Pyroptosis: A Common Feature of Immune Cells of Haemodialysis Patients
Abstract
1. Introduction
2. Results
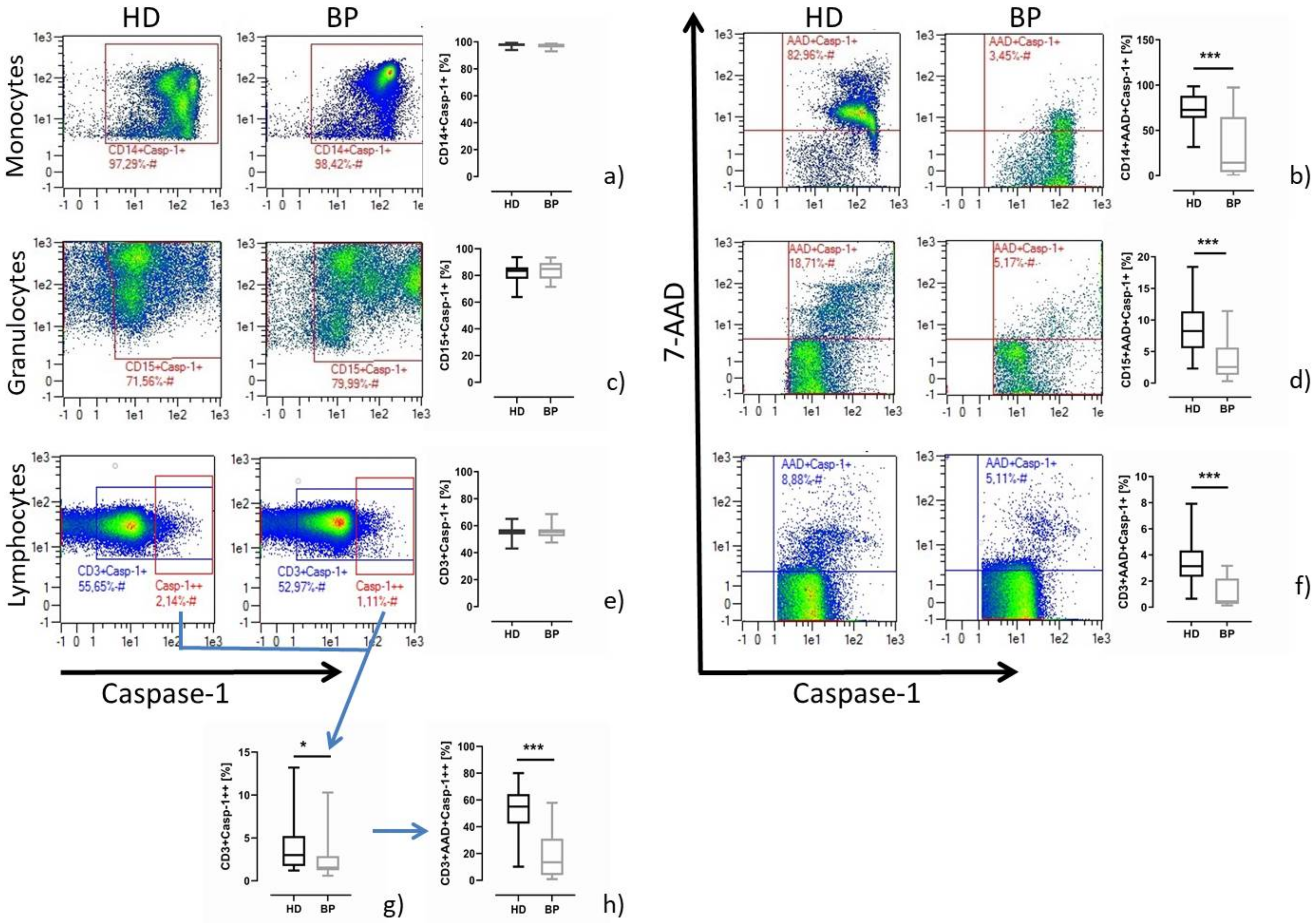
2.1. Pyroptosis: A Common Feature of Immune Cells of HD Patients
2.2. Pyroptosis in Patients after In Vitro Stimulation with LPS/Nigericin
2.3. Caspase-1 Activity and T Helper Cell Differentiation
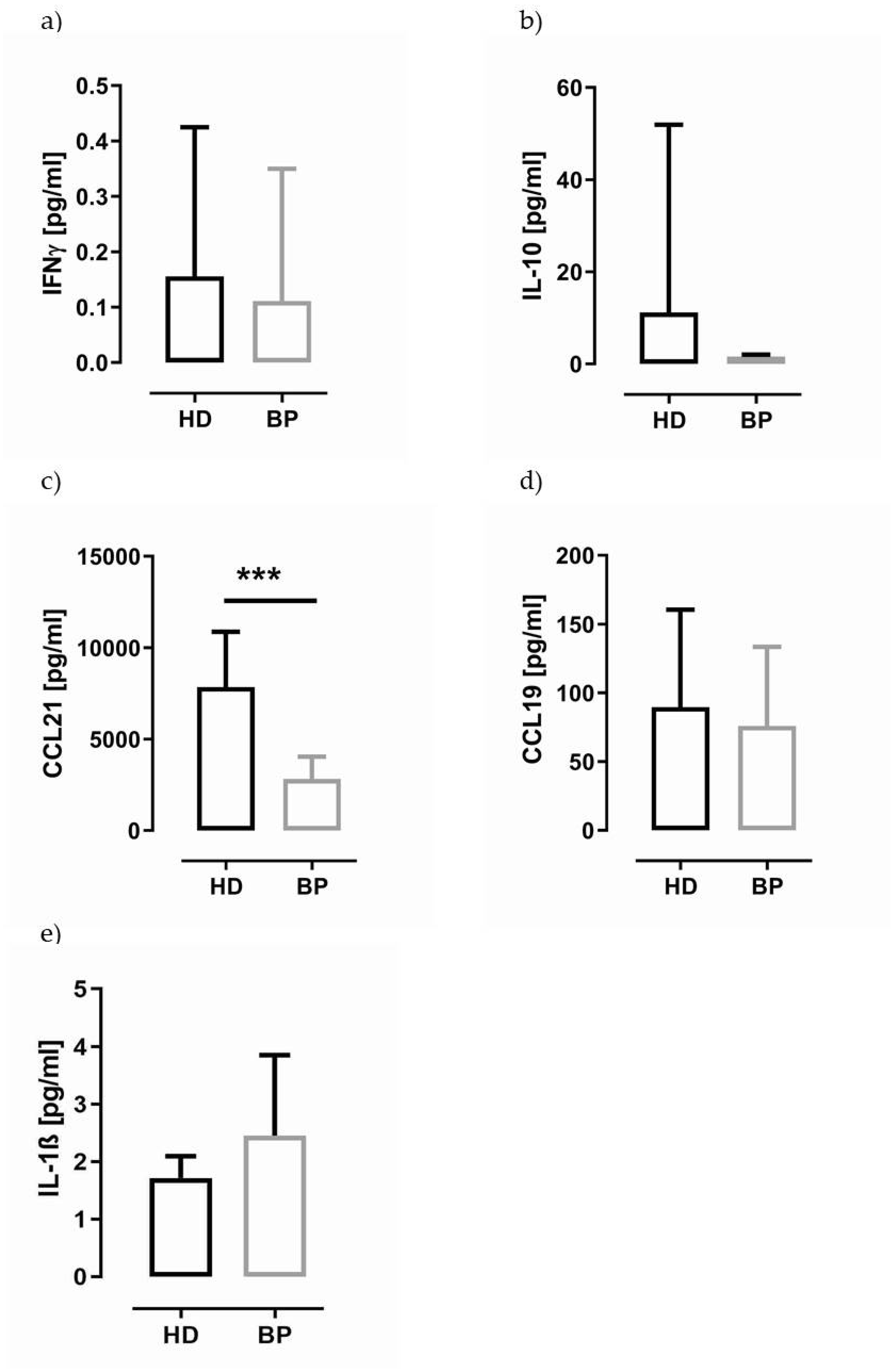
2.4. IL-1β in Supernatants of PBMCs and Granulocytes of HD and BP Patients
2.5. Indoxyl Sulfate Can Induce Pyroptosis
2.6. IS Induces the Transcription of Caspase-1, Caspase-4 and IL-1β in Differentiated THP-1 Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Population
5.2. PBMC/Granulocyte Isolation
5.3. NLRP3 Inflammasome Stimulation Model
5.4. Antibodies for Flow Cytometry
5.5. Determination of Active Caspase-1 by Flow Cytometry
5.6. THP-1 Cell Culture
5.7. Determination of Active Caspase-1 by Caspase-Glo®1 Inflammasome Assay
5.8. RNA/cDNA/qPCR
5.9. IL-1β Determination Using HEK-Blue™ IL-1β Indicator Cells
5.10. Cytokine Analysis
5.11. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacKenzie, A.; Wilson, H.L.; Kiss-Toth, E.; Dower, S.K.; North, R.A.; Surprenant, A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 2001, 15, 825–835. [Google Scholar] [CrossRef]
- Piccioli, P.; Rubartelli, A. The secretion of IL-1β and options for release. Semin. Immunol. 2013, 25, 425–429. [Google Scholar] [CrossRef]
- Qu, Y.; Franchi, L.; Nunez, G.; Dubyak, G.R. Nonclassical IL-1 beta secretion stimulated by P2 × 7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 2007, 179, 1913–1925. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Walle, L.V.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Chen, K.W.; Demarco, B.; Heilig, R.; Shkarina, K.; Boettcher, A.; Farady, C.J.; Pelczar, P.; Broz, P. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. EMBO J. 2019, 38, e101638. [Google Scholar] [CrossRef]
- Orning, P.; Lien, E.; Fitzgerald, K.A. Gasdermins and their role in immunity and inflammation. J. Exp. Med. 2019, 216, 2453–2465. [Google Scholar] [CrossRef]
- Matsuo, K.; Yamamoto, S.; Wakamatsu, T.; Takahashi, Y.; Kawamura, K.; Kaneko, Y.; Goto, S.; Kazama, J.J.; Narita, I. Increased Proinflammatory Cytokine Production and Decreased Cholesterol Efflux Due to Downregulation of ABCG1 in Macrophages Exposed to Indoxyl Sulfate. Toxins 2015, 7, 3155–3166. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Yamamoto, S.; Ito, T.; Sato, Y.; Matsuo, K.; Takahashi, Y.; Kaneko, Y.; Goto, S.; Kazama, J.J.; Gejyo, F.; et al. Indoxyl Sulfate Promotes Macrophage IL-1β Production by Activating Aryl Hydrocarbon Receptor/NF-κ/MAPK Cascades, but the NLRP3 inflammasome Was Not Activated. Toxins 2018, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Yndestad, A.; Finsen, A.V.; Ueland, T.; Husberg, C.; Dahl, C.P.; Øie, E.; Vinge, L.E.; Sjaastad, I.; Sandanger, Ø.; Ranheim, T.; et al. The Homeostatic Chemokine CCL21 Predicts Mortality and May Play a Pathogenic Role in Heart Failure. PLoS ONE 2012, 7, e33038. [Google Scholar] [CrossRef]
- Boini, K.M.; Hussain, T.; Li, P.-L.; Koka, S. Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction. Cell. Physiol. Biochem. 2017, 44, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, M.; Simpson, D.S.; Hempel, A.; Frank, D.; Petrie, E.; Vince, A.; Feltham, R.; Murphy, J.; Chatfield, S.M.; Salvesen, G.S.; et al. The Pyroptotic Cell Death Effector Gasdermin D Is Activated by Gout-Associated Uric Acid Crystals but Is Dispensable for Cell Death and IL-1β Release. J. Immunol. 2019, 203, 736–748. [Google Scholar] [CrossRef]
- Ulrich, C.; Wildgrube, S.; Fiedler, R.; Seibert, E.; Kneser, L.; Fick, S.; Schäfer, C.; Markau, S.; Trojanowicz, B.; Girndt, M. NLRP3 Inflammasome Activation in Hemodialysis and Hypertensive Patients with Intact Kidney Function. Toxins 2020, 12, 675. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, C.; Chen, Z.; Liu, L.; Jiang, J.; Wu, Z.; Zhao, M.; Chen, Y. NLRP3: A Novel Mediator in Cardiovascular Disease. J. Immunol. Res. 2018, 2018, 5702103. [Google Scholar] [CrossRef]
- Bruchard, M.; Rebé, C.; Derangère, V.; Togbé, D.; Ryffel, B.; Boidot, R.; Humblin, E.; Hamman, A.; Chalmin, F.; Berger, H.; et al. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat. Immunol. 2015, 16, 859–870. [Google Scholar] [CrossRef]
- Arbore, G.; West, E.E.; Spolski, R.; Robertson, A.A.B.; Klos, A.; Rheinheimer, C.; Dutow, P.; Woodruff, T.M.; Yu, Z.X.; O’Neill, L.A.; et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4⁺ T cells. Science 2016, 352, aad1210. [Google Scholar] [CrossRef] [PubMed]
- Menning, A.; Höpken, U.E.; Siegmund, K.; Lipp, M.; Hamann, A.; Huehn, J. Distinctive role of CCR7 in migration and functional activity of naive- and effector/memory-like Treg subsets. Eur. J. Immunol. 2007, 37, 1575–1583. [Google Scholar] [CrossRef]
- Akhavanpoor, M.; Gleissner, C.A.; Gorbatsch, S.; Doesch, A.O.; Akhavanpoor, H.; Wangler, S.; Jahn, F.; Lasitschka, F.; Katus, H.A.; Erbel, C. CCL19 and CCL21 modulate the inflammatory milieu in atherosclerotic lesions. Drug Des. Devel. Ther. 2014, 8, 2359–2371. [Google Scholar] [CrossRef][Green Version]
- Hsu, H.-J.; Yen, C.-H.; Wu, I.-W.; Hsu, K.-H.; Chen, C.-K.; Sun, C.-Y.; Chou, C.-C.; Chen, C.-Y.; Tsai, C.-J.; Wu, M.-S.; et al. The association of uremic toxins and inflammation in hemodialysis patients. PLoS ONE 2014, 9, e102691. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S. Molecular mechanisms underlying uremic toxin-related systemic disorders in chronic kidney disease: Focused on β2-microglobulin-related amyloidosis and indoxyl sulfate-induced atherosclerosis-Oshima Award Address 2016. Clin. Exp. Nephrol. 2019, 23, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Imazu, M.; Fukuda, H.; Kanzaki, H.; Amaki, M.; Hasegawa, T.; Takahama, H.; Hitsumoto, T.; Tsukamoto, O.; Morita, T.; Ito, S.; et al. Plasma indoxyl sulfate levels predict cardiovascular events in patients with mild chronic heart failure. Sci. Rep. 2020, 10, 16528. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-J.; Wu, C.-J.; Pan, C.-F.; Chen, Y.-C.; Sun, F.-J.; Chen, H.-H. Serum protein-bound uraemic toxins and clinical outcomes in haemodialysis patients. Nephrol. Dial. Transpl. 2010, 25, 3693–3700. [Google Scholar] [CrossRef]
- Moradi, H.; Sica, D.A.; Kalantar-Zadeh, K. Cardiovascular Burden Associated with Uremic Toxins in Patients with Chronic Kidney Disease. Am. J. Nephrol. 2013, 38, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- O’Brien, M.; Moehring, D.; Muñoz-Planillo, R.; Núñez, G.; Callaway, J.; Ting, J.; Scurria, M.; Ugo, T.; Bernad, L.; Cali, J.; et al. A bioluminescent caspase-1 activity assay rapidly monitors inflammasome activation in cells. J. Immunol. Methods 2017, 447, 1–13. [Google Scholar] [CrossRef]
- Thornberry, N.A.; Rano, T.A.; Peterson, E.P.; Rasper, D.M.; Timkey, T.; Garcia-Calvo, M.; Houtzager, V.M.; Nordstrom, P.A.; Roy, S.; Vaillancourt, J.P.; et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 1997, 272, 17907–17911. [Google Scholar] [CrossRef]
- Julien, O.; Wells, J.A. Caspases and their substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.A.; Song, Z. Some commonly used caspase substrates and inhibitors lack the specificity required to monitor individual caspase activity. Biochem. Biophys. Res. Commun. 2008, 377, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ladrech, S.; Pujol, R.; Brabet, P.; van de Water, T.R.; Puel, J.-L. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res. 2004, 64, 9217–9224. [Google Scholar] [CrossRef]
- Nakano, T.; Katsuki, S.; Chen, M.; Decano, J.L.; Halu, A.; Lee, L.H.; Pestana, D.V.S.; Kum, A.S.T.; Kuromoto, R.K.; Golden, W.S.; et al. Uremic Toxin Indoxyl Sulfate Promotes Proinflammatory Macrophage Activation Via the Interplay of OATP2B1 and Dll4-Notch Signaling. Circulation 2019, 139, 78–96. [Google Scholar] [CrossRef]
- Ito, S.; Osaka, M.; Edamatsu, T.; Itoh, Y.; Yoshida, M. Crucial Role of the Aryl Hydrocarbon Receptor (AhR) in Indoxyl Sulfate-Induced Vascular Inflammation. J. Atheroscler. Thromb. 2016, 23, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Funatake, C.J.; Marshall, N.B.; Steppan, L.B.; Mourich, D.V.; Kerkvliet, N.I. Cutting edge: Activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J. Immunol. 2005, 175, 4184–4188. [Google Scholar] [CrossRef]
- Quintana, F.J.; Basso, A.S.; Iglesias, A.H.; Korn, T.; Farez, M.F.; Bettelli, E.; Caccamo, M.; Oukka, M.; Weiner, H.L. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008, 453, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Fuller, D.S.; Komaba, H.; Nomura, T.; Massy, Z.A.; Bieber, B.; Robinson, B.; Pisoni, R.; Fukagawa, M. Serum total indoxyl sulfate and clinical outcomes in hemodialysis patients: Results from the Japan Dialysis Outcomes and Practice Patterns Study. Clin. Kidney J. 2021, 14, 1236–1243. [Google Scholar] [CrossRef]
- Leong, S.C.; Sirich, T.L. Indoxyl Sulfate—Review of Toxicity and Therapeutic Strategies. Toxins 2016, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Nagata, D.; Yoshizawa, H. Pharmacological Actions of Indoxyl Sulfate and AST-120 That Should Be Recognized for the Strategic Treatment of Patients with Chronic Kidney Disease. IJNRD 2020, 13, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shin, E.A.; Lee, J.H.; Ahn, D.; Kim, C.G.; Kim, J.-H.; Kim, S.-H. Caspase inhibitors: A review of recently patented compounds (2013–2015). Expert Opin. Ther. Pat. 2018, 28, 47–59. [Google Scholar] [CrossRef] [PubMed]



| HD | BP | p-Value | |
|---|---|---|---|
| CD4 (%) | 56.5 ± 16.6 | 57.3 ± 15.3 | 0.874 |
| CD8 (%) | 31.9 ± 18.8 | 32.1 ± 13.9 | 0.735 |
| Th1 (CD4+CXCR3+CCR6-) | 37.1 ± 18.1 | 44.8 ± 18.3 | 0.194 |
| Th2 (CD4+CXCR3-CCR6-) | 61.0 ± 18.4 | 53.5 ± 18.1 | 0.227 |
| Th17 (CD4+CXCR3-CCR6+) | 1.2 ± 0.9 | 1.1 ± 0.8 | 0.563 |
| Tregs (CD4+CD25+CD127low) | 4.9 ± 2.0 | 2.6 ± 1.7 | 00.004 |
| %CD4+CD28+CD45RO-CCR7+ (CD4+CCR7+ naive cells) | 24.4 ± 16.7 | 27.4 ± 16.5 | 0.650 |
| %CD4+28+45RO+CCR7+ (CD4+ central memory cells) | 56.8 ± 18.3 | 58.4 ± 16.0 | 0.840 |
| HD | BP | Statistics | |
|---|---|---|---|
| uP: IL-1β (pg/mL) | 4.6 ± 6.6 | 17.4 ± 29.7 | 0.001 |
| sP: IL-1β (pg/mL) | 5409 ± 3166 | 5507 ± 4620 | 0.678 |
| uG: IL-1β (pg/mL) | 2.5 ± 0.6 | 7.1 ± 5.7 | 0.001 |
| sG: IL-1β (pg/mL) | 275 ± 199 | 890 ± 1564 | 0.198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulrich, C.; Kneser, L.; Fiedler, R.; Beckert, J.; Wildgrube, S.; Seibert, E.; Fick, S.; Schäfer, C.; Markau, S.; Trojanowicz, B.; et al. Pyroptosis: A Common Feature of Immune Cells of Haemodialysis Patients. Toxins 2021, 13, 839. https://doi.org/10.3390/toxins13120839
Ulrich C, Kneser L, Fiedler R, Beckert J, Wildgrube S, Seibert E, Fick S, Schäfer C, Markau S, Trojanowicz B, et al. Pyroptosis: A Common Feature of Immune Cells of Haemodialysis Patients. Toxins. 2021; 13(12):839. https://doi.org/10.3390/toxins13120839
Chicago/Turabian StyleUlrich, Christof, Leonie Kneser, Roman Fiedler, Julia Beckert, Susann Wildgrube, Eric Seibert, Sylvia Fick, Christoph Schäfer, Silke Markau, Bogusz Trojanowicz, and et al. 2021. "Pyroptosis: A Common Feature of Immune Cells of Haemodialysis Patients" Toxins 13, no. 12: 839. https://doi.org/10.3390/toxins13120839
APA StyleUlrich, C., Kneser, L., Fiedler, R., Beckert, J., Wildgrube, S., Seibert, E., Fick, S., Schäfer, C., Markau, S., Trojanowicz, B., & Girndt, M. (2021). Pyroptosis: A Common Feature of Immune Cells of Haemodialysis Patients. Toxins, 13(12), 839. https://doi.org/10.3390/toxins13120839





