Observation of Bothrops atrox Snake Envenoming Blister Formation from Five Patients: Pathophysiological Insights
Abstract
:1. Introduction
2. Results
2.1. Clinical Observations
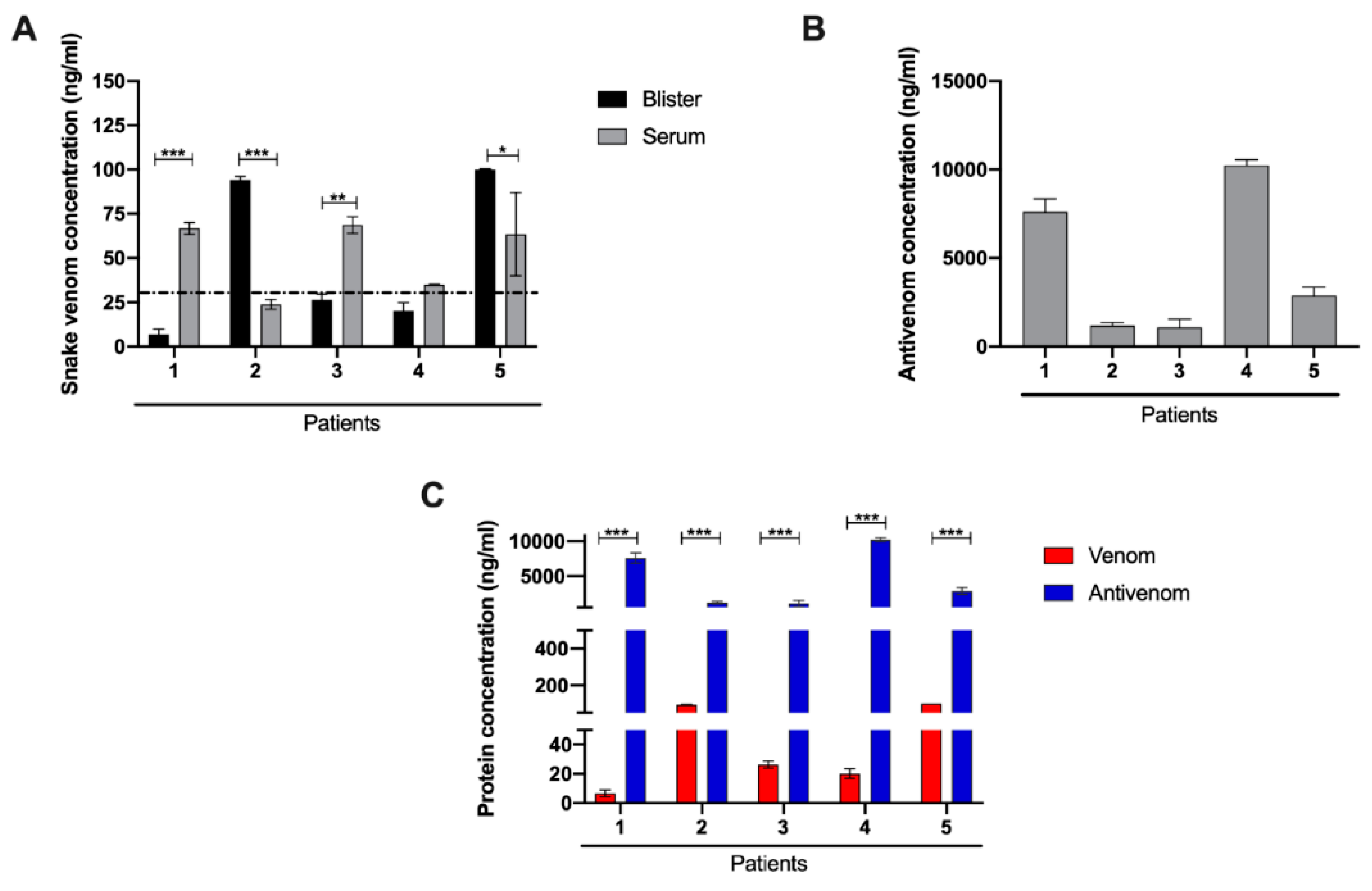
2.2. Laboratory Characterization for the Presence of Venom and Antivenom in Patient Serum and Blister Fluid
2.3. Proteomic Characterization of Patient Blister Fluids
3. Discussion
4. Materials and Methods
4.1. Ethical Statement and Clinical Data Collection
4.2. ELISA Quantification of Antivenom and Venom in Blister Contents
4.3. Western Blot of Antivenom in Blister Contents
4.4. Proteomic Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- WHO. Rabies and Envenomings. A Neglected Public Health Issue; World Health Organization: Geneva, Switzerland, 2007; ISBN 978-92-4-156348-2. [Google Scholar]
- Feitosa, E.L.; Sampaio, V.S.; Salinas, J.L.; Queiroz, A.M.; da Silva, I.M.; Gomes, A.A.; Sachett, J.; Siqueira, A.M.; Ferreira, L.C.; Dos Santos, M.C.; et al. Older Age and Time to Medical Assistance Are Associated with Severity and Mortality of Snakebites in the Brazilian Amazon: A Case-Control Study. PLoS ONE 2015, 10, e0132237. [Google Scholar] [CrossRef]
- Magalhães, S.F.V.; Peixoto, H.M.; Moura, N.; Monteiro, W.M.; de Oliveira, M.R.F. Snakebite envenomation in the Brazilian Amazon: A descriptive study. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 143–151. [Google Scholar] [CrossRef]
- Feitosa, E.S.; Sampaio, V.; Sachett, J.; Castro, D.B.; Noronha, M.; Lozano, J.L.; Muniz, E.; Ferreira, L.C.; Lacerda, M.V.; Monteiro, W.M. Snakebites as a largely neglected problem in the Brazilian Amazon: Highlights of the epidemiological trends in the State of Amazonas. Rev. Soc. Bras. Med. Trop. 2015, 48 (Suppl. 1), 34–41. [Google Scholar] [CrossRef]
- Alves, E.C.; Sachett, J.A.G.; Sampaio, V.S.; Sousa, J.D.B.; Oliveira, S.S.; Nascimento, E.F.D.; Santos, A.D.S.; da Silva, I.M.; da Silva, A.M.M.; Wen, F.H.; et al. Predicting acute renal failure in Bothrops snakebite patients in a tertiary reference center, Western Brazilian Amazon. PLoS ONE 2018, 13, e0202361. [Google Scholar] [CrossRef]
- Monteiro, W.M.; Contreras-Bernal, J.C.; Bisneto, P.F.; Sachett, J.; Mendonça da Silva, I.; Lacerda, M.; Guimarães da Costa, A.; Val, F.; Brasileiro, L.; Sartim, M.A.; et al. Bothrops atrox, the most important snake involved in human envenomings in the amazon: How venomics contributes to the knowledge of snake biology and clinical toxinology. Toxicon X 2020, 6, 100037. [Google Scholar] [CrossRef]
- Oliveira, S.S.; Alves, E.C.; Santos, A.S.; Pereira, J.P.T.; Sarraff, L.K.S.; Nascimento, E.F.; de-Brito-Sousa, J.D.; Sampaio, V.S.; Lacerda, M.V.G.; Sachett, J.A.G.; et al. Factors Associated with Systemic Bleeding in a Tertiary Hospital in the Brazilian Amazon. Toxins 2019, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Moura-da-Silva, A.M.; Contreras-Bernal, J.C.; Cirilo Gimenes, S.N.; Freitas-de-Sousa, L.A.; Portes-Junior, J.A.; da Silva Peixoto, P.; Kei Iwai, L.; Mourão de Moura, V.; Ferreira Bisneto, P.; Lacerda, M.; et al. The relationship between clinics and the venom of the causative Amazon pit viper (Bothrops atrox). PLoS Negl. Trop. Dis. 2020, 14, e0008299. [Google Scholar] [CrossRef]
- Wen, F.H.; Monteiro, W.M.; Moura da Silva, A.M.; Tambourgi, D.V.; da Silva, I.M.; Sampaio, V.S.; dos Santos, M.C.; Sachett, J.; Ferreira, L.C.L.; Kalil, J.; et al. Snakebites and Scorpion Stings in the Brazilian Amazon: Identifying Research Priorities for a Largely Neglected Problem. PLoS Negl. Trop. Dis. 2015, 9, e0003701. [Google Scholar] [CrossRef] [Green Version]
- Escalante, T.; Rucavado, A.; Pinto, A.F.M.; Terra, R.M.S.; Gutierrez, J.M.; Fox, J.W. Wound Exudate as a Proteomic Window to Reveal Different Mechanisms of Tissue Damage by Snake Venom Toxins. J. Proteome Res. 2009, 8, 5120–5131. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Koch, M.; Krieger, A.; Brachvogel, B.; Kreft, S.; Bruckner-Tuderman, L.; Krieg, T.; Shannon, J.D.; Fox, J.W. Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J. Proteome Res. 2010, 9, 4758–4766. [Google Scholar] [CrossRef]
- Macêdo, J.K.A.; Joseph, J.K.; Menon, J.; Escalante, T.; Rucavado, A.; Gutiérrez, J.M.; Fox, J.W. Proteomic Analysis of Human Blister Fluids Following Envenomation by Three Snake Species in India: Differential Markers for Venom Mechanisms of Action. Toxins 2019, 11, 246. [Google Scholar] [CrossRef] [Green Version]
- Rucavado, A.; Escalante, T.; Kalogeropoulos, K.; Camacho, E.; Gutiérrez, J.M.; Fox, J.W. Analysis of wound exudates reveals differences in the patterns of tissue damage and inflammation induced by the venoms of Daboia russelii and Bothrops asper in mice. Toxicon 2020, 186, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Núñez, J.; Gutiérrez, J.M. Blister formation and skin damage induced by BaP1, a haemorrhagic metalloproteinase from the venom of the snake Bothrops asper. Int. J. Exp. Pathol. 1998, 79, 245–254. [Google Scholar] [PubMed]
- Fox, J.W.; Serrano, S.M.T. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon 2005, 45, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Clissa, P.B.; Laing, G.D.; Theakston, R.D.G.; Mota, I.; Taylor, M.J.; Moura-da-Silva, A.M. The effect of jararhagin, a metalloproteinase from Bothrops jararaca venom, on pro-inflammatory cytokines released by murine peritoneal adherent cells. Toxicon 2001, 39, 1567–1573. [Google Scholar] [CrossRef]
- Rucavado, A.; Escalante, T.; Teixeira, C.F.P.; Fernandes, C.M.; Diaz, C.; Gutierrez, J.M. Increments in cytokines and matrix metalloproteinases in skeletal muscle after injection of tissue-damaging toxins from the venom of the snake Bothrops asper. Mediat. Inflamm. 2002, 11, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Moura-da-Silva, A.M.; Butera, D.; Tanjoni, I. Importance of snake venom metalloproteinases in cell biology: Effects on platelets, inflammatory and endothelial cells. Curr. Pharm. Des. 2007, 13, 2893–2905. [Google Scholar] [CrossRef]
- Baramova, E.N.; Shannon, J.D.; Bjarnason, J.B.; Fox, J.W. Degradation of extracellular matrix proteins by hemorrhagic metalloproteinases. Arch. Biochem. Biophys. 1989, 275, 63–71. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Rucavado, A.; Chaves, F.; Diaz, C.; Escalante, T. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon 2009, 54, 958–975. [Google Scholar] [CrossRef]
- Rucavado, A.; Nicolau, C.A.; Escalante, T.; Kim, J.; Herrera, C.; Gutiérrez, J.M.; Fox, J.W. Viperid Envenomation Wound Exudate Contributes to Increased Vascular Permeability via a DAMPs/TLR-4 Mediated Pathway. Toxins 2016, 8, 349. [Google Scholar] [CrossRef] [Green Version]
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Coordenação-Geral de Desenvolvimento da Epidemiologia em Serviços. Guia de Vigilância em Saúde. Chapter 11. p. 69. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_4ed.pdf (accessed on 8 September 2021).
- Pardal, P.P.O.; Souza, S.M.; Monteiro, M.R.C.C.; Fan, H.W.; Cardoso, J.L.C.; Franca, F.O.S.; Tomy, S.C.; Sano-Martins, I.S.; Sousa-e-Silva, M.C.C.; Colombini, M.; et al. Clinical trial of two antivenoms for the treatment of Bothrops and Lachesis bites in the north eastern Amazon region of Brazil. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 28–42. [Google Scholar] [CrossRef]
- Sousa, L.F.; Nicolau, C.A.; Peixoto, P.S.; Bernardoni, J.L.; Oliveira, S.S.; Portes-Junior, J.A.; Mourao, R.H.V.; Lima-dos-Santos, I.; Sano-Martins, I.S.; Chalkidis, H.M.; et al. Comparison of Phylogeny, Venom Composition and Neutralization by Antivenom in Diverse Species of Bothrops Complex. PLoS Negl. Trop. Dis. 2013, 7, e2442. [Google Scholar] [CrossRef] [Green Version]
- Stone, S.F.; Isbister, G.K.; Shahmy, S.; Mohamed, F.; Abeysinghe, C.; Karunathilake, H.; Ariaratnam, A.; Jacoby-Alner, T.E.; Cotterell, C.L.; Brown, S.G. Immune response to snake envenoming and treatment with antivenom; complement activation, cytokine production and mast cell degranulation. PLoS Negl. Trop. Dis. 2013, 7, e2326. [Google Scholar] [CrossRef]
- Riani, M.; Le Jan, S.; Plée, J.; Durlach, A.; Le Naour, R.; Haegeman, G.; Bernard, P.; Antonicelli, F. Bullous pemphigoid outcome is associated with CXCL10-induced matrix metalloproteinase 9 secretion from monocytes and neutrophils but not lymphocytes. J. Allergy Clin. Immunol. 2017, 139, 863–872. [Google Scholar] [CrossRef] [Green Version]
- Hiroyasu, S.; Turner, C.T.; Richardson, K.C.; Granville, D.J. Proteases in Pemphigoid Diseases. Front. Immunol. 2019, 10, 1454. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- Bickler, P.E. Amplification of Snake Venom Toxicity by Endogenous Signaling Pathways. Toxins 2020, 12, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niimi, Y.; Pawankar, R.; Kawana, S. Increased expression of matrix metalloproteinase-2, matrix metalloproteinase-9 and matrix metalloproteinase-13 in lesional skin of bullous pemphigoid. Int. Arch. Allergy Immunol. 2006, 139, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Voelcker, V.; Gebhardt, C.; Averbeck, M.; Saalbach, A.; Wolf, V.; Weih, F.; Sleeman, J.; Anderegg, U.; Simon, J. Hyaluronan fragments induce cytokine and metalloprotease upregulation in human melanoma cells in part by signalling via TLR4. Exp. Dermatol. 2008, 17, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, N.; Escalante, T.; Gutierrez, J.M.; Rucavado, A. Skin pathology induced by snake venom metalloproteinase: Acute damage, revascularization, and re-epithelization in a mouse ear model. J. Investig. Dermatol. 2008, 128, 2421–2428. [Google Scholar] [CrossRef]
- Zomer, H.D.; Trentin, A.G. Skin wound healing in humans and mice: Challenges in translational research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Clinical Data/Patient | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|---|
| Envenomation severity | Moderate | Moderate | Moderate | Severe | Severe | |
| Gender | Male | Female | Male | Male | Male | |
| Bite site | Foot | Foot | Foot | Leg | Foot | |
| Tourniquet use | No | No | No | Yes | No | |
| Time until hospital admission | 5 h | 2 h | 6 h | 11 h | 4 h | |
| Time from hospital admission to blister appearance | 58 h | 135 h | 77 h | 111 h | 80 h | |
| Time from envenomation to blister appearance | 63 h | 137 h | 83 h | 122 h | 84 h | |
| Blister site * | Foot | Foot | Foot | Leg | Foot | |
| Pain | Time 0 | 10 | 8 | 7 | 10 | 10 |
| Time 24 | 4 | 7 | 5 | 0 | 5 | |
| (0 to 10 scale) ** | Time 48 | 0 | 5 | 4 | 0 | 0 |
| Time 72 | 0 | 5 | 3 | 0 | 7 | |
| Time 144 | 0 | 4 | 2 | 8 | 0 | |
| Edema *** | Time 0 | Moderate | Moderate | Mild | Moderate | Moderate |
| Time 24 | Moderate | Moderate | Moderate | Moderate | Severe | |
| Time 48 | Moderate | Moderate | Moderate | Moderate | Severe | |
| Time 72 | Moderate | Moderate | Moderate | Moderate | Moderate | |
| Time 144 | Moderate | Mild | Mild | Moderate | Moderate | |
| Ecchymosis | No | After 48 h | No | No | No | |
| Necrosis | No | No | No | No | No | |
| Infection | No | Yes | Yes | Yes | Yes | |
| Lactic Dehydrogenase # | Time 0 | 464 | 234 | 715 | 935 | 264 |
| Time 24 | 335 | 221 | 501 | 305 | 255 | |
| Time 48 | 308 | 115 | 281 | 286 | 344 | |
| Time 72 | 313 | 283 | 302 | 328 | 255 | |
| Time 144 | 285 | 263 | 289 | 230 | 289 | |
| C-reactive protein ## | Time 0 | 6,5 | 6,5 | 48 | 96 | 6,5 |
| Time 24 | 96 | 96 | 48 | 96 | 48 | |
| Time 48 | 96 | 96 | 192 | 24 | 48 | |
| Time 72 | 24 | 96 | 96 | 48 | 96 | |
| Time 144 | 48 | 96 | 96 | 12 | 48 | |
| Identified Proteins | Ac. Number | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Control Serum |
|---|---|---|---|---|---|---|---|
| Complement C3 | P01024 | 106 | 128 | 69 | 264 | 101 | 122 |
| Complement factor B | P00751 | 2 | 4 | 4 | 4 | 5 | 56 |
| Complement C4-B | P0C0L5 | 0 | 0 | 3 | 28 | 2 | 37 |
| Complement component C7 | P10643 | 0 | 0 | 0 | 0 | 1 | 25 |
| Complement component C9 | P02748 | 0 | 0 | 0 | 0 | 1 | 21 |
| Complement factor H | Q03591 | 0 | 0 | 5 | 0 | 5 | 20 |
| Complement factor I | P05156 | 0 | 0 | 1 | 0 | 0 | 16 |
| Complement C1s | P09871 | 0 | 1 | 2 | 3 | 1 | 16 |
| Complement component C8 | P07357 | 0 | 0 | 2 | 0 | 1 | 15 |
| Complement C1r | Q9NZP8 | 1 | 0 | 0 | 4 | 0 | 13 |
| Complement factor H-related protein 1 | Q03591 | 0 | 0 | 0 | 0 | 1 | 2 |
| Complement C2 | Q8SQ75 | 4 | 5 | 0 | 22 | 2 | 0 |
| Fibrinogen alpha chain | P02671 | 32 | 37 | 11 | 30 | 14 | 13 |
| Fibrinogen gamma chain | P02679 | 1 | 3 | 9 | 7 | 12 | 0 |
| Fibrinogen beta chain | P02675 | 0 | 2 | 12 | 6 | 11 | 0 |
| Serum amyloid A-4 | P35542 | 0 | 0 | 2 | 1 | 2 | 9 |
| Proteoglycan 4 | Q92954 | 0 | 2 | 0 | 0 | 0 | 2 |
| Heat shock 70 kDa protein 1B | P0DMV9 | 6 | 25 | 18 | 6 | 37 | 0 |
| Heat shock protein HSP 90-alpha | P07900 | 0 | 0 | 4 | 0 | 3 | 0 |
| Heat shock protein HSP 90-beta | P08238 | 0 | 0 | 1 | 0 | 2 | 0 |
| Basement membrane-specific heparan sulfate proteoglycan core protein | Q05793 | 0 | 0 | 0 | 1 | 0 | 0 |
| Putative histone H2B type 2-C | Q6DN03 | 0 | 0 | 4 | 2 | 0 | 0 |
| Histone H4 | P35059 | 0 | 8 | 30 | 15 | 0 | 0 |
| Myosin regulatory light chain sqh | P40423 | 0 | 0 | 1 | 0 | 1 | 0 |
| Myosin-9 | P35579 | 0 | 6 | 50 | 6 | 84 | 0 |
| Protein S100-A4 | P26447 | 11 | 10 | 6 | 4 | 4 | 0 |
| Protein S100-A6 | P06703 | 3 | 4 | 2 | 0 | 5 | 0 |
| Protein S100-A8 | P05109 | 2 | 5 | 6 | 8 | 23 | 0 |
| Protein S100-A9 | P06702 | 0 | 10 | 19 | 11 | 44 | 0 |
| Protein S100-A12 | P80511 | 0 | 6 | 6 | 6 | 17 | 0 |
| Protein S100-A11 | P31949 | 2 | 3 | 7 | 4 | 8 | 0 |
| Protein S100-P | P25815 | 3 | 5 | 3 | 3 | 8 | 0 |
| Annexin A1 | P04083 | 0 | 0 | 0 | 1 | 4 | 0 |
| Annexin A3 | P12429 | 0 | 5 | 0 | 4 | 56 | 0 |
| Annexin A5 | Q5R1W0 | 0 | 0 | 0 | 0 | 19 | 0 |
| Annexin A6 | P08133 | 0 | 0 | 0 | 0 | 4 | 0 |
| Alpha-2-HS-glycoprotein | P02765 | 30 | 15 | 5 | 25 | 3 | 39 |
| Vitronectin | P04004 | 2 | 6 | 2 | 4 | 6 | 32 |
| Fibronectin | P04937 | 0 | 1 | 4 | 0 | 2 | 17 |
| Lumican | P51884 | 7 | 0 | 1 | 14 | 0 | 10 |
| Proteoglycan 4 | Q92954 | 0 | 2 | 0 | 0 | 0 | 2 |
| EGF-containing fibulin-like extracellular matrix protein 1 | O35568 | 0 | 0 | 1 | 1 | 0 | 2 |
| Collagen alpha-1(III) chain | P02461 | 0 | 0 | 0 | 1 | 0 | 0 |
| Collagen alpha-1(I) chain | P02452 | 1 | 0 | 2 | 12 | 3 | 0 |
| Collagen alpha-1(VI) chain | P12109 | 0 | 0 | 0 | 4 | 0 | 0 |
| Collagen alpha-1(XXVII) chain | Q5QNQ9 | 0 | 0 | 1 | 0 | 0 | 0 |
| Collagen alpha-3(VI) chain | P12111 | 0 | 0 | 1 | 0 | 0 | 0 |
| Matrix metalloproteinase-9 | P14780 | 0 | 1 | 6 | 1 | 13 | 0 |
| Olfactomedin-4 | Q6UX06 | 0 | 0 | 0 | 4 | 21 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimenes, S.N.C.; Sachett, J.A.G.; Colombini, M.; Freitas-de-Sousa, L.A.; Ibiapina, H.N.S.; Costa, A.G.; Santana, M.F.; Park, J.-J.; Sherman, N.E.; Ferreira, L.C.L.; et al. Observation of Bothrops atrox Snake Envenoming Blister Formation from Five Patients: Pathophysiological Insights. Toxins 2021, 13, 800. https://doi.org/10.3390/toxins13110800
Gimenes SNC, Sachett JAG, Colombini M, Freitas-de-Sousa LA, Ibiapina HNS, Costa AG, Santana MF, Park J-J, Sherman NE, Ferreira LCL, et al. Observation of Bothrops atrox Snake Envenoming Blister Formation from Five Patients: Pathophysiological Insights. Toxins. 2021; 13(11):800. https://doi.org/10.3390/toxins13110800
Chicago/Turabian StyleGimenes, Sarah N. C., Jacqueline A. G. Sachett, Mônica Colombini, Luciana A. Freitas-de-Sousa, Hiochelson N. S. Ibiapina, Allyson G. Costa, Monique F. Santana, Jeong-Jin Park, Nicholas E. Sherman, Luiz C. L. Ferreira, and et al. 2021. "Observation of Bothrops atrox Snake Envenoming Blister Formation from Five Patients: Pathophysiological Insights" Toxins 13, no. 11: 800. https://doi.org/10.3390/toxins13110800
APA StyleGimenes, S. N. C., Sachett, J. A. G., Colombini, M., Freitas-de-Sousa, L. A., Ibiapina, H. N. S., Costa, A. G., Santana, M. F., Park, J.-J., Sherman, N. E., Ferreira, L. C. L., Wen, F. H., Monteiro, W. M., Moura-da-Silva, A. M., & Fox, J. W. (2021). Observation of Bothrops atrox Snake Envenoming Blister Formation from Five Patients: Pathophysiological Insights. Toxins, 13(11), 800. https://doi.org/10.3390/toxins13110800









