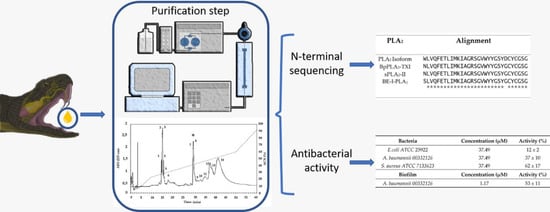
Antibiofilm Activity of Acidic Phospholipase Isoform Isolated from Bothrops erythromelas Snake Venom
Abstract
1. Introduction
2. Results
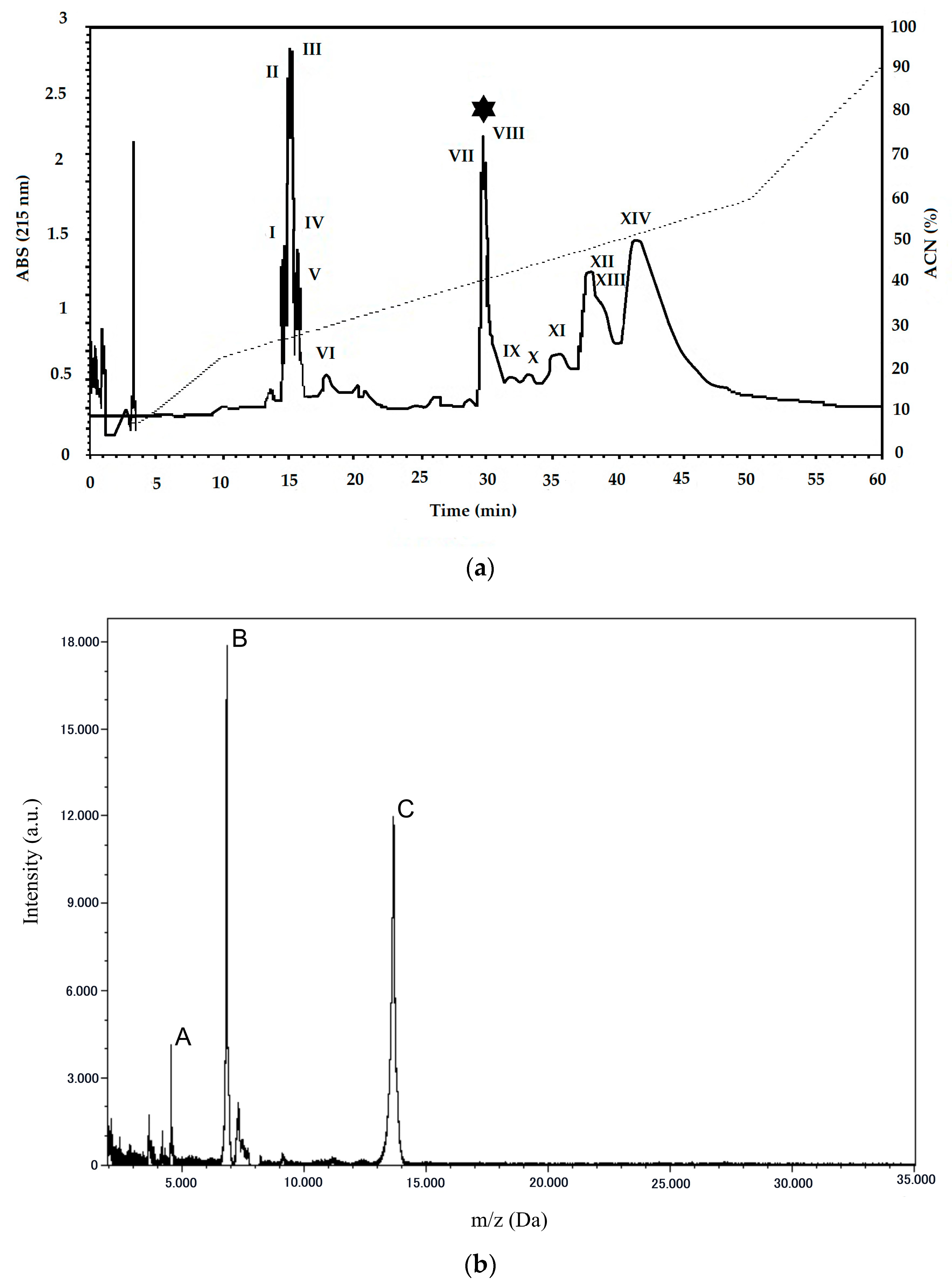
2.1. Purification and Characterization of PLA2 from B. erythromelas
2.2. Hemolytic Activity Assays
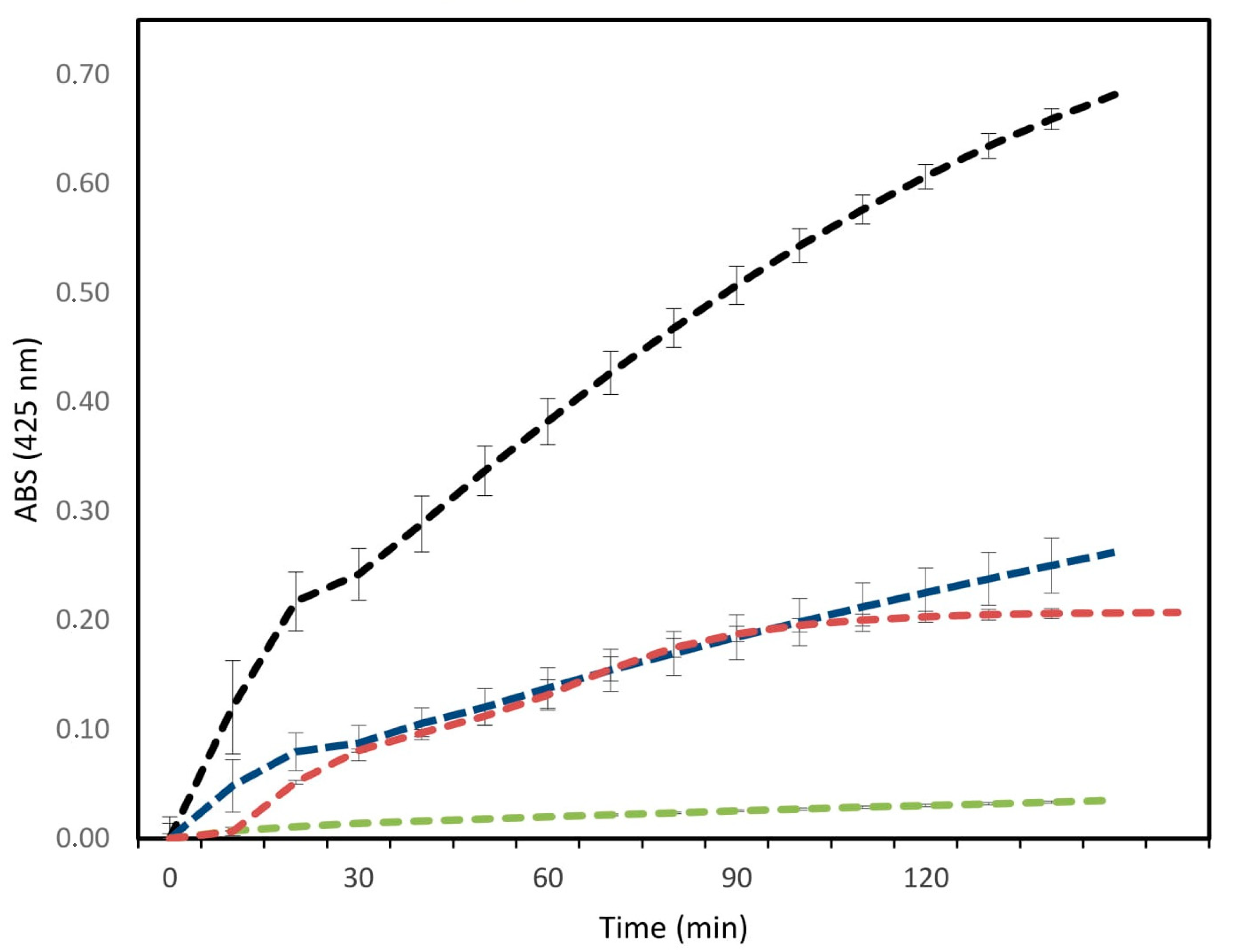
2.3. Antibacterial and Antibiofilm Activity
3. Discussion
4. Conclusions
5. Methods
5.1. B. erythromelas Venom Extraction
5.2. Quantification of Venom Proteins
5.3. Purification of Venom Proteins
5.4. Phospholipase Activity
5.5. Mass Spectrometry
5.6. Amino-Terminal Sequencing of PLA2 from B. erythromelas
5.7. Hemolysis Test
5.8. Antibacterial Activity
5.9. Antibiofilm Activity
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Livermore, D.M. Minimizing antibiotic resistance. Lancet Infect. Dis. 2005, 5, 450–459. [Google Scholar] [CrossRef]
- Loureiro, R.J.; Roque, F.; Rodrigues, A.T.; Herdeiro, M.T.; Ramalheira, E. O uso de antibióticos e as resistências bacterianas: Breves notas sobre a sua evolução. Rev. Port. Saúde Pública 2016, 34, 77–84. [Google Scholar] [CrossRef]
- World Health Organization. Containing Antimicrobial Resistance; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/antimicrobial-resistance/en/ (accessed on 23 June 2019).
- Prevenção e Controle de Infecções e de Resistência aos Antimicrobianos–2017: Programa de Prevenção e Controle de Infecções e de Resistência aos Antimicrobianos 45. Available online: https://www.sns.gov.pt/wp-content/uploads/2017/12/DGS_PCIRA_V8.pdf (accessed on 15 January 2019).
- Costerton, J.W.; Irvin, R.T.; Cheng, K.J. The Bacterial Glycocalyx in Nature and Disease. Annu. Rev. Microbiol. 1981, 35, 299–324. [Google Scholar] [CrossRef]
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.M.M.; Brugnera, D.F.; Piccoli, R.H. Biofilmes microbianos na indústria de alimentos: Uma revisão. Rev. Inst. Adolfo Lutz 2010, 69, 277–284. [Google Scholar]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [PubMed]
- West, S.A.; Winzer, K.; Gardner, A.; Diggle, S.P. Quorum sensing and the confusion about diffusion. Trends Microbiol. 2012, 20, 586–594. [Google Scholar] [CrossRef]
- Trentin, D.; Giordani, R.; Macedo, A. Biofilmes bacterianos patogênicos: Aspectos gerais, importância clínica e estratégias de combate. Rev. Lib. 2013, 14, 213. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targests for developing antibiofim agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Costerton, L.W.; Lewandowski, Z.; Debeer, D.; Caldwell, D.; Korber, D.; James, G. Biofilms, the customized microniche. J. Bacteriol. 1994, 176, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Mukherjee, P.K. Candida Biofilms: Development, Architecture, and Resistance. Microbiol. Spectr. 2015, 3, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, T.J.; Graham, G.G.; Scott, K.F. Antibacterial actions of secreted phospholipases A2. Rev. Biochim. Biophys. Acta 2008, 1781, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Samy, R.P.; Gopalakrishnakone, P.; Chow, V.T.K.; Ho, B. Viper metalloproteinase (Agkistrodon halyspallas) with antimicrobial activity against multi-drug resistant human pathogens. J. Cell Physiol. 2008, 216, 54–68. [Google Scholar] [CrossRef]
- Moreira, V.; De Castro Souto, P.C.; Ramirez Vinolo, M.A.; Lomonte, B.; Gutiérrez, J.M.; Curi, R.; Teixeira, C. A catalytically-inactive snake venom Lys49 phospholipase A2 homolog induces expression of cyclooxygenase-2 and production of prostaglandins through selected signaling pathways in macrophages. Eur. J. Pharmacol. 2013, 708, 68–79. [Google Scholar] [CrossRef]
- Dias, R.G.; Sampaio, S.C.; Sant’anna, M.B.; Cunha, F.Q.; Gutiérrez, J.M.; Lomonte, B.; Cury, Y.; Picolo, G. Articular inflammation induced by an enzymatically-inactive Lys49 phospholipase A2: Activation of endogenous phospholipases contributes to the pronociceptive effect. J. Venom. Anim. Toxins Trop. Dis. 2017, 23, 1–13. [Google Scholar] [CrossRef]
- Vindas, J.; Carrera, Y.; Lomonte, B.; Gutiérrez, J.M.; Calvete, J.J.; Sanz, L.; Fernández, J. A novel pentameric phospholipase A2myotoxin (PophPLA2) from the venom of the pit viper porthidium ophryomegas. Int. J. Biol. Macromol. 2018, 118, 1–8. [Google Scholar] [CrossRef]
- Scott, D.L.; White, S.P.; Otwinowski, Z.; Yuan, W.; Gelb, M.H.; Singler, P.B. Interfacial catalysis: The mechanism of phospholipase A2. Science 1990, 250, 1541–1546. [Google Scholar] [CrossRef]
- Schaloske, R.H.; Dennis, E.A. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta 2006, 1761, 1246–1259. [Google Scholar] [CrossRef]
- De Maria, L.; Vind, J.; Oxenboll, K.M.; Svendsen, A.; Patkar, S. Phospholipases and their industrial applications. Appl. Microbiol. Biotechnol. 2007, 74, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Chaves, F.; Díaz, C.; Escalante, T. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon 2009, 54, 958–975. [Google Scholar] [CrossRef]
- Jorge, R.J.B.; Monteiro, H.S.; Gonçalves-Machado, L.; Guarnieri, M.C.; Ximenes, R.M.; Borges-Nojosa, D.M.; Luna, K.P.; Zingali, R.B.; Corrêa-Netto, C.; Gutiérrez, J.M.; et al. Venomics and antivenomics of Bothrops erythromelas from five geographic populations within the Caatinga ecoregion of northeastern Brazil. J. Proteom. 2015, 30, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Nery, N.M.; Luna, K.P.O.; Fernandes, C.F.C.; Zuliani, J.P. An overview of Bothrops erythromelas venom. Rev. Soc. Bras. Med. Trop. 2016, 49, 680–686. [Google Scholar] [CrossRef]
- Kini, R.M.; Evans, H.J. A model to explain the pharmacological effects of snake venom phospholipases A2. Toxicon 1989, 27, 613–635. [Google Scholar] [CrossRef]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef]
- Soares, A.; Fontes, M.; Giglio, J. Phospholipase A2 Myotoxins from Bothrops Snake Venoms: Structure-Function Relationship. Curr. Org. Chem. 2004, 8, 1677–1690. [Google Scholar] [CrossRef]
- Montecucco, C.O.; Rossetto, O.; Caccin, P.; Rigoni, M.; Carli, L.; Morbiato, L.; Muraro, L.; Paoli, M. Different mechanisms of inhibition of nerve terminals by botulinum and snake presynaptic neurotoxins. Toxicon 2009, 54, 561–564. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 2013, 62, 27–39. [Google Scholar] [CrossRef]
- Rodrigues, R.S.; Boldrini-França, J.; Fonseca, F.P.; De La Torre, P.; Henrique-Silva, F.; Sanz, L.; Calvete, J.J.; Rodrigues, V.M. Combined snake venomics and venom gland transcriptomic analysis of Bothropoides pauloensis. J. Proteom. 2012, 75, 2707–2720. [Google Scholar] [CrossRef]
- Yunes Quartino, P.J.; Barra, J.L.; Fidelio, G.D. Cloning and functional expression of secreted phospholipases A2 from Bothrops diporus (Yarará Chica). Biochem. Biophys. Res. Commun. 2012, 427, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Modesto, J.C.A.; Spencer, P.J.; Fritzen, M.; Valença, R.C.; Oliva, M.L.V.; Silva, M.B.; Chudzinski-Tavassi, A.M.; Guarnieri, M.C. BE-I-PLA2, a novel acidic phospholipase A2 from Bothrops erythromelas venom: Isolation, cloning and characterization as potent anti-platelet and inductor of prostaglandin I2 release by endothelial cells. Biochem. Pharmacol. 2006, 72, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Garcia Denegri, M.E.; Acosta, O.C.; Huancahuire-Veja, S.; Martins-De-Souza, D.; Marangoni, S.; Maruñak, S.L.; Teibler, G.P.; Leiva, L.C.; Ponce-Soto, L.A. Isolation and functional characterization of a new acidic PLA2 Ba SpII RP4 of the Bothrops alternatus snake venom from Argentina. Toxicon 2010, 56, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Pereanez, J.A.; Quintana, J.C.; Alarcón, J.C.; Núñez, V. Isolation and functional characterization of a basic phospholipase A2 from Colombian Bothrops asper venom. Vitae Rev. La Fac. Química Farm. 2014, 21, 38–48. [Google Scholar]
- Corrêa, E.A.; Kayano, A.M.; Diniz-Sousa, R.; Setúbal, S.S.; Zanchi, F.B.; Zuliani, J.P.; Matos, N.B.; Almeida, J.R.; Resende, L.M.; Marangoni, S.; et al. Isolation, structural and functional characterization of a new Lys49 phospholipase A2 homologue from Bothrops neuwiedi urutu with bactericidal potential. Toxicon 2016, 115, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Menaldo, D.L.; Jacob-Ferreira, A.L.; Bernardes, C.P.; Cintra, A.C.O.; Sampaio, S.V. Purification procedure for the isolation of a P-I metalloprotease and an acidic phospholipase A2 from Bothrops atrox snake venom. J. Venom. Anim. Toxins Trop. Dis. 2015, 21, 1–14. [Google Scholar] [CrossRef]
- Cedro, R.C.A.; Menaldo, D.L.; Costa, T.R.; Zoccal, K.F.; Sartim, M.A.; Santos-Filho, N.A.; Faccioli, S.H.; Sampaio, S.V. Cytotoxic and inflammatory potential of a phospholipase A2 from Bothrops jararaca snake venom. J. Venom. Anim. Toxins Trop. Dis. 2018, 24, 1–14. [Google Scholar] [CrossRef]
- De Roodt, A.; Fernández, J.; Solano, D.; Lomonte, B. A myotoxic Lys49 phospholipase A2-homologue is the major component of the venom of Bothrops cotiara from Misiones, Argentine. Toxicon 2018, 148, 143–148. [Google Scholar] [CrossRef]
- Fagundes, F.H.R.; Aparicio, R.; Dos Santos, M.L.; Diz, E.B.S.; Oliveira, S.C.B.; Toyama, D.O.; Toyama, M.H. A Catalytically Inactive Lys49 PLA2 Isoform from Bothrops jararacussu venom that Stimulates Insulin Secretion in Pancreatic Beta Cells. Protein Pept. Lett. 2011, 18, 1133–1139. [Google Scholar] [CrossRef]
- Galbiatti, C.; Rocha, T.; Randazzo-Moura, P.; Ponce-Soto, L.A.; Marangoni, S.; Cruz-Höfling, M.A.; Rodrigues-Simioni, L. Pharmacological and partial biochemical characterization of Bmaj-9 isolated from Bothrops marajoensis snake venom. J. Venom. Anim. Toxins Trop. Dis. 2012, 18, 62–72. [Google Scholar] [CrossRef][Green Version]
- Fernandes, C.A.H.; Borges, R.J.; Lomonte, B.; Fontes, M.R.M. A structural-based proposal for a comprehensive myotoxic mechanism of phospholipase A2-like proteins from viperid snake venoms. Biochim. Biophys. Acta 2014, 1844, 2265–2276. [Google Scholar] [CrossRef]
- Silveira, L.B.; Marchi-Salvador, D.P.; Santos-Filho, N.A.; Silva, F.P., Jr.; Marcussi, S.; Fuly, A.L.; Nomizo, A.; Da Silva, S.L.; Stábeli, R.G.; Arantes, E.C.; et al. Isolation and expression of a hypotensive and anti-platelet acidic phospholipase A2 from Bothrops moojeni snake venom. J. Pharm. Biomed. Anal. 2013, 73, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.J.; Londoño, M.; Quintana, J.C.; Rua, C.; Segura, C.; Lomonte, B.; Núñez, V. An acidic phospholipase A2 with antibacterial activity from Porthidium nasutum snake venom. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 161, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.L.; Quartino, P.Y.; Arce-Bejarano, R.; Fernadez, J.; Camacho, L.F.; Gutiérrez, J.M.; Kuemmel, D.; Fidelio, G.; Lomonte, B. Intravascular hemolysis induced by phospholipase A2 from the venom of the Eastern coral snake, Micrurus fulvius: Functional profiles of hemolytic and non-hemolytic isoforms. Toxicol. Lett. 2018, 286, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Costa-Torres, A.F.; Dantas, R.T.; Toyama, M.H.; Diz Filho, E.; Zara, F.J.; Rodrigues De Queiroz, M.G.; Pinto Nogueira, N.A.; Rosa De Oliveira, M.; De Oliveira Toyama, D.; Monteiro, H.S.; et al. Antibacterial and antiparasitic effects of Bothrops marajoensis venom and its fractions: Phospholipase A2 and L-aminoacid oxidase. Toxicon 2010, 55, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Sousa, R.; Caldeira, C.A.S.; Kayano, A.M.; Paloschi, M.V.; Pimenta, D.C.; Simôes-Silva, R.; Ferreira, A.S.; Zanchi, F.B.; Matos, N.B.; Grabner, F.P.; et al. Identification of the Molecular Determinants of the Antibacterial Activity of LmutTX, a Lys49 Phospholipase A2 Homologue Isolated from Lachesis muta muta Snake Venom (Linnaeus, 1766). Basic Clin. Pharm. Toxicol. 2018, 122, 413–423. [Google Scholar] [CrossRef]
- Sudharshan, S.; Dhananjaya, B.L. Antibacterial potential of a basic phospholipase A2 (VRV-PL-VIIIa) from Daboia russelii pulchella (Russell’s viper) venom. J. Venom. Anim. Toxins Trop. Dis. 2015, 21, 1–8. [Google Scholar] [CrossRef][Green Version]
- Yan, X.M.; Zhang, S.Q.; Chang, Q.; Liu, P.; Xu, J.S. Antibacterial and antifungal effects of Agkistrodon halys Pallas: Purification of its antibacterial protein-LAO. Shi Yan Sheng Wu Xue Bao 2000, 33, 309–316. [Google Scholar]
- Soares, A.M.; Guerra-Sa, R.; Borja-Oliveira, C.R.; Rodrigues, V.M.; Rodrigues-Simioni, L.; Rodrigues, V.; Fontes, M.R.M.; Lomonte, B.; Gutierrez, J.M.; Giglio, J.R. Structural and functional characterization of BnSP-7, a Lys49 myotoxic phospholipase A2 homologue from Bothrops neuwiedi pauloensis venom. Arch. Biochem. Biophys. 2000, 378, 201–209. [Google Scholar] [CrossRef]
- Furtado, M.F.D. Biological and immunological properties of the venom of Bothrops alcatraz, and endemic species of pitviper from Brazil. Comp. Biochem. Physiol. 2005, 141, 117–123. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide Endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- De Nemacetin-Anvisa, B. Available online: http://www.anvisa.gov.br/datavisa/fila_bula/frmVisualizarBula.asp?pNuTransacao=21331052016&pIdAnexo=3776379 (accessed on 10 September 2019).
- Villa, F.; Cappitelli, F. Plant-derived bioactive compounds at sub-lethal concentrations towards smart biocide-free antibiofilm strategies. Phytochem. Rev. 2013, 12, 245–254. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigsten, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- O’gara, J.P. Ica and beyond: Biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 2007, 270, 179–188. [Google Scholar] [CrossRef]
- Bocian, A.; Ciszkowicz, E.; Hus, K.K.; Buczkowicz, J.; Lecka-Szlachta, K.; Pietrowska, M.; Petrilla, V.; Petrillova, M.; Legath, L.; Legáth, J. Antimicrobial Activity of Protein Fraction from Naja ashei Venom against Staphylococcus epidermidis. Molecules 2020, 25, 293. [Google Scholar] [CrossRef]
- Blower, R.J.; Barksdale, S.M.; Van Hoek, M.L. Snake Cathelicidin NA-CATH and Smaller Helical Antimicrobial Peptides Are Effective against Burkholderia thailandensis. PLoS Negl. Trop. Dis. 2015, 9, 1–16. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Akhavan, M.M.; Fallah, F.; Karimi, A. A Recombinant Snake Cathelicidin Derivative Peptide: Antibiofilm Properties and Expression in Escherichia coli. Biomolecules 2018, 8, 118. [Google Scholar] [CrossRef]
- Mohamed, F.M.; Brezden, A.; Mohammad, H.; Chmielewski, J.; Seleem, M.N. A short D-enantiomeric antimicrobial peptide with potent immunomodulatory and antibiofilm activity against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Klein, R.C.; Fabres-Klein, M.H.; De Oliveira, L.L.; Feio, R.N.; Malouin, F.; Ribon, A.D.O.B. A C-Type Lectin from Bothrops jararacussu Venom Disrupts Staphylococcal Biofilms. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef]
- Canhas, I.N.; Heneine, L.G.D.; Fraga, T.; Assis, D.C.S.; Borges, M.H.; Chartone-Souza, E.; Nascimento, A.M.A. Antibacterial activity of different types of snake venom from the Viperidae family against Staphylococcus aureus. Acta Sci. Biol. Sci. 2017, 39, 309–319. [Google Scholar] [CrossRef][Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Holzer, M.; Mackessy, S. An aqueous endpoint assay of snake venom phospholipase A2. Toxicon 1996, 34, 1149–1155. [Google Scholar] [CrossRef]
- Serino-Silva, C.; Morais-Zani, K.; Toyama, M.H.; Toyama, D.O.; Gaeta, H.H.; Rodrigues, C.F.B.; Aguiar, W.S.; Tashima, A.K.; Grego, K.F.; Tanaka-Azevedo, A.M. Purification and characterization of the first γ-phospholipase inhibitor (γPLI) from Bothrops jararaca snake serum. PLoS ONE 2018, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.H.; Ribeiro, S.M.; Nolasco, D.O.; De La Fuente-Núñez, C.; Felício, M.R.; Gonçalves, S.; Mattos, O.C.; Liao, L.M.; Santos, N.C.; Hancock, R.E.W.; et al. A polyalanine peptide derived from polar fish with anti-infectious activities. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Hecht, D.W.; Citron, D.M.; Dzink-Fox, J.; Gregory, W.W.; Jacobus, N.V.; Jenkins, S.G.; Rosenblatt, J.E.; Schuetz, A.N.; Wexler, H. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, Approved Standard, 8th ed.; CLSI Document M11-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA; Annapolis Junction, MD, USA, 2012; pp. 1–39. [Google Scholar]
- De La Fuente-Núnez, C.; Reffuveille, F.; Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response. PLoS Pathog. 2014, 10, 1–12. [Google Scholar] [CrossRef]
- De La Fuente-Núnez, C.; Reffuveille, F.; Mansour, S.C.; Reckseidler-Zenteno, S.L.; Hernández, D.; Brackman, G.; Coenye, T.; Hancock, R.E. D-enantiomeric peptides that eradicate wild-type and multi-drug resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem. Biol. 2015, 22, 196–205. [Google Scholar] [CrossRef]
- Naves, P.; Del Prado, G.; Huelves, L.; Gracia, M.; Ruiz, V.; Blanco, J.; Soriano, F. Correlation between virulence factors and in vitro biofilm formation by Escherichia coli strains. Microb. Pathog. 2008, 45, 86–91. [Google Scholar] [CrossRef]
| Species | Access Number | PLA2 | Alignment | Homology (%) | Charge |
|---|---|---|---|---|---|
| B. erythromelas | - | PLA2 Isoform | WLVQFETLIMKIAGRSGVWYYGSYDCYCGSG | - | 0 |
| B. pauloensis | D0UGJ0.1 | BpPLA2-TXI | NLVQFETLIMKIAGRSGVWYYGSYGCYCGSG | 96 | +1 |
| B. diporus | AFJ79208.1 | sPLA2-II | NLVQFETLIMKIAGRSGVWYYGSYGCYCGSG | 96 | +1 |
| B. erythromelas | Q2HZ28.1 | BE-I-PLA2 | SLVQFETLIMKIAGRSGVWYYGSYGCYCGSG | 96 | +1 |
| ***********************.****** |
| Bacteria | Concentration (µM) | Ciprofloxacin (%) | Activity PLA2 (%) | IC50 PLA2 (µM) |
|---|---|---|---|---|
| E. coli ATCC 25922 | 37.4 | 97 ± 16 | 12 ± 20 | - |
| S. aureus ATCC 7133623 | 37.4 | 90 ± 18 | 62 ± 17 | 30.2 |
| A. baumannii 00332126 | 37.4 | 0 | 37 ± 10 | - |
| Biofilm | ||||
| A. baumannii 00332126 | 1.17 | 7 ± 6 | 53 ± 11 | 1.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, E.; Frihling, B.; Barros, E.; de Oliveira, C.; Verbisck, N.; Flores, T.; de Freitas Júnior, A.; Franco, O.; de Macedo, M.; Migliolo, L.; et al. Antibiofilm Activity of Acidic Phospholipase Isoform Isolated from Bothrops erythromelas Snake Venom. Toxins 2020, 12, 606. https://doi.org/10.3390/toxins12090606
Nunes E, Frihling B, Barros E, de Oliveira C, Verbisck N, Flores T, de Freitas Júnior A, Franco O, de Macedo M, Migliolo L, et al. Antibiofilm Activity of Acidic Phospholipase Isoform Isolated from Bothrops erythromelas Snake Venom. Toxins. 2020; 12(9):606. https://doi.org/10.3390/toxins12090606
Chicago/Turabian StyleNunes, Ellynes, Breno Frihling, Elizângela Barros, Caio de Oliveira, Newton Verbisck, Taylla Flores, Augusto de Freitas Júnior, Octávio Franco, Maria de Macedo, Ludovico Migliolo, and et al. 2020. "Antibiofilm Activity of Acidic Phospholipase Isoform Isolated from Bothrops erythromelas Snake Venom" Toxins 12, no. 9: 606. https://doi.org/10.3390/toxins12090606
APA StyleNunes, E., Frihling, B., Barros, E., de Oliveira, C., Verbisck, N., Flores, T., de Freitas Júnior, A., Franco, O., de Macedo, M., Migliolo, L., & Luna, K. (2020). Antibiofilm Activity of Acidic Phospholipase Isoform Isolated from Bothrops erythromelas Snake Venom. Toxins, 12(9), 606. https://doi.org/10.3390/toxins12090606