Practical Application of Novel Test Methods to Evaluate the Potency of Botulinum Toxin: A Comparison Analysis among Widely Used Products in Korea
Abstract
:1. Introduction
2. Results
2.1. Estimated Potency for Each BoNT/A Product via LD50 Assay
2.2. Measurement of Total BoNT/A Protein Amounts by Sandwich ELISA
2.3. FRET-Based Potency Assay
2.4. SPR-Based Potency Assay
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Toxin Preparations
5.2. LD50 Assay
5.3. Sandwich ELISA
5.4. FRET-Based Potency Assay
5.5. SPR Binding Analysis
5.5.1. Preparation of SNAP-25 Chips
5.5.2. Endoprotease Activity Using SPR Analysis
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, T.J.; Hill, K.K.; Raphael, B.H. Historical and current perspectives on Clostridium botulinum diversity. Res. Microbiol. 2015, 166, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Tehran, D.A.; Pirazzini, M. Novel botulinum neurotoxins: Exploring underneath the iceberg tip. Toxins 2018, 10, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zornetta, I.; Azarnia Tehran, D.; Arrigoni, G.; Anniballi, F.; Bano, L.; Leka, O.; Zanotti, G.; Binz, T.; Montecucco, C. The first non Clostridial botulinum-like toxin cleaves VAMP within the juxtamembrane domain. Sci. Rep. 2016, 6, 30257. [Google Scholar] [CrossRef]
- Zhang, S.; Masuyer, G.; Zhang, J.; Shen, Y.; Lundin, D.; Henriksson, L.; Miyashita, S.I.; Martinez-Carranza, M.; Dong, M.; Stenmark, P. Identification and characterization of a novel botulinum neurotoxin. Nat. Commun. 2017, 8, 14130. [Google Scholar] [CrossRef]
- Orsini, M.; Leite, M.A.A.; Chung, T.M.; Bocca, W.; de Souza, J.A.; de Souza, O.G.; Moreira, R.P.; Bastos, V.H.; Teixeira, S.; Oliveira, A.B.; et al. Botulinum neurotoxin type A in neurology: Update. Neurol. Int. 2015, 7, 31–35. [Google Scholar] [CrossRef]
- Nepal, M.R.; Jeong, T.C. Alternative methods for testing botulinum toxin: Current status and future perspectives. Biomol. Ther. 2020, 28, 302–310. [Google Scholar] [CrossRef]
- Dressler, D.; Adib Saberi, F. Botulinum toxin: Mechanisms of action. Eur. Neurol. 2005, 53, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Halevi, S.; Melman, P.; Schwartz, J.; Cai, S.; Singh, B.R. A novel surface plasmon resonance biosensor for the rapid detection of botulinum neurotoxins. Biosensors 2017, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.L.; Maslanka, S.; Johnson, E.; Goodnough, M. Detection of botulinal neurotoxins A, B, E, and F by amplified enzyme-linked immunosorbent assay: Collaborative study. J. AOAC Int. 2003, 86, 314–331. [Google Scholar] [CrossRef] [Green Version]
- Kalb, S.R.; Moura, H.; Boyer, A.E.; McWilliams, L.G.; Pirkle, J.L.; Barr, J.R. The use of Endopep-MS for the detection of botulinum toxins A, B, E, and F in serum and stool samples. Anal. Biochem. 2006, 351, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Yadirgi, G.; Stickings, P.; Rajagopal, S.; Liu, Y.; Sesardic, D. Immuno-detection of cleaved SNAP-25 from differentiated mouse embryonic stem cells provides a sensitive assay for determination of botulinum A toxin and antitoxin potency. J. Immunol. Methods 2017, 451, 90–99. [Google Scholar] [CrossRef]
- Dong, M.; Tepp, W.H.; Johnson, E.A.; Chapman, E.R. Using fluorescent sensors to detect botulinum neurotoxin activity in vitro and in living cells. Proc. Natl. Acad. Sci. USA 2004, 101, 14701–14706. [Google Scholar] [CrossRef] [Green Version]
- Frevert, J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type a products. Drugs R D 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frevert, J.; Ahn, K.Y.; Park, M.Y.; Sunga, O. Comparison of botulinum neurotoxin type a formulations in Asia. Clin. Cosmet. Investig. Dermatol. 2018, 11, 327–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schellekens, H. How to predict and prevent the immunogenicity of therapeutic proteins. Biotechnol. Annu. Rev. 2008, 14, 191–202. [Google Scholar] [PubMed]
- Samadzadeh, S.; Urer, B.; Brauns, R.; Rosenthal, D.; Lee, J.I.; Albrecht, P.; Hefter, H. Clinical implications of difference in antigenicity of different botulinum neurotoxin type a preparations: Clinical take-home messages from our research pool and literature. Toxins 2020, 12, 499. [Google Scholar] [CrossRef]
- Frevert, J. Content of botulinum neurotoxin in Botox(R)/Vistabel(R), Dysport(R)/Azzalure(R), and Xeomin(R)/Bocouture(R). Drugs R D 2010, 10, 67–73. [Google Scholar] [CrossRef]
- Yoon, J.S.; Kim, J.C.; Lee, S.Y. Double-blind, randomized, comparative study of Meditoxin versus Botox in the treatment of essential blepharospasm. Korean J. Ophthalmol. 2009, 23, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Ferracci, G.; Miquelis, R.; Kozaki, S.; Seagar, M.; Leveque, C. Synaptic vesicle chips to assay botulinum neurotoxins. Biochem. J. 2005, 391, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Eivazzadeh-Keihan, R.; Pashazadeh-Panahi, P.; Baradaran, B.; de la Guardia, M.; Hejazi, M.; Sohrabi, H.; Mokhtarzadeh, A.; Maleki, A. Recent progress in optical and electrochemical biosensors for sensing of Clostridium botulinum neurotoxin. TrAC Trends Anal. Chem. 2018, 103, 184–197. [Google Scholar] [CrossRef]
- Eisele, K.H.; Fink, K.; Vey, M.; Taylor, H.V. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon 2011, 57, 555–565. [Google Scholar] [CrossRef]
- Dressler, D.; Pan, L.; Adib Saberi, F. Antibody-induced failure of botulinum toxin therapy: Re-start with low-antigenicity drugs offers a new treatment opportunity. J. Neural Transm. 2018, 125, 1481–1486. [Google Scholar] [CrossRef]
- Naumann, M.; Boo, L.M.; Ackerman, A.H.; Gallagher, C.J. Immunogenicity of botulinum toxins. J. Neural Transm. 2013, 120, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.Y.; Jeong, G.J.; Kwon, T.R.; Kim, J.H.; Li, K.; Kim, B.J. Efficacy and safety of a novel botulinum toxin A for masseter reduction: A randomized, double-blind, placebo-controlled, optimal dose-finding study. Dermatol. Surg. 2021, 47, e5–e9. [Google Scholar] [CrossRef] [PubMed]
- Hefter, H.; Rosenthal, D.; Moll, M. High botulinum toxin-neutralizing antibody prevalence under long-term cervical dystonia treatment. Mov. Disord. Clin. Pract. 2016, 3, 500–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheon, H.I.; Jung, N.; Won, C.H.; Kim, B.J.; Lee, Y.W. Efficacy and Safety of Prabotulinumtoxin A and Onabotulinumtoxin A for Crow’s Feet: A Phase 3, Multicenter, Randomized, Double-Blind, Split-Face Study. Dermatol. Surg. 2019, 45, 1610–1619. [Google Scholar] [CrossRef]
- Wanitphakdeedecha, R.; Ungaksornpairote, C.; Kaewkes, A.; Sathaworawong, A.; Vanadurongwan, B.; Lektrakul, N. A pilot study comparing the efficacy of two formulations of botulinum toxin type A for muscular calves contouring. J. Cosmet. Dermatol. 2018, 17, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.Y.; Chang, H.J.; Lee, S.J.; Lee, S.; Park, J.H.; Kwon, J.Y. Efficacy of Repeated Botulinum Toxin Type A Injections for Spastic Equinus in Children with Cerebral Palsy—A Secondary Analysis of the Randomized Clinical Trial. Toxins 2017, 9, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, G.J.; Kim, J.H.; Oh, H.J.; Park, K.Y.; Seo, S.J. Potency and persistence of reconstituted botulinum neurotoxin type A: Mouse IP LD50 assay. Dermatol. Surg. 2020, 46, e78–e81. [Google Scholar] [CrossRef]
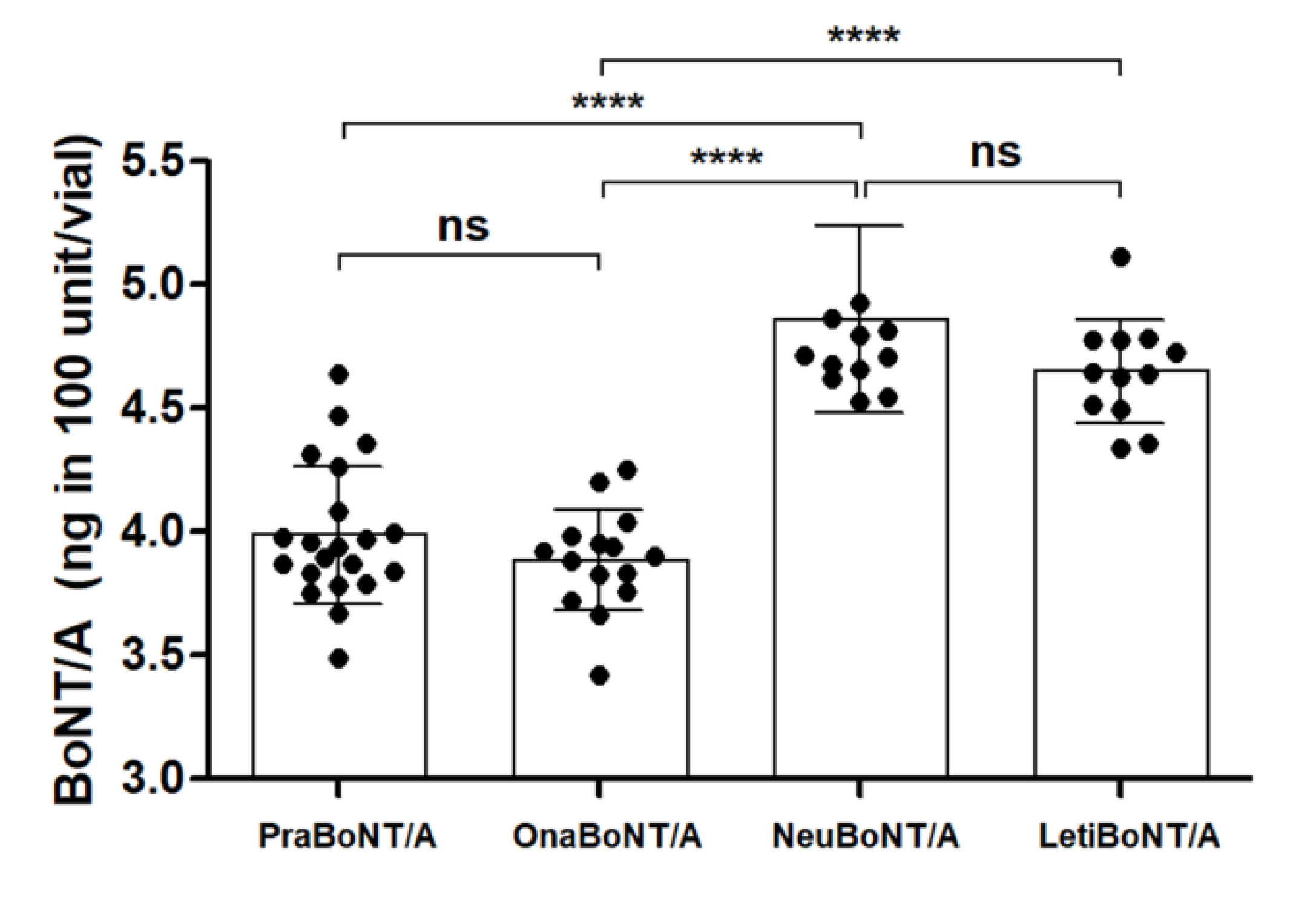
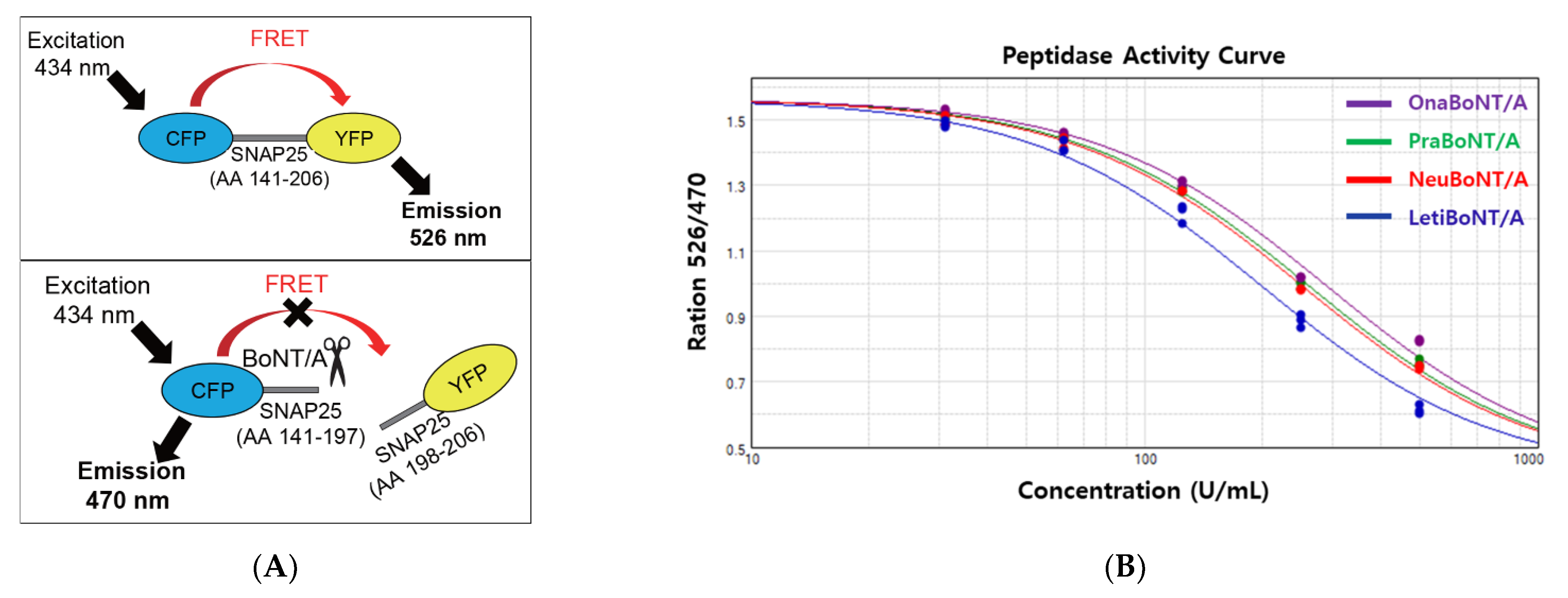
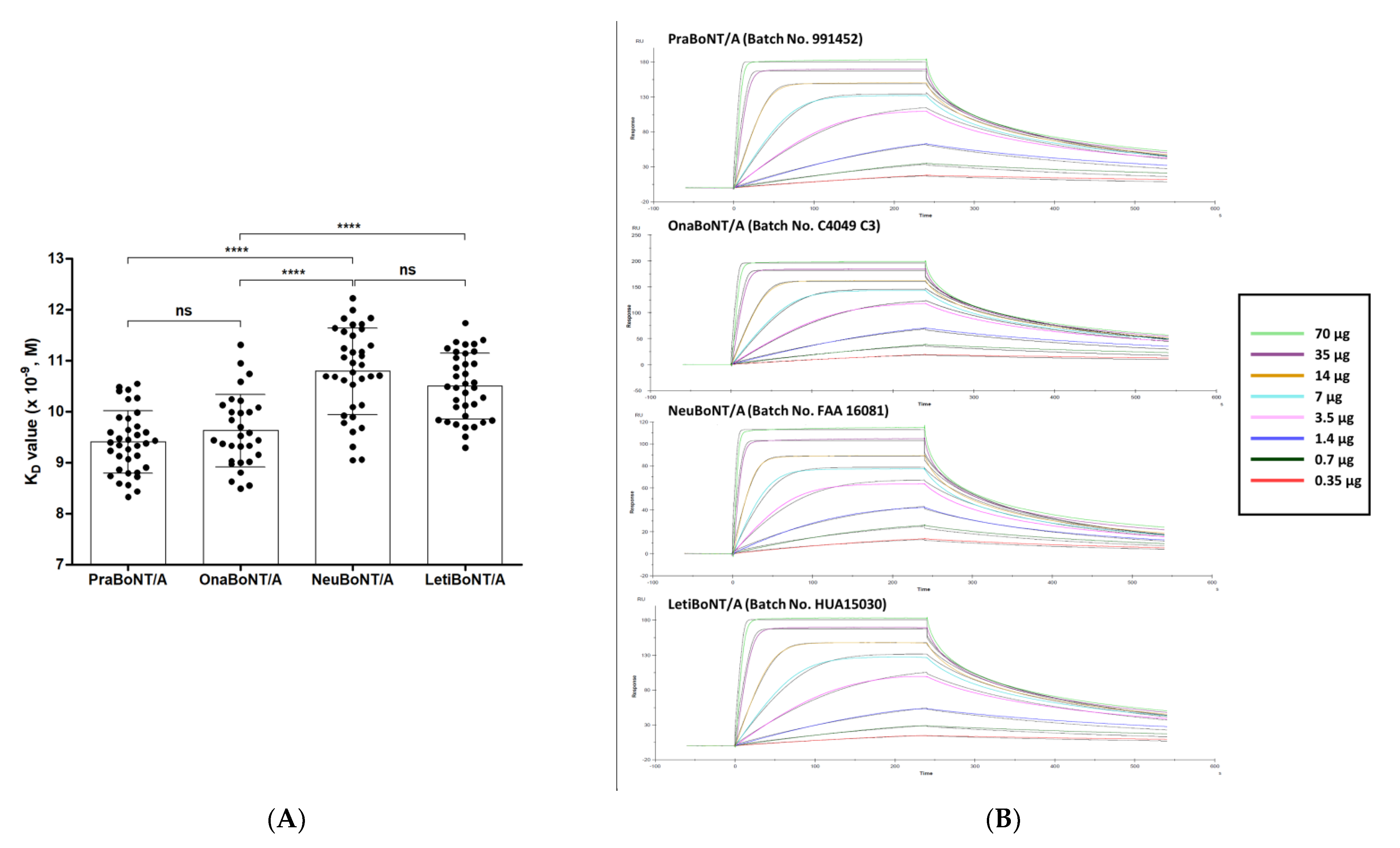
| Product Name | ELISA (ng/100 U) | FRET (Efficacy Relative to 100 U OnaBoNT/A) | Potency (%) | KD (×10−9, M) * |
|---|---|---|---|---|
| Prabotulinumtoxin A | 3.98 ± 0.28 | 111.0 ± 0.033 | 99.87 ± 5.29% | 9.40 ± 0.61 |
| Onabotulinumtoxin A | 3.88 ± 0.20 | 100.0 (Standard) | 101.23 ± 10.90% | 9.62 ± 0.71 |
| Neubotulinumtoxin A | 4.86 ± 0.37 | 115.4 ± 0.034 | 105.03 ± 9.29% | 10.79 ± 0.85 |
| Letibotulinumtoxin A | 4.64 ± 0.21 | 146.4 ± 0.043 | 118.87 ± 9.65% | 10.50 ± 0.64 |
| Product Name | Brand Name | Drying Method | Dosage (unit) | Composition | Excipients | Storage |
|---|---|---|---|---|---|---|
| PraBoNT/A | NABOTA® | Vacuum-dried | 100 U | BoNT/A 900 kD complex | 0.5 mg human serum albumin, 0.9 mg NaCl | 2~8 °C |
| OnaBoNT/A | BOTOX® | Vacuum-dried | ||||
| NeuBoNT/A | Meditoxin® | Freeze-dried | ||||
| LetiBoNT/A | Botulax® | Freeze-dried |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.-Y.; Kim, J.-H.; Jin, J.-E.; Shin, S.-H.; Park, K.-Y. Practical Application of Novel Test Methods to Evaluate the Potency of Botulinum Toxin: A Comparison Analysis among Widely Used Products in Korea. Toxins 2021, 13, 833. https://doi.org/10.3390/toxins13120833
Hong J-Y, Kim J-H, Jin J-E, Shin S-H, Park K-Y. Practical Application of Novel Test Methods to Evaluate the Potency of Botulinum Toxin: A Comparison Analysis among Widely Used Products in Korea. Toxins. 2021; 13(12):833. https://doi.org/10.3390/toxins13120833
Chicago/Turabian StyleHong, Ji-Yeon, Jong-Hee Kim, Jung-Eun Jin, Sun-Hye Shin, and Kui-Young Park. 2021. "Practical Application of Novel Test Methods to Evaluate the Potency of Botulinum Toxin: A Comparison Analysis among Widely Used Products in Korea" Toxins 13, no. 12: 833. https://doi.org/10.3390/toxins13120833
APA StyleHong, J.-Y., Kim, J.-H., Jin, J.-E., Shin, S.-H., & Park, K.-Y. (2021). Practical Application of Novel Test Methods to Evaluate the Potency of Botulinum Toxin: A Comparison Analysis among Widely Used Products in Korea. Toxins, 13(12), 833. https://doi.org/10.3390/toxins13120833





