
- Nutt, J.G.; Muenter, M.D.; Aronson, A.; Kurland, L.T.; Melton, J., III. Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov. Disord. 1988, 3, 188–194. [Google Scholar] [CrossRef]
- Duffey, P.O.; Butler, A.G.; Hawthorne, M.R.; Barnes, M.P. The epidemiology of the primary dystonias in the north of England. Adv. Neurol. 1998, 78, 121–125. [Google Scholar]
- Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group. A prevalence study of primary dystonia in eight European countries. J. Neurol. 2000, 247, 787–792. [Google Scholar] [CrossRef]
- Konkiewitz, E.C.; Trender-Gerhard, I.; Kamm, C.; Warner, T.; Ben-Shlomo, Y.; Gasser, T.; Conrad, B.; Ceballos-Baumann, A.O. Service-based survey of dystonia in Munich. Neuroepidemiology 2002, 21, 202–206. [Google Scholar] [CrossRef] [Green Version]
- Pekmezović, T.; Ivanović, N.; Svetel, M.; Nalić, D.; Smiljković, T.; Raičević, R.; Kostić, V.S. Prevalence of primary late-onset focal dystonia in the Belgrade population. Mov. Disord. 2003, 18, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Asgeirsson, H.; Jakobsson, F.; Hjaltason, H.; Jonsdottir, H.; Sveinbjornsdottir, S. Prevalence study of primary dystonia in Iceland. Mov. Disord. 2006, 21, 293–298. [Google Scholar] [CrossRef] [PubMed]
- National Spasmodic Dysphonia Association: Spasmodic Dysphonia. Available online: https://dysphonia.org/about-sd/what-is-spasmodic-dysphonia/onset-diagnosis-sd/ (accessed on 24 November 2021).
- Yamazaki, R. Epidemiological Investigation on Spasmodic Dysphonia—Investigation by Questionnaire. Jpn. J. Logop. Phoniatr. 2001, 2, 343–347. [Google Scholar] [CrossRef]
- Yanagida, S.; Nishizawa, N.; Hatakeyama, H.; Mizoguchi, K.; Homma, A.; Fukuda, S. Investigative study on spasmodic dysphonia. Jpn. J. Logop. Phoniatr. 2016, 57, 391–397. [Google Scholar] [CrossRef]
- Tisch, S.H.; Brake, H.M.; Law, M.; Cole, I.E.; Darveniza, P. Spasmodic dysphonia: Clinical features and effects of botulinum toxin therapy in 169 patients –an Australian experience. J. Clin. Neurosci. 2003, 10, 434–438. [Google Scholar] [CrossRef]
- Tanner, K.; Roy, N.; Merrill, R.M.; Sauder, C.; Houtz, D.R.; Smith, M.E. Spasmodic dysphonia: Onset, course, socioemotional effects, and treatment response. Ann. Otol. Rhinol. Laryngol. 2011, 120, 465–473. [Google Scholar] [CrossRef]
- Creighton, F.X.; Hapner, E.; Klein, A.; Rosen, A.; Jinnah, H.A.; Johns, M.M. Diagnostic delays in spasmodic dysphonia: A call for clinician education. J. Voice 2015, 29, 592–594. [Google Scholar] [CrossRef] [Green Version]
- Ludlow, C.L.; Adler, C.H.; Berke, G.S.; Bielamowicz, S.A.; Blitzer, A.; Bressman, S.B.; Hallett, M.; Jinnah, H.A.; Juergens, U.; Martin, S.B.; et al. Research priorities in spasmodic dysphonia. Otolaryngol. Head Neck Surg. 2008, 139, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Faham, M.; Ahmadi, A.; Silverman, E.; Harouni, G.G.; Dabirmoghaddam, P. Quality of life after botulinum toxin injection in patients with adductor spasmodic dysphonia; a systematic review and meta-analysis. J. Voice 2021, 35, 271–283. [Google Scholar] [CrossRef]
- Stewart, C.F.; Sinclair, C.F.; Kling, I.F.; Diamond, B.E.; Blitzer, A. Adductor focal laryngeal Dystonia: Correlation between clinicians’ ratings and subjects’ perception of Dysphonia. J. Clin. Mov. Disord. 2017, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Morzaria, S.; Damrose, E.J. A comparison of the VHI, VHI-10, and V-RQOL for measuring the effect of botox therapy in adductor spasmodic dysphonia. J. Voice 2012, 26, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Hogikyan, N.D.; Wodchis, W.P.; Spak, C.; Kileny, P.R. Longitudinal effects of botulinum toxin injections on voice-related quality of life (V-RQOL) for patients with adductory spasmodic dysphonia. J. Voice 2001, 15, 576–586. [Google Scholar] [CrossRef] [Green Version]
- Cannito, M.P.; Doiuchi, M.; Murry, T.; Woodson, G.E. Perceptual structure of adductor spasmodic dysphonia and its acoustic correlates. J. Voice 2012, 26, 818.e5–818.e13. [Google Scholar] [CrossRef]
- Langeveld, T.P.; Drost, H.A.; Frijns, J.H.; Zwinderman, A.H.; Baatenburg de Jong, R.J. Perceptual characteristics of adductor spasmodic dysphonia. Ann. Otol. Rhinol. Laryngol. 2000, 109, 741–748. [Google Scholar] [CrossRef]
- Sapienza, C.M.; Walton, S.; Murry, T. Acoustic variations in adductor spasmodic dysphonia as a function of speech task. J. Speech Lang. Hear. Res. 1999, 42, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, S.; Nishizawa, N.; Hashimoto, R.; Mizoguchi, K.; Hatakeyama, H.; Homma, A.; Fukuda, S. Reliability and Validity of Speech Evaluation in Adductor Spasmodic Dysphonia. J. Voice 2018, 32, 585–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stachler, R.J.; Francis, D.O.; Schwartz, S.R.; Damask, C.C.; Digoy, G.P.; Krouse, H.J.; McCoy, S.J.; Ouellette, D.R.; Patel, R.R.; Reavis, C.C.W.; et al. Clinical Practice Guideline: Hoarseness (Dysphonia) (Update). Otolaryngol. Head Neck Surg. 2018, 158 (Suppl. 1), S1–S42. [Google Scholar] [CrossRef] [Green Version]
- Sulica, L. Contemporary management of spasmodic dysphonia. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 543–548. [Google Scholar] [CrossRef]
- Novakovic, D.; Waters, H.H.; D’Elia, J.B.; Blitzer, A. Botulinum toxin treatment of adductor spasmodic dysphonia: Longitudinal functional outcomes. Laryngoscope 2011, 121, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Watts, C.; Nye, C.; Whurr, R. Botulinum toxin for treating spasmodic dysphonia (laryngeal dystonia): A systematic Cochrane review. Clin. Rehabil. 2006, 20, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Troung, D.; Rontal, M.; Rolnick, M.; Aronson, A.E.; Mistura, K. Double blind controlled study of botulinum toxin in adductor spasmodic dysphonia. Laryngoscope 1991, 101, 630–634. [Google Scholar] [CrossRef]
- Hyodo, M.; Nagao, A.; Asano, K.; Sakaguchi, M.; Mizoguchi, K.; Omori, K.; Tada, Y.; Hatakeyama, H.; Oridate, N.; Naito, K.; et al. Botulinum toxin injection into the intrinsic laryngeal muscles to treat spasmodic dysphonia: A multicenter, placebo-controlled, randomized, double-blinded, parallel-group comparison/open-label clinical trial. Eur. J. Neurol. 2021, 28, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Kumada, M.; Kobayashi, T.; Kosaki, H.; Niimi, S. ‘Mora method’ for objective evaluation of severity of spasmodic dysphonia. Jpn. J. Logop. Phoniatr. 1997, 38, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Kumada, M.; Bell-Berti, F.; Kobayashi, T.; Makiyama, K.; Niimi, S. The syllable method: Proportion of impaired syllables as an indicator of spasmodic dysphonia severity. Folia Phoniatr. Logop. 2001, 53, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Omori, K. Diagnosis of Voice Disorders. JMAJ 2011, 54, 248–253. [Google Scholar]
- Yamaguchi, H.; Shrivastav, R.; Andrews, M.L.; Niimi, S. A comparison of voice quality ratings made by Japanese and American listeners using the GRBAS scale. Folia Phoniatr. Logop. 2003, 55, 147–157. [Google Scholar] [CrossRef]
- Jacobson, B.H.; Johnson, A.; Grywalski, C.; Silbergleit, A.; Jacobson, G.; Benninger, M.S.; Newman, C.W. The Voice Handicap Index (VHI): Development and validation. Am. J. Speech Lang. Pathol. 1997, 6, 66–70. [Google Scholar] [CrossRef]
- Taguchi, A.; Mise, K.; Nishikubo, K.; Hyodo, M.; Shiromoto, O. Japanese version of voice handicap index for subjective evaluation of voice disorder. J. Voice 2012, 26, 668.e15–668.e19. [Google Scholar] [CrossRef]
- Hirose, K.; Asano, K.; Sakaguchi, M.; Nagao, A.; Nakahira, M.; Doi, N.; Kobayashi, T.; Hyodo, M. Post-treatment clinical course following botulinum toxin injection therapy for adductor spasmodic dysphonia: Analysis of data from a placebo-controlled, randomized, double-blinded clinical trial in Japan. Laryngoscope Investig. Otolaryngol. 2021, 6, 1088–1095. [Google Scholar] [CrossRef]
- Blitzer, A.; Brin, M.F.; Stewart, C.; Aviv, J.E.; Fahn, S. Abductor laryngeal dystonia: A series treated with botulinum toxin. Laryngoscope 1992, 102, 163–167. [Google Scholar] [CrossRef]
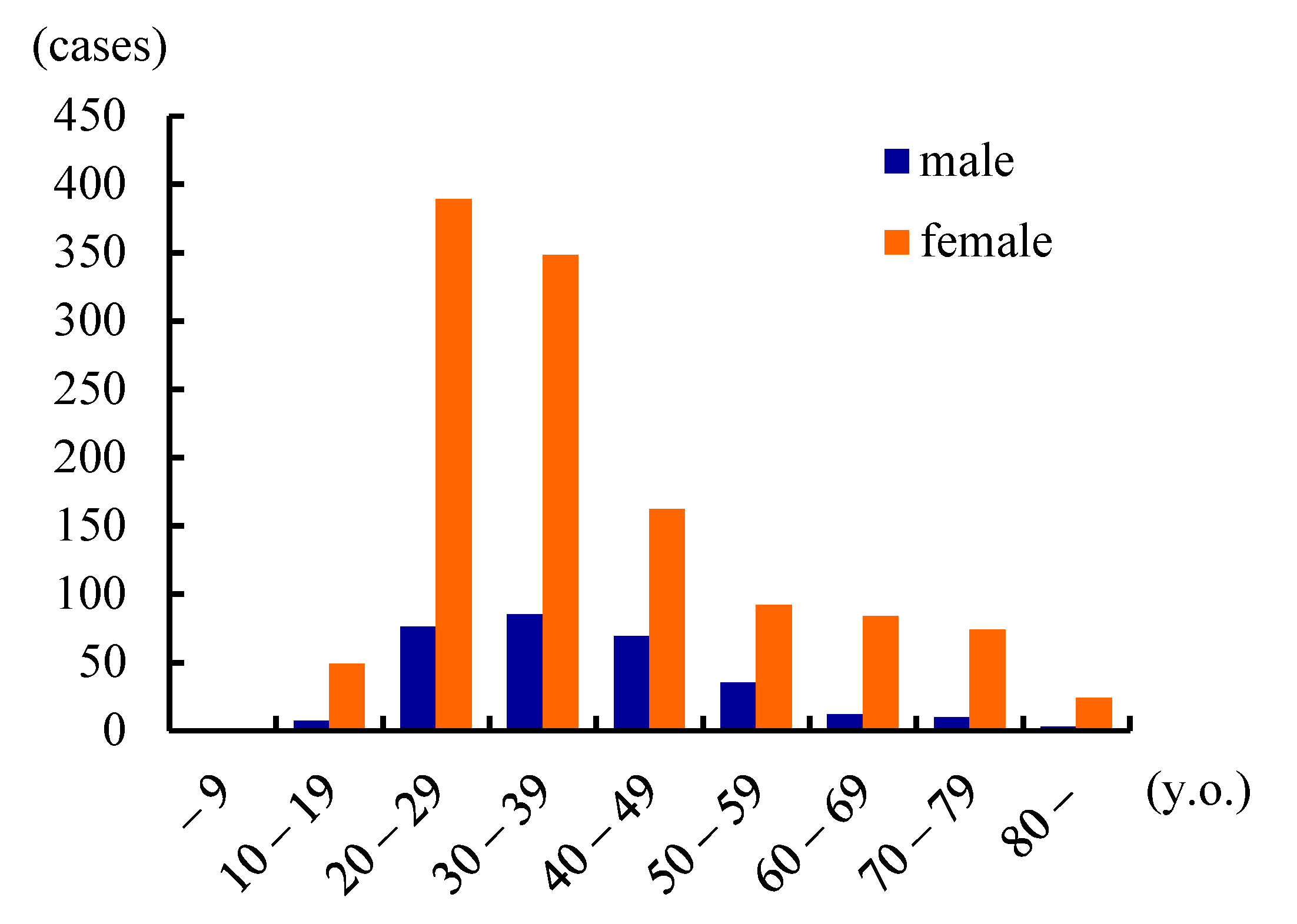
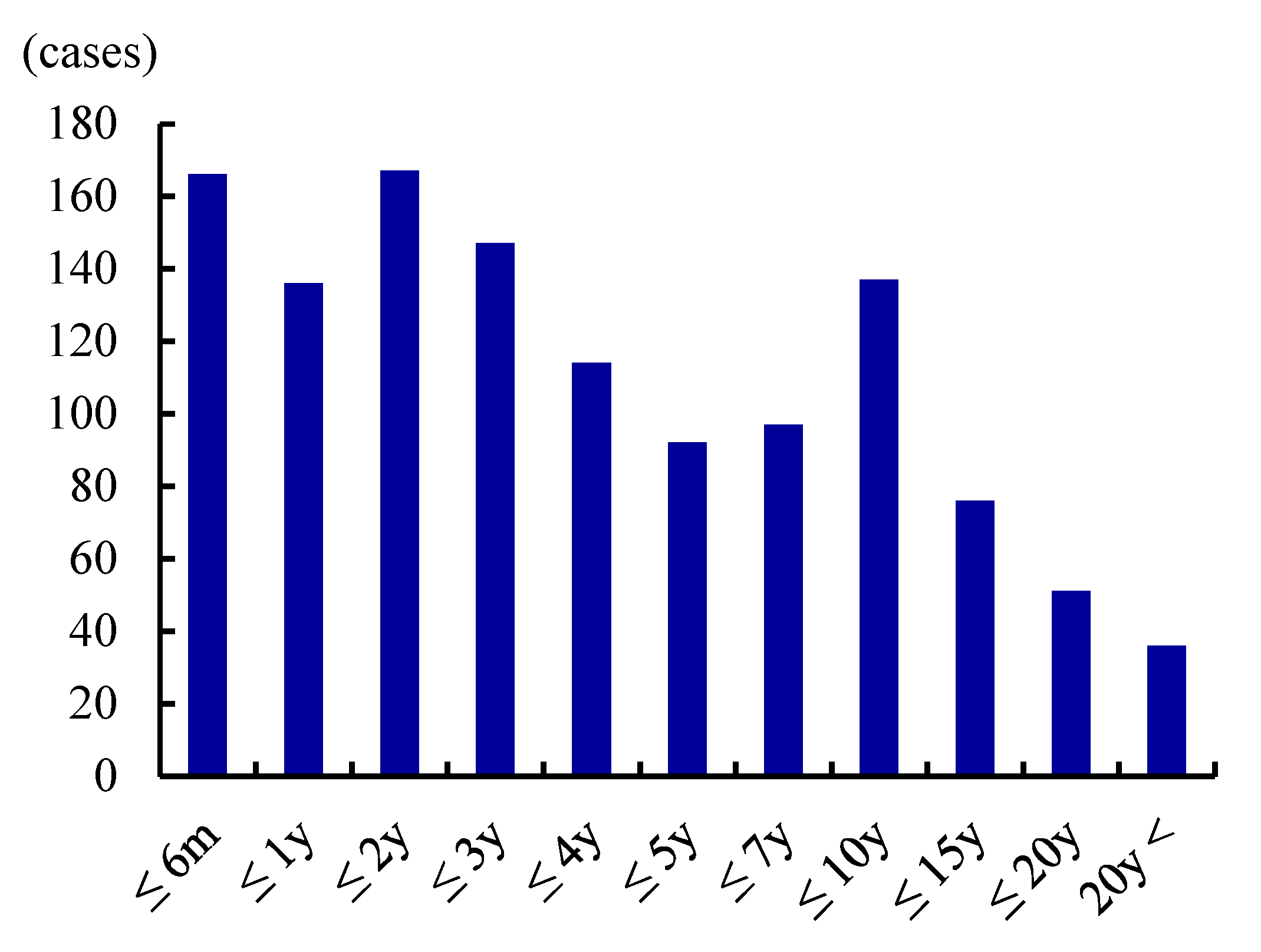
| Publications | Prevalence (/100,000) | Male:Female | Age of Onset (y.o.) |
|---|---|---|---|
| Nutt, et al. (Rochester, USA) (1988) | 5.2 (1.1–15.1) | 1:1 | 35 |
| Duffey, et al. (Northern England) (1998) | 0.8 (0.5–1.3) | N/A | N/A |
| ESDE (Europe) (2000) | 0.7 (0.5–0.9) | N/A | N/A |
| Castelon Konkiewitz, et al. (Munich, Germany) (2002) | 1.0 (0.4–1.5) | 1:1.3 | 48.0 |
| Pekmezović, et al. (Belgrade, Yugoslavia) (2003) | 1.1 (0.6–1.9) | 1:1.6 | 46.3 |
| Asgeirsson, et al. (Iceland) (2006) | 5.9 (3.4–9.4) | 1:2.4 | 50.1 |
| NSDA (North America) (2019) | 13.7 | N/A | N/A |
| Yamazaki (Japan) (2001) | 0.9 | 1:4.4 | 36.7 |
| Yanagida, et al. (Hokkaido, Japan) (2016) | 1.6 | 1:4.3 | 32 |
| Hyodo, et al. (Japan) (2016) | 3.5–7.0 | 1:4.1 | 30.9 |
| Requirements (All Mandatory). |
| (1) Voice symptoms persist for ≥6 months; |
| (2) No organic lesion or paralysis of the phonatory organs; |
| (3) No apparent abnormality in laryngeal function in terms of breathing or swallowing; |
| (4) No apparent physical or psychological cause prior to disease onset; |
| (5) No neurological or muscular disease except dystonia. |
| 1. Main Symptoms: Symptoms that appear during speech in conjunction with a normal voice. |
| ADSD |
| (1) Involuntary and intermittent strained or strangled voice; |
| (2) Involuntary and intermittent voice breaks; |
| (3) Aperiodic voice tremor; |
| (4) Effortful phonation. |
| ABSD |
| (1) Involuntary and intermittent breathy hoarseness; |
| (2) Involuntary and intermittent aphonia; |
| (3) Involuntary and intermittent falsetto voice; |
| (4) Voiceless phonation. |
| Mixed Type |
| Combination of the symptoms of ADSD and ABSD |
| 2. Accompanying Symptoms |
| (1) Certain words are difficult to pronounce (e.g., words that begin with a vowel by ADSD patients, unvoiced consonants by ABSD patients); |
| (2) Voice symptoms are reduced or disappear when the voice is high-pitched; |
| (3) Voice symptoms are reduced or disappear when laughing, crying, whispering, or singing; |
| (4) Voice symptoms worsen in strained or stressful situations, such as talking on the telephone or during business discussions. |
| 3. Findings during Phonation |
| (1) Laryngoscopic findings |
| Involuntary and intermittent adduction/abduction of the vocal folds that are synchronized with the voice symptoms. |
| (2) Findings other than vocal fold findings |
| Involuntary (unusual) descent or elevation of the larynx, or an abnormal cervical position. |
| (3) The sensory trick |
| Voice symptoms are alleviated by touching the neck with a hand, when chewing gum, when tilting the neck, or on topical anesthesia of the laryngeal mucosa. |
| 4. Treatment Response |
| (1) Trial injection of BT into the thyroarytenoid/posterior cricoarytenoid muscle improves the major symptoms; |
| (2) Systematic voice therapy does not completely resolve the symptoms. |
| 5. Differential Diagnoses |
| (1) Essential or secondary voice tremor; |
| (2) Muscle tension dysphonia; |
| (3) Psychogenic dysphonia; |
| (4) Stuttering. |
| <Definite> |
| (1) ≥3 main symptoms and all of “5. Differential diagnoses” lacking; |
| (2) ≥3 main symptoms and ≥3 items of “2. Accompanying symptoms” or “3. Findings during phonation”. |
| <Possible> |
| (1) ≥3 main symptoms, but at least one of “5. Differential diagnoses” is possible; |
| (2) 2 main symptoms and ≥2 items of any of “2. Accompanying symptoms”, “3. Findings during phonation” or “4. Treatment response”. |
| Subjective grading | |
| 1.Voice Handicap Index (VHI) | Score |
| (1) 0–24 | 0 |
| (2) 25–49 | 1 |
| (3) 50–74 | 2 |
| (4) 75–120 | 3 |
| 2. Socio-psychological impairment | |
| (1) Normal social life without difficulty in daily conversation; | 0 |
| (2) Normal social life with mild difficulty in daily conversation; | 1 |
| (3) Moderate impairment of social life because of difficulty in daily conversation, such as difficulty when talking on the telephone or in business discussions; | 2 |
| (4) Apparent impairment of social life because of difficulty in daily conversation, such as avoiding talking or socializing with others, quitting a job, becoming fired, or forsaking employment | 3 |
| Objective severity grade | |
| Physicians listen to free talk or recitations of standardized sentences. | |
| (1) Smooth and clear in conversation and recitation; | 0 |
| (2) Mild difficulty in conversation or recitation; | 1 |
| (3) Moderate difficulty in conversation and recitation, and sometimes hard to hear; | 2 |
| (4) Severe difficulty in conversation and recitation, and often very hard to hear. | 3 |
Overall disease severity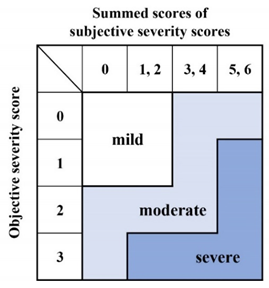 | |
| (Mean ± SE) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Change Value | |||||||||
| 2 w | 4 w | 8 w | 12 w | |||||||
| number of aberrant morae | BT | 19.2 ± 1.36 | −9.9 ± 2.66 | ** # | −7.0 ± 2.30 | * # | −6.3 ± 1.90 | * # | −3.5 ± 1.42 | * # |
| Placebo | 21.3 ± 1.86 | −1.1 ± 0.68 | −0.2 ± 0.46 | −0.3 ± 0.62 | 0.4 ± 0.43 | |||||
| (S) in GRBAS | BT | 2.1 ± 0.21 | −1.18 ± 0.33 | ** # | −0.91 ± 0.37 | * | −0.73 ± 0.36 | * | −0.36 ± 0.24 | * |
| Placebo | 1.9 ± 0.34 | −0.18 ± 0.18 | −0.27 ± 0.36 | −0.00 ± 0.19 | −0.27 ± 0.30 | |||||
| VHI | BT | 78.5 ± 5.69 | −14.6 ± 7.35 | −24.0 ± 9.63 | * | −20.6 ± 9.91 | * | −16.7 ± 7.59 | * | |
| Placebo | 72.5 ± 5.01 | −9.8 ± 3.32 | −5.3 ± 3.43 | −8.0 ± 3.52 | −5.7 ± 4.90 | |||||
| VAS | BT | 71.9 ± 5.39 | −11.6 ± 8.67 | −20.5 ± 8.74 | −18.6 ± 10.53 | −15.6 ± 8.68 | ||||
| Placebo | 72.9 ± 5.45 | −2.0 ± 4.09 | −6.2 ± 4.67 | −0.2 ± 4.70 | −3.2 ± 3.95 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyodo, M.; Asano, K.; Nagao, A.; Hirose, K.; Nakahira, M.; Yanagida, S.; Nishizawa, N. Botulinum Toxin Therapy: A Series of Clinical Studies on Patients with Spasmodic Dysphonia in Japan. Toxins 2021, 13, 840. https://doi.org/10.3390/toxins13120840
Hyodo M, Asano K, Nagao A, Hirose K, Nakahira M, Yanagida S, Nishizawa N. Botulinum Toxin Therapy: A Series of Clinical Studies on Patients with Spasmodic Dysphonia in Japan. Toxins. 2021; 13(12):840. https://doi.org/10.3390/toxins13120840
Chicago/Turabian StyleHyodo, Masamitsu, Kento Asano, Asuka Nagao, Kahori Hirose, Maya Nakahira, Saori Yanagida, and Noriko Nishizawa. 2021. "Botulinum Toxin Therapy: A Series of Clinical Studies on Patients with Spasmodic Dysphonia in Japan" Toxins 13, no. 12: 840. https://doi.org/10.3390/toxins13120840
APA StyleHyodo, M., Asano, K., Nagao, A., Hirose, K., Nakahira, M., Yanagida, S., & Nishizawa, N. (2021). Botulinum Toxin Therapy: A Series of Clinical Studies on Patients with Spasmodic Dysphonia in Japan. Toxins, 13(12), 840. https://doi.org/10.3390/toxins13120840





